Gender effects on cortical thickness and the influence of scaling
Abstract
Using magnetic resonance imaging and well-validated computational cortical pattern matching methods in a large and well-matched sample of healthy subjects (n = 60), we analyzed the regional specificity of gender-related cortical thickness differences across the lateral and medial cortices at submillimeter resolution. To establish the influences of brain size correction on gender effects, comparisons were performed with and without applying affine transformations to scale each image volume to a template. We revealed significantly greater cortical thickness in women compared to men, after correcting for individual differences in brain size, while no significant regional thickness increases were observed in males. The pattern and direction of the results were similar without brain size correction, although effects were less pronounced and a small cortical region in the lateral temporal lobes showed greater thickness in males. Our gender-specific findings support a dimorphic organization in male and female brains that appears to involve the architecture of the cortical mantle and that manifests as increased thickness in female brains. This sexual dimorphism favoring women, even without correcting for brain size, may have functional significance and possibly account for gender-specific abilities and/or behavioral differences between sexes. Hum Brain Mapp, 2005. © 2005 Wiley-Liss, Inc.
INTRODUCTION
Advances in imaging methods and analysis tools have provided new insights into the sexual dimorphisms of the human brain. For example, prior brain imaging studies have identified gender-specific differences in regions of interest [Frederikse et al., 1999; Goldstein et al., 2001; Nopoulos et al., 2000; Paus et al., 1996; Schlaepfer et al., 1995] or over the whole brain or cortex using measures such as curvature, cortical complexity, volume and surface area, and gray matter (GM) volume and concentration [Barta and Dazzan, 2003; Goldstein et al., 2001; Good et al., 2001a; Luders et al., 2004; Nopoulos et al., 2000]. The thickness of the cortex reflects the underlying cyto- and myeloarchitecture of the brain (the organization of cortical layers, the size, number, and density of neuronal cell bodies and/or synaptic connections, and the myelination of fibers). The analysis of cortical thickness, although related to regional measures of cortical GM volume and concentration, may thus provide new insights into gender-related differences in brain morphology.
With novel computational image analysis algorithms [Fischl and Dale, 2000; Jones et al., 2000; Kabani et al., 2001; Lerch and Evans, 2005; Memoli et al., 2004], cortical thickness can be measured over the entire cortex, but few magnetic resonance imaging (MRI) studies have analyzed gender differences in cortical thickness specifically. Some studies, however, have examined gender effects while assessing neurodevelopmental or disease-related hypotheses [Kuperberg et al., 2003; Narr et al., 2004; Salat et al., 2004; Sowell et al., 2004]. Specifically, Narr et al. [2004] examined sex differences collapsed across groups of schizophrenia patients and healthy controls, showing thicker parietal cortices in women and thicker medial frontal regions in men. Cortical thickness differences were not measured in healthy subjects separately, and patients with schizophrenia showed decreased cortical thickness in several cortical regions, while gender interactions were present. A different study conducted to investigate the influence of aging revealed a trend toward larger global thickness in males in the left and right hemispheres [Salat et al., 2004], while another study revealed no significant differences between men and women [Nopoulos et al., 2000].
In the context of sparse and inconsistent results concerning gender differences in cortical thickness, our goal was to examine gender effects on regional thickness across the entire lateral and medial cortices with submillimeter resolution in a large and well-matched sample of healthy subjects. Young adults with a relatively narrow age range were selected to minimize the influences of age and possible interactions of age with gender. These have been found to influence brain tissue volumes and cortical thickness measures in previous studies [Courchesne et al., 2000; De Bellis et al., 2001; Good et al., 2001b; Sowell et al., 2003, 2004]. We further aimed to establish the presence and direction of gender differences in cortical thickness using both scaled (after transforming images into standard ICBM-305 space applying 12-parameter transformations) and unscaled imaging data (after applying 6-parameter rigid-body transformations). Many previous studies assessing gender differences in GM concentration using voxel-based morphometry [VBM; Ashburner and Friston, 2000] and sulcal pattern matching approaches [Thompson et al., 2004] have conducted analyses on data scaled to a template using linear or nonlinear registrations. These normalization procedures are performed to control for global shape and brain size differences. The optimized VBM procedure [Ashburner and Friston, 2000; Good et al., 2001b] contains a volume-preserving step (by modulating the intensity values in the segmented images by the Jacobian determinants, or volume expansion factors, derived from the spatial normalization). Even so, the issue of how spatial normalizations alter gender differences in morphological features has been largely neglected. We hypothesized that gender effects would parallel previous findings of global increases of GM percentages and proportions, as well as larger cortical GM volume and concentration in normalized (scaled) data in females compared to males [Goldstein et al., 2001; Good et al., 2001a; Gur et al., 1999; Luders et al., 2002]. Specifically, we expected regionally increased cortical thickness in women after brain size normalizations. In contrast, we expected gender differences to be less pronounced when examining brains in their original dimensions (without scaling).
SUBJECTS AND METHODS
Subjects and MRI Acquisition
We analyzed the brains of 60 right-handed, healthy subjects selected from a database of high-resolution anatomical MR images acquired at the Center for Neuroscientific Innovation and Technology (ZENIT), Magdeburg, Germany. Male and female subjects were matched in terms of numbers (30 women, 30 men) and age (women: 24.32 ± 4.35 years; men: 25.45 ± 4.72 years). Handedness was determined by referring to self-reports of hand preference. Subjects were volunteers and included university students from different faculties who were recruited via notice board and/or Internet advertisements. All subjects gave informed consent according to institutional guidelines (Ethics Committee of the University of Magdeburg).
Images were obtained on a 1.5 T MRI system (General Electric, Waukesha, WI) using a T1-weighted spoiled gradient echo pulse sequence with the following parameters: TR = 24 ms, TE = 8 ms, 30° flip angle, FOV = 250 × 250 mm2, matrix size = 256 × 256 × 124, voxel size = 0.98 × 0.98 × 1.5 mm.
Image Processing
Image volumes passed through a number of preprocessing steps using several manual and automated procedures implemented using the LONI (Laboratory of Neuro Imaging) Pipeline Processing Environment [Rex et al., 2003]. First, we created an intracranial mask of the brain using a brain surface extraction algorithm tool (BSE) that is based on a combination of nonlinear smoothing, edge finding, and morphologic processing [Shattuck and Leahy, 2002]. Any small errors in the masks were corrected manually to separate intracranial regions from surrounding extracranial tissue. Using these modified brain masks, all extracerebral tissues were removed from the image volumes. Brain masks and anatomical images were corrected for head alignment and individual differences in brain size by using an automatic 12-parameter linear registration [Woods et al., 1998] to transform each brain volume into the target space of the ICBM-305 average brain created by the International Consortium for Brain Mapping [Mazziotta et al., 1995]. After applying radiofrequency (RF) bias field corrections to eliminate intensity drifts due to magnetic field inhomogeneities, each image volume was segmented into different tissue types by classifying voxels based on their signal intensity values [Shattuck et al., 2001], followed by separating the left hemisphere from the right. Tissue classified brain volumes were resampled to 0.33 mm cubic voxels to improve the spatial resolution and precision of subsequent thickness measurements. These preprocessing steps are summarized and illustrated in Figure 1.
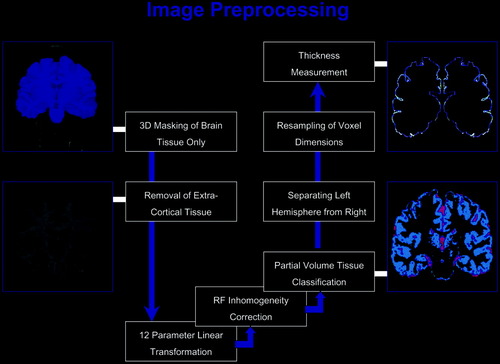
Summary of image preprocessing steps.
Cortical Thickness Measurement
Cortical thickness—defined as the 3-D distance (in mm) between the inner GM–white matter (WM) border and the closest point on the outer surface (CSF–GM border)—was calculated using the Eikonal fire equation [Memoli et al., 2004; Sapiro, 2001; Thompson et al., 2004] applied to voxels that were classified as GM. More specifically, we identified the GM–WM interface as the set of voxels classified as GM that have at least one neighboring WM voxel, setting the distance values for this layer of voxels to zero [similar to the methods used by Miller et al., 2000; Ratnanather et al., 2001; Lohmann et al., 2003]. In order to estimate cortical thickness, we coded successive layers of voxels by assigning them a value equal to the closest 3-D distance to the GM–WM interface. That is, in a series of subsequent passes over the image (from inside to outside), layers that are adjacent to the voxel layer coded in the last step will be processed and assigned a distance value. The primary reason for this wavefront propagation is to prevent distances from propagating across cerebrospinal fluid (CSF) by setting the rules for serial propagation in such a way that this is forbidden. Essentially, the process computes the shortest distance from a given GM voxel to the nearest WM voxel, avoiding solutions where this line would pass through CSF. Avoidance of WM voxels is not necessary since the existence of a shortcut across WM automatically implies that the shortest path has not yet been found [Thompson et al., 2005].
Our approach circumvents the need to find an accurate surface representation of the GM–CSF interface, which can be difficult or impossible if no CSF appears to separate gyri across the walls of a deep sulcus. The current method will code GM voxels on either side of the sulcus with increasing distance values in a series of passes over the data until they meet in the middle. This method suffers from ambiguity when two GM surfaces meet without being separated by a CSF intensity voxel, due to partial voluming. In this case, the method must effectively assume that the boundary lies at the point that produces equal thickness for both of the opposing GM surfaces. However, this has virtually no impact on the results; for example assigning both surfaces 4 mm is effectively indistinguishable from assigning 3 mm and the other 5 mm (which might be the true values) due to the subsequent smoothing [Thompson et al., 2005].
We used volumetric (3-D) smoothing for the cortical thickness values, as the local maxima of the thickness field are defined in a volumetric format. That is, a local smoothing kernel of radius 15 mm is defined at each vertex on the cortical surface model. The mean distance code is computed for all voxels in the local maxima field that lie under the 15 mm kernel, disregarding voxels that are not in the local maxima field. This is equivalent to applying a uniform spatial filter of radius 15 mm to the local maxima field, only retaining voxels with nonzero values, and reading off the resulting values at each surface vertex. Alternate approaches for surface-based smoothing are under development by our group [Memoli et al., 2004] and others [Chung et al., 2005; Lerch and Evans, 2005; Salat et al., 2004]. Lerch and Evans [2005] noted that the dimensions of the smoothing kernel can be optimized for detecting differences in cortical thickness between groups. As we filter the thickness data using only values from the local maxima field, we only filter data in the cortical surface, which is advantageous for maintaining statistical power. One theoretical benefit of surface-based smoothing is the use of the surface metric to define the kernel size and metrically covariant filters to optimize signal enhancement [see Memoli et al., 2004]. Future work will determine which filtering approach is optimal.
Cortical Pattern Matching
Cortical pattern matching methods [Thompson et al., 2004] were used to spatially relate homologous regions of cortex between subjects in order to permit interindividual comparison of cortical thickness in equivalent surface locations. For that purpose we created 3-D cortical surface models for each hemisphere based on automatically generated spherical mesh surfaces that were continuously deformed to fit a threshold intensity value that best differentiates extracortical CSF from underlying cortical GM [MacDonald et al., 1994]. The idea of this deformation process is to use a starting mesh which is deformed until its borders fit to a given intensity threshold of the respective image.
As a result of the linear transformation procedure, the generated 3-D cortical surface models correspond globally in size, orientation, and parameter space coordinates. Nevertheless, the same parameter space coordinates, within each cortical surface model, do not yet index the same anatomy across all subjects. Therefore, the cortical surface models from each individual were used to identify and manually outline 16 sulci in each lateral hemisphere as well as 12 sulci in each medial hemisphere, where raters (lateral: E.L.; medial: H.DL.) were blind to group status. The outlined lateral sulci included the Sylvian fissure, central, post-, and precentral sulcus, inferior and superior temporal sulcus (main body and ascending branch), inferior and middle frontal sulcus, intraparietal sulcus, transverse occipital sulcus, occipital-temporal sulcus, olfactory and collateral sulcus, as well as primary and secondary intermediate sulcus, which constitute the posterior borders of the supramarginal and angular gyrus, respectively. The set of medial sulci included the callosal sulcus and inferior callosal outline segment, superior and inferior rostral sulcus, paracentral sulcus, anterior and posterior segments of the cingulate sulcus, outer segment of a double parallel cingulate sulcus (when present), parieto-occipital sulcus, anterior and posterior segments of the calcarine sulcus, as well as the subparietal sulcus. Detailed anatomic protocols for delineating cortical anatomy are available at http://www.loni.ucla.edu/∼esowell/edevel/proto.html and have been previously validated, and their inter- and intrarater reliability have been reported [Blanton et al., 2001; Narr et al., 2001; Sowell et al., 2002]. Since it is not possible to compute intraclass correlations for 3-D curves (as these are not simple scalar measures), interrater reliability of manual outlining was measured by mapping the 3-D root mean square (r.m.s.) difference in millimeters between 100 equidistant points from each sulcal landmark in six test brains that were traced by E.L. (lateral sulci) and H.DL. (medial sulci) and compared to a gold standard arrived at by a consensus of raters. Intra-rater reliability was also computed by comparing the 3-D r.m.s. distance between equidistant surface points from each sulcal landmark traced six times in one test brain by the same rater (E.L. and H.DL.). Three-dimensional r.m.s. distance was <2 mm, and on average <1 mm for all landmarks within the rater and relative to the gold standard.
Surface points making up each sulcal outline were made spatially uniform and homologous sulcal surface points were matched between subjects in 3D [Thompson et al., 1996]. The manually derived sulcal landmarks were then used as anchors to drive the surrounding cortical surface anatomy of each individual into correspondence. During the surface-warping procedures, the algorithm computes a 3-D vector deformation field that records the amount of x, y, and z coordinate shift (or deformation) associating the same cortical surface locations in each subject with reference to the average anatomical pattern of the entire study group [Thompson et al., 2004]. That is, the deformation fields provide a spatial correspondence between equivalent 3-D cortical surface locations in each subject that also correspond to point locations in the images that contain the thickness information.
In summary, the cortical pattern matching step induces a re-parameterization of the original surface, i.e., a reassignment of surface coordinates to the image. Consequently, thickness values are in register with the original imaging data and represent measurements obtained from the same sulcal/gyral anatomy across data from all subjects. Averaging individual thickness maps after reassigning thickness values to a particular point in 3-D space (as a result of the cortical pattern matching) leads to different results than simply averaging thickness values based on their original x-, y-, and z-location in the original thickness file. This allows for the comparisons of cortical thickness in corresponding regions across different brains where thickness information is not altered.
Statistical Analysis
The mean values for cortical thickness obtained from each cortical surface point were calculated to provide maps of average cortical thickness across the lateral and medial surfaces. Importantly, in addition to our analyses on scaled brains (average ICBM-305 space using 12-parameter transformations), we examined cortical thickness in unscaled brains (average ICBM-305 space using 6-parameter transformations) to preserve the actual brain size of men and women while correcting for head alignment and tilt. Independent sample Student's t-tests were thus performed on scaled and unscaled data at each 3-D cortical surface location to assess the effect of gender on cortical thickness. Uncorrected two-tailed probability values (P < 0.05) from these tests were mapped directly onto the average cortical surface model of the entire sample, providing detailed and spatially accurate maps of local thickness differences between groups.
Given that statistical tests were made at thousands of cortical surface points and adjacent data points are highly correlated, permutation testing was employed using a threshold of P = 0.01, as in our prior work [Thompson et al., 2004]. For permutation testing, subjects were randomly assigned to either the male or female group 100,000 times and a new statistical test was performed at each cortical surface point for each random assignment. The number of significant results from these randomizations was then compared to the number of significant results in the true assignment to produce a corrected overall significance value for the uncorrected statistical maps, so that the statistical validity was verified even in the presence of correlations in the data.
Finally, in order to be able to relate our thickness findings to individual surface measurements, we calculated the surface area of the scaled (average ICBM-305 space using 12-parameter transformations) and unscaled mesh models (average ICBM-305 space using 6-parameter transformations). Scaled and unscaled cortical surface areas were compared between females and males using one-tailed two-sample t-tests.
RESULTS
Average Cortical Thickness
Figure 2 shows the average distribution of scaled and unscaled cortical thickness in standard 305 stereotaxic space for the whole sample. Average cortical thickness ranges from 1.5 to 3.4 mm on the lateral and medial brain surfaces (with slightly lower peaks on the upper end of thickness values in unscaled data). In both scaled and unscaled data the lowest cortical thickness on the lateral and medial surfaces appears to be located in superior portions of the postcentral gyrus and surrounding the occipital poles. The highest cortical thickness on the lateral surface seems to be located in the temporal lobes, mainly in anterior parts of the superior temporal gyrus and posterior parts of the medial and inferior temporal gyri. Higher thickness was also detected on the medial surface in the frontal lobe and covering anterior portions of the cingulate gyrus as well as partly extending into the parietal lobe in scaled data. The distributional pattern of scaled and unscaled average cortical thickness seems to be similar in males and females (not shown).
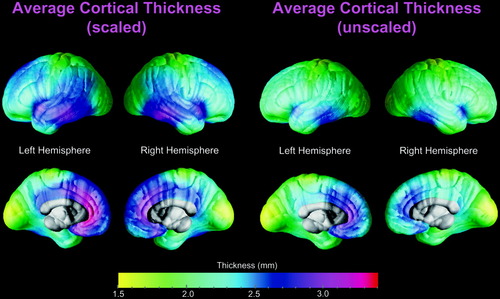
Average cortical thickness mapped for the whole sample (n = 60) in ICBM-305 space after using 12-parameter transformations (left) and after using 6-parameter transformations (right). The brain surface is color-coded according to the color bar, where thickness is shown in millimeters. Callosal, subcallosal, and midbrain regions have been excluded on the medial aspect of the surface.
Gender Differences of Cortical Thickness
Statistical analyses revealed significantly increased cortical thickness in women compared to men after interindividual differences in brain size had been removed by transforming images into standard ICBM-305 stereotaxic space using 12-parameter transformations (Fig. 3, left panel). Importantly, no regions with significantly increased cortical thickness were observed in males. Similarly, when the actual brain sizes of men and women were preserved (using only 6-parameter (rigid-body) transformations into ICBM-305 space), we revealed the same pattern and general direction of gender differences in cortical thickness, albeit significance was much less pronounced. A small cortical region in the left lateral temporal lobes showed increased thickness in males (Fig. 3, right panel). Permutation tests were highly significant for the comparison of thickness between males and females for scaled (left hemisphere: P ≤ 0.00001; right: P ≤ 0.00002) and unscaled data (left hemisphere: P ≤ 0.00268; right: P ≤ 0.01389), indicating that the observed gender effects do not occur by chance, and that thickness is greater in women than men, regardless of scaling.
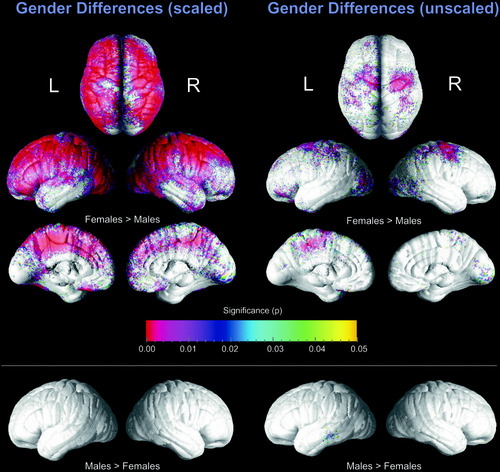
Uncorrected statistical maps of gender differences in cortical thickness in ICBM-305 space after using 12-parameter transformations (left) and after using 6-parameter transformations (right). The color bar encodes the P-value associated with the t-tests of cortical thickness performed at each cortical surface point. All colored cortical regions indicate statistically significant differences. All gray-shaded regions are not significantly different between males and females.
Brain regions demonstrating significantly higher cortical thickness in women in scaled data appear to be spread over the whole lateral brain surface and can be detected in all four lobes in each hemisphere, with temporal regions being least different. While increased female thickness in occipital and parietal lobes appears to be equally pronounced in the left and right hemisphere, gender differences are more prominent in the left frontal lobe than in the right. Similarly, the gender effect is stronger in the left hemisphere on the superior surface of the brain close to midline. In contrast, anterior portions of the temporal lobe demonstrate a stronger effect in the right hemisphere. Gender differences on the medial surfaces are less pronounced, but are evident. The most spatially extended and significant areas with sex differences were identified in the cingulate gyrus, paracentral lobe, and medial frontal lobe. Here differences in the left hemisphere were more pronounced than in the right.
In unscaled data, increased female thickness on the lateral surface was restricted bilaterally to superior pre- and postcentral regions, the occipital lobe, the most anterior tip of the temporal lobe, and predominantly in the vicinity of the left inferior and superior frontal gyrus. A very small region in the left posterior temporal lobe (surrounding the border between medial and inferior temporal gyrus) showed significantly increased thickness in male brains. Again, gender differences on the medial surfaces seem to be more concentrated in the left hemisphere, with the largest regions of increased female thickness in the paracentral lobe. Increased thickness in females in the right hemisphere was distributed more diffusely, with the most pronounced differences in occipital regions and surrounding the border between the precuneus and paracentral lobe.
The scaled surface areas of the cortex were significantly larger in females compared to males (P < 0.007, females: 1,051.41 ± 42.75 cm2, males: 1,026.07 ± 34.24 cm2) after transforming images into standard ICBM-305 space by applying 12-parameter transformations. In contrast, males had significantly larger cortex areas when comparisons were made without scaling brains (P < 0.0001, females: 811.13 ± 45.57 cm2, males: 870.64 ± 56.56 cm2).
DISCUSSION
We used an automated method to measure thickness across the cortex to characterize the average profile of cortical thickness distributions and to investigate differences between men and women in a well-matched sample of young and healthy adults. Given that allometric relationships have been demonstrated between brain volume and cerebral substructures [Jancke et al., 1997, 1999; Steinmetz et al., 1996], our analyses also extend previous findings concerning whether brain size normalizations affect the presence and direction of observed gender differences in cortical thickness.
Average Cortical Thickness
The results from this study indicate a heterogeneous distribution of cortical thickness, with different cortical regions exhibiting different thickness values ranging from 1.5–3.4 mm. These findings agree with previous reports of average thickness of 1.5–2.8 mm [Kuperberg et al., 2003], 2.0–3.3 mm [Conel, 1967], 1.0–4.5 mm [Fischl and Dale, 2000], 2.0–4.5 mm [Narr et al., 2004], 1.5–5.5 mm [Sowell et al., 2004], and 2.13–2.26 [Salat et al., 2004]. Of note, several previous analyses revealed thicker cortex in the temporal poles than in more posterior temporal regions [Sowell et al., 2004; Narr et al., 2004]. Our data do not confirm those previous reports where the cortex on the temporal poles is clearly thinner. One reason for this discrepancy in findings could be that areas of the cortex that are closed to the sinuses (such as the temporal poles, etc.) are prone to susceptibility artifacts, which can lead to a loss of contrast-to-noise in the temporal poles. Other brain regions are in line, in terms of minimum and maximum thickness, with others in the literature. For example, our findings of lowest thickness in primary visual regions agree with the results of von Economo [1929], Fischl and Dale [2000], Kruggel et al. [2003], Sowell et al. [2004], Narr et al. [2004], and Memoli et al. [2004]. Similarly, our findings of lowest thickness in somato-sensory regions correspond with reports by Kabani et al.[2001], Fischl and Dale [2000], Kruggel et al. [2003], Sowell et al. [2004], Narr et al. [2004], and Memoli et al. [2004]. Moreover, the present study revealed highest thickness in medial frontal regions that also agree with results by Fischl and Dale [2000] and Kuperberg et al. [2003]. With regard to the validation of the method, thickness analyses using independent samples in our lab [Narr et al., 2004; Sowell et al., 2004] have revealed spatial distributions of cortical thickness that agree with those based on postmortem analyses [von Economo, 1929]. Furthermore, thickness maps generated through the same approach as in the present study have been shown to be stable over time in validation studies using short-interval repeat scanning of multiple subjects [Sowell et al., 2004].
Our findings of lower average thickness values in unscaled data in the whole study group supports the assumption that cortical thickness is slightly increased through the normalization procedure using 12-parameter registrations. That is, if we scale our male and female brains to the ICBM-305 template—an average of 305 individual brains with blurred edges that therefore possess larger template dimensions than any individual brain—we increase the number of voxels that will be later classified as GM. Since scaling is a uniform procedure, the numbers of GM voxels along the cortical surface and the thickness of the cortex are both increased. Thus, scaling affects both the thickness of male and female cortices, but to a higher extent the latter group because of their typically smaller brain dimensions. In fact, extracting the scaling factor (SF) from our transformation files supports this postulation: brain size was increased on average by 27% during affine normalization, where female brains were scaled more than male brains (female SF: 1.358; male SF: 1.178).
Gender Differences in Cortical Thickness
After interindividual differences in brain size had been removed by applying linear 12-parameter transformations, numerous cortical regions showed increased cortical thickness in females compared to males, while we did not detect any region showing significantly increased thickness in males. Most interestingly, when the actual brain sizes of men and women were preserved (using only 6-parameter transformations into ICBM-305 space), we revealed the same pattern and direction of gender differences in cortical thickness, albeit the effects were much less pronounced and a small region in the temporal lobe showed significantly increased thickness in male brains. The observed larger cortical thickness in female brains may be related to our findings of larger cortical surface areas in females in scaled brains, although this relationship is clearly absent in unscaled data, where gender differences in surface area were reversed. These findings agree with previous reports, where unscaled surface areas were documented as larger in males [Nopoulos et al., 2000; Pakkenberg and Gundersen, 1997; Salat et al., 2004]. After controlling for cerebral volume, however, women were reported to have somewhat greater surface area measures, although this did not reach statistical significance [Nopoulos et al., 2000].
Scaled vs. unscaled data
Linear or nonlinear transformations of the data (required to fit the dimensions of a template) alter gender differences in cortical GM density and thickness. Understanding the effects of regional anatomic normalization on these differences has always been a challenging issue. Here we show that scaling of the data modulates the structural outcomes to some extent without significantly changing the overall direction of the gender effect. Given that one implicit function of scaling data is to correct for individual brain sizes, and given that female brains are, on average, smaller than male brains, we expected the measured cortical thickness to increase by more in females than in males by correcting for the females' smaller brain volumes. Our observations of stronger regional effects of increased thickness in females in scaled data confirmed this assumption.
However, exaggerated female thickness in scaled imaging data may also be attributable to allometric power laws, with disproportionate increases occurring in cortical thickness as compared to brain volume, as demonstrated for several brain structures [Jancke et al., 1997, 1999; Steinmetz et al., 1996]. That is, normalizing data using affine transformation might lead to disproportionate increases of cortical thickness relative to the increase that would be expected for a brain of a larger volume. Surprisingly, although several studies have examined normalized (scaled) data to control for different brain sizes between subjects, the issue of how spatial normalizations alter group differences in morphological features has been largely neglected. VBM and some former sulcal pattern matching approaches have typically employed scaled data (without systematically evaluating the effect of scaling). However, if the true relation between cortical thickness (or GM) and brain volume is sublinear or superlinear (i.e., increases more slowly or quickly than linearly with brain volume), then a linear stereotaxic correction (scaling) will leave differences in the data that in principle might be related to brain scale. As a result, morphometric differences that were detected and reported based on scaled data do not necessarily reflect true differences between groups after “taking individual brain volumes into account.” Clearly, future studies are needed to better model the possibly nonlinear relation between cortical thickness and brain volume, making it easier to identify the residual effects of gender.
A related issue is that some methodological approaches, for example, studies using VBM, evaluate group differences by filtering out the influence of scaling on GM measurements subsequent to the normalization procedure. That is, the intensity values in the normalized segmented images are modulated by the Jacobian determinants [Ashburner and Friston, 2000; Good et al., 2001a, b] to test for differences in absolute GM volumes (in contrast to relative GM volumes without the volume-preserving step). Interestingly, Good et al. [2001a] detected higher relative GM volumes in females only, while both females and males showed regions of higher absolute GM volume (as a consequence of the volume-preserving step). Thus, analyzing thickness in scaled data (like in VBM without the volume preserving step) and unscaled data, thickness findings may parallel GM findings. Nevertheless, we detected a similar pattern and direction of gender effects, with females showing greater cortical thickness in several brain regions regardless of whether the data were scaled or unscaled (albeit gender differences were less pronounced and one small cortical region in the lateral temporal lobes showed greater thickness in males in unscaled data). Although cortical thickness and GM constitute different biological measures, they are related [Narr et al., 2004]. Thus, VBM results of larger relative GM volume in females, and larger absolute GM volume in both genders (as opposed to larger thickness in females in scaled and unscaled data) might be attributable to the fact that the volume-preserving step in VBM does not adjust for the possibility of a power law (which can cause artifactual differences). Consequently, comparing VBM results with findings of other computational image analysis approaches may also be the focus of future studies.
Correspondence with other gender-specific findings
Both measures of GM density/concentration and cortical thickness might reveal features that are associated with the underlying cytoarchitecture. Notwithstanding, in contrast to the analysis of signal-based GM density, the measurement of cortical thickness provides us with an additional dimension of the morphology of the cortex not directly captured by point or sphere measures of signal intensity changes. Consequently, findings from previous GM studies and our present thickness results are only partially comparable, although prior studies report significant positive correlations across all cortical locations, with the exception of the temporal poles [Narr et al., 2004]. Nonetheless, our findings of regionally increased cortical thickness in females correspond to some earlier reports of increased GM volume (bilateral superior temporal lobe, central sulci, inferior frontal gyri, right inferior parietal and cingulate gyri) and GM concentration (bilateral frontal and parietal cortical mantle, banks of the cingulate) in a voxel-based analysis [Good et al., 2001a]. Finally, our results concur with some reports of regionally increased GM and/or cortical volumes in females in the dorsolateral prefrontal cortex [Schlaepfer et al., 1995], precentral gyrus, fronto-orbital and superior frontal cortex [Goldstein et al., 2001], inferior frontal cortex [Harasty et al., 1997], superior temporal gyrus [Harasty et al., 1997; Schlaepfer et al., 1995], parietal lobe [Nopoulos et al., 2000], and cingulate sulcus [Paus et al., 1996] as revealed through ROI (region of interest) analyses of cerebral lobes, parcellated units of the cortex or selected brain slices. There are numerous methodological differences between prior studies (e.g., using postmortem vs. imaging data and measuring GM volume vs. concentration or thickness), so our gender-specific thickness findings are not fully comparable with previous reports. However, we think it is of great interest that other automated whole-brain approaches [Good et al., 2001a] did not reveal any cortical region of increased GM concentration in men compared to women, corroborating the present results with respect to cortical thickness in scaled data. Furthermore, there is agreement between our findings of regionally increased cortical thickness in females and earlier reports of larger overall cortical volumes relative to cerebrum size in women compared to men [Goldstein et al., 2001], overall higher GM percentages or proportions in the female brain [Gur et al., 1999; Luders et al., 2002], as well as increased female cortical complexities [Luders et al., 2004].
A finer gyrification pattern in the female brain might result in increased partial volume effects, which could appear as thicker cortex in MRI. However, the thickness mapping approach is somewhat immune to partial volume effects for two reasons. First, the partial volume classifier used for GM segmentation in the present study [Shattuck et al., 2001] is one of several classifiers in which the tissue class probabilities are estimated using a Gaussian mixture model. Specific densities are fitted for the mixed GM/WM and GM/CSF tissue classes that could be present in more voxels in brains with a finer fissuration pattern. Notwithstanding, the total amount of GM should be estimated accurately, as the partial volume class is modeled explicitly from the intensity histogram of each dataset. Second, the supersampling of the data, before the thickness is measured, allows fitting of the intensities at the GM/WM interface at slightly finer resolution, so that each voxel should be classified more accurately. Finally, it is also possible that thickness and complexity are correlated for biological reasons rather than those due to image rasterization. In a separate sample of 40 normal subjects, who were controls for a study of Williams syndrome, we noted that individual differences in cortical complexity were correlated with cortical thickness in a broad right hemisphere region, including limbic, primary sensorimotor, visual, and perisylvian cortices [Thompson et al., 2005]. They were not associated in the left hemisphere in normal subjects or in either hemisphere for Williams syndrome subjects. Interestingly, although Williams syndrome subjects had overall greater complexity, they had less GM and thinner cortices overall. There is, therefore, no simple relationship between thickness and complexity, and careful study of these measures in larger samples is warranted.
Contrasting with our present findings of larger thickness in females, some previous analyses revealed a trend toward larger thickness in males [Pakkenberg and Gundersen, 1997; Salat et al., 2004]. Others, in turn, suggested that cortical thickness is similar in men and women [Nopoulos et al., 2000; Rabinowicz et al., 1999]. Thus, it is possible that thickness differences in males and females, as observed in the present study, might be due to different maturation rates or differences in cortical thinning at that particular age range sampled. Several studies have revealed significant sex by age interactions, with males having more prominent age-related brain atrophies than females [De Bellis et al., 2001; Good et al., 2001b; Murphy et al., 1996]. Nevertheless, some of these findings were conducted on samples with age ranges not applicable to the present study (e.g., 7–17 years), or based on morphometric measurements different from cortical thickness (e.g., CSF, GM). In addition, there exist conflicting results with respect to the region of decline [Cowell et al., 1994; Good et al., 2001b; Murphy et al., 1996; Xu et al., 2000]. Interestingly, a study designed to investigate age and gender effects on cortical thickness in particular detected no significant age by gender interactions, and men and women showed a similar degree of global thinning [Salat et al., 2004]. Moreover, as observed in the latter thickness study, cortical thinning was not apparent before the third decade, while gender effects in the present study were detected on subjects in their mid-20s, where male and female groups were closely age matched and standard deviations were small. Longitudinal studies (with one imaging session when subjects are in their mid-20s) remain to be conducted to empirically establish the impact of age on our gender-specific findings with respect to cortical thickness. At this point it seems rather unlikely that different stages of cortical thinning in male and female brains of subjects in their mid-20s are responsible for the observed gender effects.
Possible implications of gender-specific findings
The MRI signal strength and thus the measurement of cortical thickness is related to cellular characteristics such as cell packing density, myelination, cell size, and number of cortical neurons [Eickhoff et al., 2004; Kruggel et al., 2003]. If regional cortical thickness is associated with those microstructural factors, an increased thickness in females may have functional significance, especially in those regions that seem to show a clear sexual dimorphism favoring women in the absence of data scaling. They may also account for gender-specific abilities and/or behavioral differences between men and women, given that correlations have been found between GM and IQ, as well as spatial and verbal performances [Gur et al., 1999; Haier et al., 2004; Thompson et al., 2001]. A direct or indirect relationship between the regional thickness at a particular cortical location and the functional organization and outcome mediated by this region may occur if increased thickness reflects a regionally increased number of cortical neurons. Increased numbers of cortical neurons in functional units might be advantageous by contributing to an efficient processing of ingoing and outgoing information. However, given that negative relationships between cell density and layer thickness have been reported in deep cortical layers in some regions [Chance et al., 2004; Gittins and Harrison, 2004], a decreased cortical thickness might not be entirely caused by reductions in neuronal numbers. Furthermore, as discussed in earlier reports [Sowell et al., 2003, 2004], decreased cortical thickness might also result from myelin deposition in the cortical neuropil, smaller neuronal sizes and/or densities. Those morphologic features at the microscopic level might increase the signal intensity in certain cortical regions in male brains, which in turn would lead to the measurement of lower cortical thickness in men. In this case, less cortical thickness is not necessarily functionally disadvantageous. However, there does not appear to be any evidence to suggest that myelination is different between males and females, and previous reports instead indicate an increased rather than a decreased number and density of cortical neurons in men [Pakkenberg and Gundersen, 1997; Rabinowicz et al., 1999], although observations of increased cell packing density or number of neurons per cortical unit in women also exist [Witelson et al., 1995]. Clearly, further research unscrambling the relationships between cerebral micro- and macrostructure as well as cognitive functioning is necessary before we can relate our regional findings of increased cortical thickness in females to specific cellular differences, gender-specific abilities, and/or particular behavioral differences between men and women.
Increased cortical thickness in female brains might accompany similar or approximately equal numbers of cortical neurons in larger male and smaller female brains. Thus, it is also probable that there is no clear functional correlate to the increased regional thickness of the cortex in the female brain other than neuronal compensation. The assumption of approximately equal numbers of neurons seems to conflict with postmortem analysis, where more neurons were found in the cortex of male brains [Pakkenberg and Gundersen, 1997; Rabinowicz et al., 1999]. However, given that such histological estimations are based on tissue sections from a restricted number of cortical loci, neuronal numbers in the female cortex may be underestimated if female brains accommodate more cortex due to a higher folding complexity, as demonstrated recently [Luders et al., 2004]. Nonetheless, if increased cortical thickness simply reflects a reallocation of neurons in female brains (because of smaller brain sizes), then there remains the question of why thickness is only increased in certain regions of the female cortex and not evenly distributed (e.g., not in the temporal lobes).
With regard to the regional specificity of our findings, there might be a link to hormonal activity early in human development. The effect of testosterone and estrogen on the sexual differentiation of the brain has been examined predominantly in animals. Notwithstanding, we found an intriguing correspondence between our revealed regionally increased thickness in female brains and regions with high densities of sex steroid receptors identified from the animal literature [Goldstein et al., 2001]. As visualized by Goldstein et al., higher densities of estrogen receptors have been detected predominantly in superior frontal and fronto-orbital regions, surrounding the pre-, post-, and central sulci, the inferior parietal lobe, the occipital pole (lateral surface) and covering the cingulate, paracentral, and medial frontal gyrus (medial surface). In contrast, the temporal lobes are the most extended regions with developmentally low densities of estrogen receptors, which might be related to the apparent lack of sexual dimorphism in large regions of the temporal lobe in the present study. Further work is needed to reliably translate outcomes from cytoarchitectonical measures in animal studies to human brains [Goldstein et al., 2001]. But the striking correspondence between our thickness findings and hormonal receptor densities in animals might serve to generate hypotheses about the spatial organization of hormonal receptors in the postnatal human brain and its association with micro and macro structure in the adult brain. In addition to the modulating effect of sex steroids on gender-specific brain morphology, other factors have been suggested to direct the development and structural plasticity of the human brain, such as use-dependent influences like lifelong intensive training in specific cognitive or motor skills [Draganski et al., 2004; Gaser and Schlaug, 2003; Kochunov et al., 2003] and/or genetic influences [Thompson et al., 2001].
In conclusion, the present analysis revealed region-specific gender effects on the thickness of the cortex that might be associated with functional outcomes in which women perform or behave in general differently than men. Although gender-specific results were similar in scaled and unscaled data, spatial normalizations were shown to influence results, suggesting that these issues require attention in future studies.




