Direction-dependent visual cortex activation during horizontal optokinetic stimulation (fMRI study)
Abstract
Looking at a moving pattern induces optokinetic nystagmus (OKN) and activates an assembly of cortical areas in the visual cortex, including lateral occipitotemporal (motion-sensitive area MT/V5) and adjacent occipitoparietal areas as well as ocular motor areas such as the prefrontal cortex, frontal, supplementary, and parietal eye fields. The aim of this functional MRI (fMRI) study was to investigate (1) whether stimulus direction-dependent effects can be found, especially in the cortical eye fields, and (2) whether there is a hemispheric dominance of ocular motor areas. In a group of 15 healthy subjects, OKN in rightward and leftward directions was visually elicited and statistically compared with the control condition (stationary target) and with each other. Direction-dependent differences were not found in the cortical eye fields, but an asymmetry of activation occurred in paramedian visual cortex areas, and there were stronger activations in the hemisphere contralateral to the slow OKN phase (pursuit). This can be explained by a shift of the mean eye position of gaze (beating field) in the direction of the fast nystagmus phases of approximately 2.6 degrees, causing asymmetrical visual cortex stimulation. The absence of a significant difference in the activation pattern of the cortical eye fields supports the view that the processing of eye movements in both horizontal directions is mediated in the same cortical ocular motor areas. Furthermore, no hemispheric dominance for OKN processing was found in right-handed volunteers. Hum Brain Mapp, 2005. © 2005 Wiley-Liss, Inc.
INTRODUCTION
Optokinetic nystagmus (OKN) is a reflexive ocular motor response that holds the images of the environment steady on the retina by first driving the eyes in the direction of motion stimulation (slow phase, pursuit) and then resetting the eyes in the opposite direction (quick phase, saccade). It is elicited by pattern motion relative to a stationary observer or by self-motion when observing relative motion of the surroundings. Earlier functional MRI (fMRI) studies using frequency-spoiled single-slice fast low-angle shot (FLASH) pulse sequences and echo planar imaging (EPI) during OKN found bilateral activations in a complex sensorimotor network, especially in the visual cortex, including the motion-sensitive area MT/MST in the occipitotemporal cortex and the adjacent occipitoparietal cortex, as well as ocular motor areas such as supplementary, frontal, and parietal eye fields, and the prefrontal cortex [Bucher et al.,1997; Dieterich et al.,1998,2003a; Galati et al.,1999]. In the first study, Bucher et al. individually compared the signal intensity changes and the extent of activation of each activated area (by counting the number of voxels per cluster in only one slice) in every single subject, and an analysis for repeated measurements revealed no significant difference between rightward or leftward OKN. However, the number of activated voxels on average was smaller in the left hemisphere than in the right hemisphere, and a right hemispheric dominance in the occipitotemporal region was assumed, regardless of the direction of OKN [Bucher et al.,1997]. Using a similar type of data analysis in single subjects, the second study confirmed the assumption of a right hemispheric dominance in visual motion-sensitive areas, predominantly in the occipitotemporal cortex and ocular motor and vestibular cortex areas (posterior insula) during horizontal and vertical OKN stimulation [Dieterich et al.,1998]. In contrast, this hemispheric dominance of cortical ocular motor areas was not found in several fMRI and PET imaging studies that used statistical group analysis techniques to determine the cortical processing of other types of eye movements such as smooth pursuit or voluntary saccades [frontal eye field: Rosano et al.,2002; review by Paus,1996; eye movements in general: review by Pierrot-Deseilligny et al.,2004]. Only eye movements in the context of spatial and visual attention tasks showed a right hemispheric dominance [Gitelman et al.,1999,2002; Weber et al.,2000].
The question of hemispheric dominance for OKN processing is a subject of ongoing discussion. It is of special interest since a recent human PET study showed a significant dominance in the nondominant hemisphere during vestibular stimulation, e.g., a right hemispheric dominance of multisensory vestibular cortex areas in right-handed volunteers [Dieterich et al.,2003b]. There is a close functional connection between the vestibular system and the system generating OKN; especially, large-field visual stimulation (visual field larger than 30° in diameter with stimulation of the peripheral visual field) elicits apparent self-motion in a stationary observer [vection; Brandt et al.,1972]. Thus, the question arises whether the dominance within the vestibular system is accompanied by a hemispheric dominance within the ocular motor network during OKN.
Another open question is whether there is a direction-dependent specialization of the ocular motor areas in humans. Several animal (cat and monkey) and human studies have shown that lesions in various cortical areas lead to severe direction-selective deficits in slow eye movements (so-called directional asymmetry of the smooth pursuit and optokinetic systems) [see Hoffmann et al., 2001]. However, until now there has been no clear evidence that cortical areas have a strong bias for a particular direction of stimulus or pursuit [Albright et al.,1989; Bremmer et al.,1997a,b; Erickson and Thier,1991; Hoffmann et al., 2001; Komatsu and Wurtz,1988]. A study in monkeys using single neuron recording in a portion of the frontal eye field (FEF) revealed that each pursuit neuron has a preferred pursuit direction, indicating that pursuit direction uses a place-code type of representation in FEF [Gottlieb et al.,1994]. But since the neurons were not sorted in the FEF according to preferred direction, the FEF exhibited no strong bias for any specific direction.
The current fMRI study using echo planar imaging (EPI) with high stimulus repetition rate and statistical group analysis was conducted to address the following questions: 1) Are there direction-dependent activations during horizontal rightward or leftward OKN, especially in the ocular motor areas of the cortex? 2) Is there a hemispheric dominance for processing visual horizontal optokinetic nystagmus in right-handed volunteers similar to that found for the vestibular cortical system?
SUBJECTS AND METHODS
Subjects
Fifteen healthy volunteers (11 women, 4 men; mean age 24.9 years) without any history or complaints of neurological or neuro-otological dysfunction were included in the study. No drugs known to act on vestibular or ocular motor functions were being taken. The Laterality Quotient according to the 10-item inventory of the Edinburgh test [Oldfield,1971; Salmaso and Longoni,1985] was +100 for strong right-handedness in 14 volunteers and +40 in one volunteer. The local Ethics Committee of the Johannes-Gutenberg University approved the study and all subjects gave their informed written consent in accordance with the Declaration of Helsinki after the experimental procedure had been explained.
Recording Procedure, Optokinetic Stimulation, and Eye Tracking
Subjects lay supine, their view being directed to a projection surface in front of the scanner bore by a special mirror box of the eye movement recording system (MEyeTrack-LR, SensoMotoric Instruments, SMI, Berlin/Boston; www.smi.com) which was placed on the head coil. A computer-generated stimulus pattern of 14 vertical black-and-white stripes, which remained stationary (rest condition) or moved horizontally with 6°/s velocity in a rightward or leftward direction (stimulation condition A and B), was projected onto this screen by an LCD projector. The field of view in this setup was restricted to 20° in the horizontal and 15° in the vertical dimensions, i.e., small-field stimulation that did not induce apparent self-motion (vection) but steady optokinetic nystagmus. The subject's head was fixed by a head-holder to reduce movement artifacts and subjects wore ear protection. They were required to look at the target during the whole acquisition time. Their attention and performance of visually induced optokinetic nystagmus were monitored online in the control room by the eye-tracking system using real-time image processing. For later transformation of eye movements into degrees of horizontal and vertical axis, a calibration was performed while the subjects fixated points in defined angles to their eyes (10° horizontal and 6° in the vertical direction). Two-dimensional movement analysis was performed on the dominant eye using WinEye software. The calibrated data were low-pass filtered with a digital Gaussian filter having a band width of 20 Hz. Fast phases were automatically detected and removed from the data using a combined velocity-acceleration criterion in interactive software so that detection errors could be corrected manually [for method, see Glasauer et al., 2002]. To identify a gaze shift between the rest condition and the OKN condition, the eye position was determined for each block of stimulation (33.6 s) separately. The average eye position was calculated and compared with the average eye position during the preceding rest condition.
MRI Acquisition
Functional images were acquired on a standard clinical scanner (Siemens Vision, Erlangen, Germany) at a magnetic field strength of 1.5 T using a circularly polarized head coil and echo-planar imaging (EPI) with a T2*-weighted EPI sequence (TE = 60 ms, voxel size 3 × 3 × 4 mm, interscan interval 4.2 s). The protocol included 320 volumes, each consisting of 40 transversal slices that covered the whole brain. Alternating blocks of eight images at rest (looking at the stationary striped target) and eight images during rightward or leftward OKN were acquired in randomized order.
Data Analysis
Data processing was performed using statistical parametric mapping (SPM99b, Wellcome Department of Cognitive Neurology, London, UK; online at http://www.fil.ion.ucl.ac.uk/spm) implemented in MatLab (MathWorks, Natick, MA). The data were realigned to correct for subject motion using the first image as a reference and were spatially normalized [Friston et al.,1995a] to the Montreal Neurological Institute (MNI) template. Therefore, all coordinates given here refer to the MNI coordinate system. After normalization, the image volumes had a voxel size of 2 × 2 × 2 mm3. Images were subsequently smoothed with a Gaussian kernel of 8 × 8 × 12 mm3 (for x, y, z direction) to compensate for intersubject gyral variability and to attenuate high-frequency noise, thus increasing the signal-to-noise ratio. Statistical parametric maps (SPMs) were calculated on a voxel-by-voxel basis using hemodynamic modeling of the three experimental conditions with a general linear model [Friston et al.,1995b] and the theory of Gaussian fields [Worsley and Friston,1995]. For group analysis, functional data were collapsed to achieve one representative volume per condition per subject. These images were entered in a second level statistical analysis one-sample t-test, thereby affecting a random effects model [Frison and Pocock,1992; Woods,1996] and extending the scope of inference beyond the group of subjects to the population from which these subjects had been recruited. SPMs were computed for the comparison of each OKN condition with the rest condition (rightward OKN vs. rest, and leftward OKN vs. rest) as well as for comparison of the two OKN conditions with each other (rightward vs. leftward OKN, leftward vs. rightward OKN). Blood oxygenation level-dependent (BOLD) signal increases and decreases were calculated. Clusters exceeding a threshold of P ≤ 0.001 and a cluster size threshold of five voxels were considered significant.
To implement an analysis of laterality effects (hemispheric dominance) the subject-specific contrast images were flipped across the midline, and then the flipped and nonflipped images were entered as two groups into a paired t-test. In addition to a threshold of P ≤ 0.05 (corrected for multiple comparisons), a threshold of P ≤ 0.001 uncorrected was applied to look for smaller differences in the region of the cortical eye fields.
Furthermore, for each single subject the maximum t-value within the individual FEF activation clusters in both hemispheres was identified. All data were tested for normal distribution by the Kolmogarov-Smirnov test and entered into a one-factor ANOVA to detect significant hemispheric differences. On the basis of sulcal landmarks of the FEF subregions as described earlier [Beauchamp et al.,2001; Gagnon et al.,2002; Petit and Beauchamp2003], we also identified the t-values for FEF subregions and performed a one-factor ANOVA.
Throughout the article the nomenclature of the anatomical structures follows the atlas proposed by Talairach and Tournoux [1988].
RESULTS
Eye Movement Recording
Analysis of the eye movement recordings demonstrated that the visual stimulation steadily induced OKN, and all subjects were able to accurately perform the task during the whole acquisition time. Post-scanning data analysis revealed a gaze shift of 2.6° ± 1.6° SD during rightward OKN toward the fast phase (right) and a gaze shift of 2.7° ± 1.6° SD during leftward OKN toward the left (for illustration see Fig. 1). Mean slow phase velocity (SPV) of the nystagmus was 6.3°/s ± 0.6° SD.
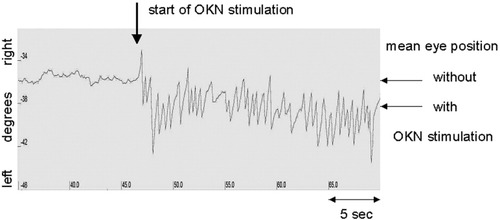
Original recording of horizontal left eye movement for single subject during fMRI scanning. The arrow indicates the start of visual optokinetic stimulation which induces an OKN with its fast phase toward the left. With OKN there is a detectable gaze shift to the left in this single subject. The mean gaze shift for the group is 2.6°.
Activation Pattern During Horizontal OKN
For more clarity, the sites of the BOLD signal increases are indicated in the form of gyri and lobes. The anatomical location of the maximum voxels of each cluster is given in MNI coordinates and Brodmann areas in Table I. OKN in both horizontal directions led to a nearly symmetrical bilateral activation pattern (Fig. 2). Compared to the rest condition, each stimulation condition showed widespread activations of the visual cortex bilaterally (cuneus, middle/superior occipital gyri) covering Brodmann areas 17, 18, and 19 and merged into the lateral occipitotemporal cortex (parts of the inferior and middle temporal gyri; BA 19/37; motion-sensitive area MT/V5) and adjacent occipitoparietal areas such as precuneus (BA 7). In addition, the lateral side of the parietal cortex along the intraparietal sulcus (inferior/superior parietal lobule, BA 40/7; partly representing the parietal eye field) was activated bilaterally in both stimulation conditions. During rightward OKN the parietal activation cluster was confluent with the large activation cluster in the right occipital cortex, whereas during leftward OKN they were separate. Both conditions showed bilateral widespread activations in the frontal lobes. These activations were located in the more anterior parts of the inferior/middle frontal gyrus (BA 46/10/44, partly covering the prefrontal cortex area) as well as in the more dorsal parts of the inferior frontal gyrus, merging into the adjacent anterior insula and precentral region (BA 44/45/9/6). Additional frontal activation clusters were located bilaterally in the lateral premotor area in the middle frontal gyrus / precentral gyrus (BA 6, representing the inferior and superior portion of the frontal eye field), in the right medial premotor area in the superior frontal gyrus (BA 6, representing the supplementary eye field), and in the left anterior cingulate gyrus (BA 31/24). During rightward OKN a small cluster (21 voxels) was found unilaterally in the right postcentral gyrus.
| Brain area | Brodmann area | Hemisphere | Left OKN | Right OKN | ||||
|---|---|---|---|---|---|---|---|---|
| MNI coordinates (x, y, z) | t | Cluster size (voxels) | MNI coordinates (x, y, z) | t | Cluster size (voxels) | |||
| Primary visual cortex merging into middle/superior occipital gyrus, precuneus, inferior/middle temporal gyrus | 17−19/37/7 | L | −6, −88, −10 | 11.41 | 18,153 | −6, −90, −12 | 13.33 | 18,575 |
| Visual cortex | R | 20, −96, 14 | 13.15 | 20, −94, 14 | 11.88 | |||
| MT/V5 | L | −42, −78, 4 | 9.47 | −50, −74, −8 | 6.32 | |||
| R | 46, −66, −2 | 10.18 | 46, −68, −2 | 11.60 | ||||
| Inferior/superior parietal lobule | 40/7 | L | −60, −40, 44 | 4.32 | 12 | −44, −36, 30 | 5.93 | 68 |
| PEF | L | −30, −58, 62 | 6.37 | 210 (18,153) | −30, −56, 60 | 6.79 | 573 | |
| R | 24, −54, 54 | 6.65 | 62, −32, 42 | 4.29 | 44 | |||
| 38, −36, 38 | 3.84 | 36, −32, 42 | 4.44 | 7 | ||||
| Middle frontal gyrus/precentral gyrus | 6 | R | 40, 4, 44 | 7.46 | 1,915 | 44, 4, 46 | 5.55 | 331 |
| 24, −4, 50 | 9.75 | 44, −6, 52 | 4.71 | |||||
| FEF | L | −44, −2, 56 | 4.47 | 49 | −30, −4, 50 | 5.24 | 83 (712) | |
| −28, −6, 48 | 4.37 | 19 | −52, 2, 44 | 5.32 | ||||
| Superior frontal gyrus pars medialis | 6 | R | 8, 8, 54 | 4.50 | 23 | 12, 6, 56 | 4.15 | 7 |
| SEF | L | −10, 14, 46 | 3.99 | 83 | ||||
| Middle/inferior frontal gyrus | 46/10/44 | L | −40, 42, 4 | 4.08 | 5 | −44, 50, 8 | 5.22 | 64 |
| 9/46 | R | 46, 52, 0 | 7.10 | 541 | 44, 42, −4 | 4.14 | 11 | |
| Partly PFC | 42, 48, 28 | 6.70 | 40, 48, 20 | 3.32 | 21 | |||
| Inferior frontal gyrus/precentral gyrus, partly including anterior insula | 44/45 | R | 36, 18, 6 | 6.67 | (1915) | 62, 16, 2 | 5.16 | 177 |
| 9/44 | R | 46, 6, 24 | 4.17 | (1915) | 62, 16, 8 | 5.13 | ||
| 44/9 | L | −44, 6, 20 | 5.33 | 635 | −60, 16, 10 | 5.83 | 712 | |
| 9/6 | L | −52, 6, 30 | 5.82 | −44, 4, 20 | 4.96 | |||
| Anterior insula | L | −30, 18, 3 | 4.35 | 19 | ||||
| Anterior cingulate gyrus | 31 | L | −14, −20, 42 | 4.45 | 18 | −14, −20, 46 | 4.80 | 25 |
| (24) | −12, 30, −2 | 4.80 | 10 | |||||
| Postcentral gyrus | Somatosensory | R | 58, −16, 48 | 5.64 | 21 | |||
- Areas with significant BOLD signal increases during rightward and leftward OKN visually induced by a computer-generated pattern of horizontally moving vertical black and white stripes were compared to the rest condition (stationary pattern) obtained by statistical group analysis (n = 15; P ≤ 0.001). The exact t values, corresponding Brodmann areas, MNI coordinates, and cluster sizes are given. To illustrate the symmetric distribution a few additional clusters at a lower threshold of P ≤ 0.005 uncorrected are given (in boldface type).
- OKN, optokinetic nystagmus; MNI, Montreal Neurological Institute template; PEF, parietal eye field; FEF, frontal eye field; SEF, supplementary eye field; PFC, prefrontal cortex; BOLD, blood oxygen level dependent.
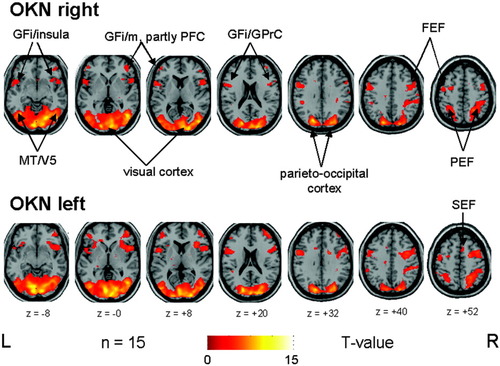
OKN during rightward (upper row) and leftward (lower row) small-field visual stimulation vs. rest condition (stationary screen) in a group of 15 healthy right-handed volunteers elicited very similar bilateral activations of the visual cortex, which merged into the adjacent occipitotemporal (motion-sensitive area MT/V5) and parietooccipital areas including the parietal eye field (PEF) along the intraparietal sulcus. Additional activations were located nearly symmetrically in the anterior insular region and adjacent parts of the inferior frontal gyri (GFi) as well as in different ocular motor structures such as the prefrontal cortex (PFC, GFm = middle frontal gyrus), frontal (FEF), and supplementary eye fields (SEF). For illustrative purposes, voxels above a threshold of P ≤ 0.005 uncorrected are shown.
Deactivation Pattern During Horizontal OKN
Horizontal OKN led in both directions to a similar nearly symmetrical bilateral pattern of BOLD signal decreases (“deactivations”) compared to the control condition. Decreases were located in the posterior part of the corpus callosum and the neighboring lower posterior cingulate gyrus (BA 24/31), partly merging into the hippocampus, and in the optic radiation/tapetum. Additional bilateral deactivations were found in the central sulcus region, predominantly in the postcentral gyri, which could be best attributed to the somatosensory cortex and adjacent parietal areas. Small unilateral signal decreases were located during both OKN directions in the frontal-most and medial part of the right middle frontal gyrus (BA 8). Decreases in the posterior insula region containing the human homolog of the parieto-insular vestibular cortex as described earlier [Dieterich et al.,2003a] were found bilaterally only at a significance level of P ≤ 0.005 (Table II, Fig. 3).
| Brain area | Brodmann area | Hemisphere | Left OKN | Right OKN | ||||
|---|---|---|---|---|---|---|---|---|
| MNI coordinates (x, y, z) | t | Cluster size (voxels) | MNI coordinates (x, y, z) | t | Cluster size (voxels) | |||
| Posterior corpus callosum, partly merging into posterior cingulate gyrus, hippocampus, optic radiation/tapetum | 24/31 | R | 26, −60, 20 | 7.95 | 568 | 26, −44, 22 | 6.82 | 670 |
| L | −22, −50, 8 | 7.02 | 487 | −4, −6, 22 | 4.61 | 27 | ||
| 0, −30, 12 | 4.82 | 24 | ||||||
| Middle frontal gyrus | 8 | R | 24, 32, 42 | 5.72 | 81 | 26, 32, 36 | 4.44 | 17 |
| Postcentral gyrus/parietal cortex | R | 18, −46, 72 | 5.27 | 6929 | 20, −42, 64 | 5.75 | 100 | |
| R | 18, −40, 64 | 4.37 | ||||||
| L | −20, −42, 74 | 4.12 | −22, −24, 66 | 4.17 | 12 | |||
| Posterior insula | R | 44, −2, −12 | 4.12 | 789 | 42, −14, 0 | 3.25 | 11 | |
| L | −38, −24, 2 | 3.28 | 11 | −38, −20, 4 | 3.63 | 39 | ||
- Areas with significant BOLD signal decreases (deactivations) during rightward and leftward OKN visually induced by a computer- generated pattern of horizontally moving vertical black and white stripes were compared to the rest condition (stationary pattern) obtained by statistical group analysis (n = 15; P ≤ 0.001). The exact t values, corresponding Brodmann areas, MNI coordinates, and cluster sizes are given. To illustrate the symmetrical distribution of deactivations, a few additional clusters at a lower threshold of P ≤ 0.005 uncorrected are given (in boldface type).
- OKN, optokinetic nystagmus; MNI, Montreal Neurological Institute template; PEF, parietal eye field; FEF, frontal eye field; SEF, supplementary eye field; PFC, prefrontal cortex; BOLD, blood oxygen level dependent.
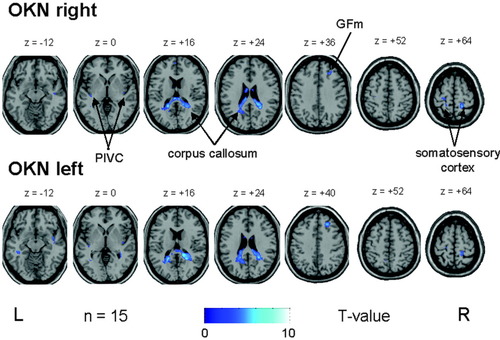
OKN during rightward (upper row) and leftward (lower row) small-field visual stimulation in a group of 15 healthy right-handed volunteers caused deactivations in the posterior corpus callosum which partly merged into adjacent parts of the posterior cingulate gyrus and optic radiation. Additional bilateral deactivations were found in the parieto-insular vestibular cortex (PIVC) in the posterior insula, in the central sulcus region (best attributed to the somatosensory cortex), and in the frontal-most and medial part of the right middle frontal gyrus (BA 8, GFm). For illustrative purposes, voxels above a threshold of P ≤ 0.005 uncorrected are shown.
Comparison of Rightward and Leftward OKN
The contrast in rightward vs. leftward OKN showed significant statistical differences in the right paramedian visual cortex (parts of the cuneus and precuneus, 17/18/31, x/y/z = 14/−90/30, 166 voxels, T = 5.06), whereas the contrast in leftward vs. rightward OKN showed a cluster in the left paramedian visual cortex (parts of the lingual gyrus, cuneus, precuneus, middle occipital gyrus; BA 17/−19/31, x/y/z = −4/−74/14, 676 voxels, T = 5.08). In addition, rightward vs. leftward OKN showed a second small cluster located in the left medial occipital gyrus (BA 19, x/y/z = −28/−86/16, T = 5.55) and leftward vs. rightward OKN in the right medial occipital gyrus (BA 19, x/y/z = 32/−78/16, T = 4.55). No statistically significant differences were found for ocular motor areas in the parietal, frontal, and prefrontal cortices or in other occipitotemporal regions at a threshold of P ≤ 0.001 (Fig. 4). There were only small additional clusters for the contrast leftward vs. rightward OKN in the right inferior / middle frontal gyrus (BA 47/6; x/y/z = 40/4/60, T = 4.79) and for rightward vs. leftward OKN in the left inferior / superior parietal lobule (BA 7; x/y/z = −22/−70/60, T = 6.36). Thus, these small areas at different sites in different hemispheres (irregular activations) do not give evidence for stimulus-direction dependency.

For the contrast of leftward vs. rightward OKN (upper row) or vice versa (lower row), the group analysis of healthy volunteers showed significant differences only in parts of the paramedian visual cortex in the hemisphere contralateral to the slow phase of OKN (pursuit) (P ≤ 0.001 uncorrected).
Comparison of Both Hemispheres (Hemispheric Dominance)
The analysis of laterality effects found no significant clusters during rightward or leftward OKN above a threshold of P ≤ 0.05 (corrected for multiple comparisons). Also at a lower significance level of P ≤ 0.001 (uncorrected), there was no hemispheric dominance for the cortical eye fields or vestibular areas. Only small areas were located within the right temporo-occipital cortex corresponding to the motion-sensitive areas MT/V5 (BA 19/37; x/y/z = 46/−58/−4) and within the visual cortex (BA 19) according to the gaze shift.
Evaluation of the data for single subjects revealed that both OKN directions in all subjects showed activation of the FEF in both hemispheres. The maximum of the individual clusters varied considerably in their location: ±20 < x < ±56, −14 < y < +14, +36 < z < +56. This wide range can be attributed to the fact that two subregions of the frontal eye field are known [Rosano et al.,2002; review by Pierrot-Deseilligny et al.,2004] to be activated independently. The one-factor ANOVA of the maximum t-values within the individual FEF activation clusters in both hemispheres revealed no significant differences (OKN bilateral P = 0.441, OKN right P = 0.628, OKN left P = 0.162) (Fig. 5). This was also the case for the analyses of the FEF subregions.
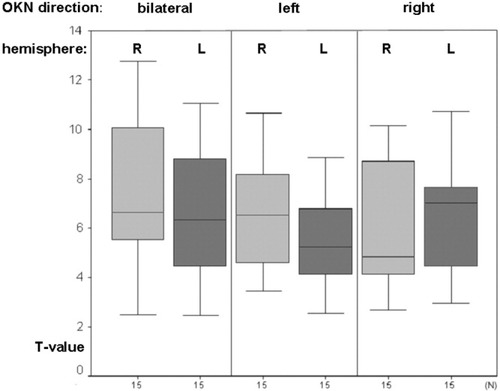
Results of the evaluation of the maximum t-values of the right- and left-hemispheric activation of the frontal eye field (FEF) in 15 healthy volunteers. The activation of the right FEF tends to be stronger than of the left FEF, but in the one-factor ANOVA no significant difference was found.
DISCUSSION
- 1
The activation-deactivation pattern induced by horizontal small-field optokinetic stimulation compared to the stationary condition was consistent with those of previous studies [Bucher et al.,1997; Dieterich et al.,1998,2003; Galati et al.,1999] and revealed significant bilateral activations of the visual cortex and ocular motor areas, thus providing a good basis for statistical comparison between the two horizontal stimulation directions.
- 2
Comparison of rightward vs. leftward OKN showed direction-dependent differences during horizontal OKN only in paramedian visual cortex areas and stronger activations in the hemisphere contralateral to the slow phase of OKN (pursuit), respectively, ipsilateral to the fast OKN phase.
- 3
No differences in the activation pattern occurred in the cortical eye fields or other cortical or subcortical brain regions involved in OKN processing.
- 4
The group analysis gave no evidence for a hemispheric dominance of the activations during OKN processing as the single subject analysis did for the subregions of the frontal eye fields.
Rightward and leftward OKN in our study showed bilateral fMRI activations of the visual cortex, including lateral occipitotemporal (motion-sensitive area MT/V5) and adjacent occipitoparietal areas, as well as activations of ocular motor areas such as the frontal, supplementary, and parietal eye fields. Further activations were found in the lateral parts of the prefrontal cortex bilaterally. These correspond best to the dorsolateral prefrontal cortex (DLPFC), which is known to be involved in saccadic inhibition, short-term memory, and decisional processes [Pierrot-Deseilligny et al.,2004]. However, in contrast to the earlier fMRI studies on OKN with FLASH sequences in single subjects [Bucher et al.,1997; Dieterich et al.,1998], the statistical group analysis of 15 right-handed subjects based on EPI sequences did not confirm a right-hemispheric dominance for OKN processing in ocular motor or vestibular cortex areas. There was only a small activation in the right temporo-occipital cortex area representing the motion-sensitive area MT/V5. However, our failure to find a hemispheric dominance in the right hemisphere in the group analysis does not generally allow us to draw a conclusion for a single subject. The question of hemispheric dominance of the nondominant hemisphere arises, because of the special functional connection between the optokinetic and the vestibular systems and because of recent finding of a significant preference of the nondominant hemisphere for the processing of vestibular information [Dieterich et al.,2003b]. A PET study with activated water during caloric vestibular stimulation showed a stronger activation in the multisensory vestibular cortex (posterior insula, inferior parietal lobule, anterior cingulum) in the right hemisphere in right-handed volunteers and a significantly stronger activation of the same areas within the left hemisphere in left-handed volunteers [Dieterich et al.,2003b]. Tendentially, this hemispheric dominance was also observed in right-handers during galvanic vestibular stimulation [Bense et al.,2001] and caloric stimulation in fMRI [Fasold et al.,2002; Suzuki et al.,2001]. Monkey studies have shown that OKN cannot be simply defined as an eye movement that combines the initial response of a smooth pursuit component with a large resetting saccade. There is a more complex system underlying optokinetic stimulation, e.g., vestibular-optokinetic system [Leigh and Zee,1999], which seems to be especially associated with the indirect component of OKN. This component is the slower build-up related to the vestibular nuclei complex and is thus responsible for vection. The close connection between the vestibular and optokinetic systems was also demonstrated in vestibular nuclei that responded to monkey head rotations and that were also driven by optokinetic stimuli [Boyle et al.,1985]. These connections are especially relevant for inducing vection, for which a large-field stimulation is more effective than a small-field stimulation [Brandt et al.,1972]. In humans the close interaction between the optokinetic system and the vestibular system was demonstrated during large-field [Brandt et al.,1998] and small-field [Dieterich et al.,2003a] optokinetic stimulation. Both showed activations of visual and ocular motor areas and simultaneous deactivations of vestibular cortex areas. Since we used small-field OKN for technical reasons, our failure to find hemispheric dominance might be due to one of two reasons: either the stimulus induces a “pure” ocular motor response without vection or there is no hemispheric dominance within the cortical ocular motor system, only within the vestibular system. The latter explanation is in agreement with our finding of signal decreases (deactivations) in the posterior insula region bilaterally, the area known to represent the human homolog of the parieto-insular vestibular cortex in monkeys [Grüsser et al.,1990a,b].
Recent fMRI studies on other types of eye movements such as smooth pursuit and saccades did not report a hemispheric specialization of ocular motor areas in the cortex [frontal eye field: Rosano et al.,2002; review by Paus,1996; eye movements in general: review by Pierrot-Deseilligny et al.,2004]. Only eye movements in the context of spatial and visual attention tasks showed a right hemispheric dominance [Gitelman et al.,1999,2002; Weber et al.,2000], suggesting that these spatial orientation effects are caused by the vestibular system.
The subtraction analysis between rightward and leftward OKN showed no direction-specific activation of the ocular motor areas such as the frontal, prefrontal, and parietal eye field or other cortical or subcortical areas. Although earlier monkey studies reported that almost all “pursuit neurons” in the FEF [Gottlieb et al.,1994] and MT/MST had a preferred direction of motion, neurons with different direction preferences obviously lay intermingled within the frontal eye fields and MT/V5 region [Komatsu and Wurtz,1988; for review, see Hoffmann et al., 2001]. Functional imaging studies have not yet been able to monitor these effects for single neurons, most probably due to insufficient spatial resolution; however, they were able to demonstrate direction-selective imbalance in the area of MT+ [Huk et al.,2001]. In conclusion, the absence of a significant difference in the activation pattern of the cortical eye fields supports the view that the processing of eye movements in both horizontal directions is mediated in the same cortical ocular motor areas.
Subtraction analysis revealed only one small cluster in paramedian visual cortex areas in the hemisphere contralateral to the slow phase of OKN (pursuit), respectively, ipsilateral to the fast OKN phase. This asymmetric activation of the visual cortex in our study can be explained by shifts of the mean eye position (beating field) of about 2.6° in the direction of the fast phases, which we could measure by simultaneous eye movement recordings with the infrared eye-tracker. The shift of the beating field leads to an asymmetric activation of the visual cortex in both hemispheres, with a stronger activation in the hemisphere contralateral to the “longer”-lasting pursuit phase. This is in agreement with a recent activation study on 10° lateral gaze compared to fixation straight ahead [Deutschländer et al.,2005]. During lateral fixation, activations occurred in the cuneus, lingual, and fusiform gyrus of the hemisphere contralateral to the fixation target. The shift of the beating field during OKN is a phenomenon known to exist in humans and animals [for overview, see Watanabe et al.,2001]. A recent psychophysical study during optokinetic visual stimulation (large-field 30 × 30°) demonstrated that it is due to an automatic shift of visual attention toward the incoming visual field [Watanabe et al.,2001]. Thus, our data fit the hypothesis that even small gaze shifts into one visual hemifield lead to transitions of visual processing, predominantly in the hemisphere contralateral to the direction of gaze in head coordinates.
Acknowledgements
We thank Judy Benson for critically reading the manuscript and Christoph Best and Thomas Stephan for support with the statistical analysis.




