Astrocyte immunosenescence and deficits in interleukin 10 signaling in the aged brain disrupt the regulation of microglia following innate immune activation
Funding information: National Institute of Dental and Craniofacial Research, Grant/Award Number: T32-DE014320; National Institute on Aging, Grant/Award Number: R01-AG051902
Abstract
Microglia, the innate immune cells of the brain, develops a pro-inflammatory, “primed” profile with age. Using single-cell RNA-sequencing, we confirmed hippocampal microglia of aged mice (18 m.o.) had an amplified (4 h) and prolonged (24 h) neuroinflammatory response to peripheral lipopolysaccharide (LPS) challenge compared to adults (2 m.o.). Overall, there were several unique cell-, age-, and time-dependent differences in the clusters of microglia identified. Analysis of upstream regulators and canonical pathways revealed impaired regulation of an activated, neuroinflammatory state within microglia. Moreover, microglia in the aged hippocampus failed to turn over during the resolving phase of neuroinflammation. Concomitantly, astrocytes in the aged hippocampus were “immunosenescent” both 4 and 24 h after LPS challenge. For example, aged astrocytes had reduced anti-inflammatory signaling and cholesterol biosynthesis, two pathways by which astrocytes regulate the inflammatory profile of microglia. One of the pathways reduced in the aged hippocampus was interleukin (IL)-10 signaling. This pathway increases astrocytic expression of transforming growth factor (TGF)-β, an anti-inflammatory cytokine with abundant receptor expression on microglia. Therefore, transgenic astrocytic Il10raKO mice were generated to determine if impaired IL-10R/TGFβ signaling within astrocytes caused an amplified microglial neuroinflammatory response. Astrocytic Il10raKO caused exaggerated sickness behavior and a prolonged neuroinflammatory response to peripheral LPS, including increased social avoidance with amplified microglial Il1b and Tnf mRNA expression. In summary, astrocytes had an immunosenescent profile with age and, in response to peripheral LPS, had IL-10R signaling deficits and a lack of cholesterol biosynthesis, both leading to the inability to resolve microglial activation.
1 INTRODUCTION
Aging is the primary risk factor for several neurodegenerative disorders and cognitive decline in humans (Ahmed et al., 2014; Bishop et al., 2010; Mattson & Magnus, 2006; Norden & Godbout, 2013). Increased inflammation is a factor in these disorders, and this process is often referred to as “inflammaging” (Franceschi et al., 2000). Microglia are unique macrophages of the central nervous system (CNS) and are key mediators of inflammatory signaling (Dantzer et al., 2008; Henry et al., 2009; Nguyen et al., 2002; Norden et al., 2014). For example, microglia interpret peripheral inflammatory signals and propagate these signals in the CNS (Colonna & Butovsky, 2017). This communication between the CNS and immune system is critical for normal physiological and behavioral responses to infection (Dantzer, 2001; Dunn et al., 2005). With age, however, there are alterations within both the CNS and immune system that may impair this bidirectional communication (Lupo et al., 2019; Müller et al., 2019). Indeed, bacterial infections, like urinary tract infections, often present in the elderly as acute cognitive impairment (Rowe & McKoy, 2017). Moreover, the elderly have an increased risk for progressive dementia and cognitive decline post-infection (Iwashyna et al., 2010). Therefore, a better understanding of the cellular dynamics involved in this age-associated neuroinflammation is critical.
One age-associated imbalance in neuro-immune communication is the “priming” of microglia. Microglia is long-lived cells with limited turnover and is highly susceptible to the effects of aging (Ajami et al., 2007). Priming is characterized by increased expression of several immune mediators, including CD11b, CD11c, CD68, CD86, major histocompatibility complex class II subunits, and toll-like receptors (Frank et al., 2006; Godbout et al., 2005; Griffin et al., 2006; Lucin & Wyss-Coray, 2009; Stichel & Luebbert, 2007). Primed microglia also have a de-ramified morphology (Choi et al., 2007), increased inflammatory cytokine expression (Godbout et al., 2005; Sierra et al., 2007; Xie et al., 2003), and decreased anti-inflammatory gene expression (Maher et al., 2005; Wynne et al., 2010; Ye & Johnson, 2001; Youm et al., 2013). Functionally these primed microglia respond to innate immune activation with exaggerated production of inflammatory cytokines (Chung et al., 2009; Robert Dantzer, 2001; Haroon et al., 2012; Henry et al., 2009; Norden & Godbout, 2013). For example, peripheral stimulation with Escherichia coli (E. coli) lipopolysaccharide (LPS) caused amplified interleukin (IL)-1β production by microglia in the aged brain (Henry et al., 2009) and prolonged sickness with lethargy, hypophagia, and social withdrawal in aged mice (O'Neil et al., 2018). Other groups have reported that this heightened reactivity of microglia after immune challenge resulted in amplified neuroinflammation and cognitive impairment in aged rodents and humans (Barnes, 1979; Barrientos et al., 2006; Barrientos et al., 2010; Bishop et al., 2010).
Recent single-cell RNA-sequencing studies show unique microglial profiles in neurodegenerative disease, traumatic brain injury, and advanced age (Keren-Shaul et al., 2017; Witcher et al., 2021). Thus, it is important to understand these unique microglial profiles and to determine the extent to which they can be manipulated or reversed. As an attempt to reverse priming in the aged brain, we and others forced turnover of microglia with the colony-stimulating factor 1 receptor antagonist PLX5622 (Elmore et al., 2018; O'Neil et al., 2018). This turnover resulted in microglia with an intermediate RNA profile and reduced lipofuscin burden (O'Neil et al., 2018). Nonetheless, forced turnover of microglia in the aged brain did not reverse their amplified neuroinflammatory response to LPS. We interpret these data to indicate that the microenvironment of the aged brain influences the profile of microglia.
Microglia are tightly regulated by the cells within their microenvironment (e.g., neurons, astrocytes, and other microglia), and these cells in turn influence microglial function (Badimon et al., 2020). Anti-inflammatory pathways using IL-10 and transforming growth factor (TGF)-β are key in the regulation of microglia (Norden et al., 2014; Norden, Trojanowski, Walker, et al., 2016). For instance, microglial IL-10 stimulates astrocytes to produce TGFβ, which in turn exerts anti-inflammatory effects on microglia (Norden et al., 2014; Norden, Trojanowski, Walker, et al., 2016). There is also evidence of autocrine regulation by microglial IL-10 (Shemer et al., 2020). Not surprisingly, an “inflammatory” profile has been detected in astrocytes with age (Cotrina & Nedergaard, 2002; Morgan et al., 1999; Norden, Trojanowski, Villanueva, et al., 2016). For instance, aged astrocytes are more fibrous, have higher expression of glial fibrillary acidic protein (GFAP), and express a unique RNA profile compared to adult astrocytes (Clarke et al., 2018). In addition, “ribotagged” astrocytes from the aged hypothalamus (24 m.o.) had increased expression of genes associated with inflammation and synapse elimination and decreased cholesterol biosynthesis compared to 4-m.o. controls (Boisvert et al., 2018). Cholesterol/apolipoprotein E (APOE) biosynthesis by astrocytes is relevant because cholesterol and APOE support metabolic, neuroprotective, and anti-inflammatory effects throughout the CNS (Loera-Valencia et al., 2019). Therefore, impairments in these microglia-astrocyte dynamics with age may underlie the prolonged neuroinflammatory response to peripheral immune challenge.
Thus, the purpose of this study was to further delineate the impaired communication between microglia and astrocytes in the aged brain following activation of the innate immune system. Here, we used single-cell RNA-sequencing to show evidence of distinct and unique transcriptional profiles of both microglia and astrocytes in the hippocampus with age and LPS challenge. Age-associated deficits were evident in the interactions between astrocytes and microglia with reductions in IL-10 signaling, microglial turnover and cholesterol biosynthesis after LPS. Evidence of astrocyte immunosenescence with age led us to develop an astrocyte-specific knockout of the IL-10 receptor. This model resulted in impaired astrocytic regulation of microglia and a prolonged neuroinflammatory response to LPS. Overall, astrocytes in the aged hippocampus were unable to resolve microglial activation following LPS challenge.
2 MATERIALS AND METHODS
2.1 Mouse strains and housing
Adult (2 months old) and aged (18–20 months old) male BALB/c mice (Mus musculus) were obtained from Charles River (Wilmington, MA) and were individually housed. Transgenic male and female mice were generated in-house and were housed 2–5 mice per cage. All mice were kept under a 12/12 h light/dark cycle with ad libitum access to rodent chow and water. To generate an Aldh1l1 reporter (tdTomato) mouse line, Aldh1l1-Cre/ERT2 mice (JAX 029655; FVB/N) were crossed with Ai9(RCL-tdT) mice (JAX 007909; C57BL/6J). In addition, Aldh1l1-Cre/ERT2 mice were crossed with IL-10Rαflox (JAX 028146; C57BL/6) mice to generate the inducible astrocyte-specific Il10raKO line. Mice were genotyped at Transnetyx (Cordova, TN) for Cre, Il10raWT, and Il10rafl expression. Cre− mice were used as Il10rafl/fl controls. Four-weeks prior to experimentation, all transgenic adult (6–14 weeks old) mice were administered 1.5 mg intraperitoneal (i.p.) tamoxifen (10 mg/ml in corn oil) daily for 5 days. This produced mice with the IL-10 receptor knocked out specifically in astrocytes (Ast-Il10raKO). For the single-cell RNA-sequencing (scRNA-Seq) and “AAV-TGFβ” experiments, only male mice were used. Female mice were evaluated in the Ast-Il10raKO experiments, but there was no effect of LPS on the control female mice; therefore, females were excluded from the study. CO2 asphyxiation was used for euthanasia in all experiments. All procedures were performed in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals and were approved by The Ohio State University Institutional Animal Care and Use Committee.
2.2 Injection of LPS
Mice received a single i.p. injection of saline or E. coli LPS (serotype 0127:B8; Millipore Sigma). Dosage was strain-specific and was selected to elicit a pro-inflammatory cytokine response in the brain resulting in a transient sickness response in adult mice without mortality in aged mice (Berg et al., 2004; Godbout et al., 2005; Wohleb et al., 2012). BALB/c mice received 0.33 mg/kg LPS (O'Neil et al., 2018), and transgenic mice received 0.50 mg/kg LPS (Corona et al., 2013).
2.3 Single-cell isolation and RNA-sequencing
Mice were sacrificed and transcardially perfused with ice-cold phosphate-buffered saline (PBS, pH 7.4). The brain was removed and the hippocampus was microdissected. The hippocampus was then dissociated using the Adult Brain Dissociation Kit (Miltenyi Biotec) according to manufacturer's instructions. In brief, hippocampi from two mice per group were pooled, and tissues were enzymatically dissociated in C-tubes at 37°C using a gentleMACS dissociator. Density centrifugation was used to remove myelin debris, remaining red blood cells were lysed, and cell viability and concentration were determined using the LUNA-FL Dual Fluorescence Cell Counter with Acridine Orange/Propidium Iodide Cell Viability Kit (Logos Biosystems). Approximately 2 × 106 live cells were recovered from each homogenate. This experiment was performed in two replicates, such that four mice were used for each experimental group.
Dissociated cells were loaded into a 10X Genomics Chromium Controller using Chromium Single Cell 3′ v3 reagents, and sequencing libraries were prepared according to the manufacturer's instructions (10X Genomics). Library quality was determined by Agilent High Sensitivity DNA BioAnalyzer chip. The resulting libraries were sequenced using an Illumina NovaSeq 6000 platform with a paired-end 150-bp sequencing strategy at Novogene (Sacramento, CA). Raw sequence data was processed using Cell Ranger software (10X Genomics) to align sequences to the mm10 reference genome, annotate genes, and count reads. Cells with high-mitochondrial gene expression were excluded from analysis.
2.4 Single-cell RNA-sequencing analysis
Raw counts were normalized and cells were clustered by expression patterns using the Seurat package in R (Stuart et al., 2019). Plots were generated using 12 separate cDNA libraries (two independent experiments). Identification of cell types clustered together using Uniform Manifold Approximation and Projection (UMAP) and t-distributed stochastic neighbor embedding (t-SNE) was established by using cell type-specific markers: astrocytes (Gja1), microglia (P2ry12), oligodendrocytes (Plp1), endothelial cells (Cldn5), choroid plexus cells (Kl), neurons (Meg3), leukocytes (Ptprc), oligodendrocyte progenitor cells (Pdgfra), and ependymal cells (Foxj1). Differential gene expression was determined using variance-stabilizing transformation followed by analysis with the Wilcoxon Rank Sum test with Bonferroni's correction for multiple comparisons. Pathway and upstream regulator analysis was performed using Ingenuity Pathway Analysis (IPA; QIAGEN). Pathways and Upstream Regulators were considered significant if p < .05 and |z| ≥ 2. Cell–cell communication network analysis was conducted using the Cell Chat package in R. Cell Chat calculates the communication probability between two cell populations based on expression of ligand-receptor pairs (Jin et al., 2021).
2.5 AAV2/5-mediated expression of TGFβ in astrocytes
To augment TGFβ production by astrocytes, a 2/5 pseudotype adeno-associated virus (AAV2/5) was used. This AAV2/5 vector was generated by Vector Biolabs (Malvern, PA) to express a biologically active form of the mouse Tgfb1 gene (RefSeq#: NM_011577) and green fluorescent protein (eGFP) using a modified GFAP(0.7) promoter (i.e., AAV2/5-GFAP(0.7)-mTGFb1(C223S/C225S)-IRES-eGFP; “AAV-TGFβ”). A control “AAV-GFP” was generated without the Tgfb1 gene (AAV2/5-GFAP(0.7)-eGFP; Vector Biolabs). Two microliters of either AAV-TGFβ or AAV-GFP were injected bilaterally into the hippocampi of aged BALB/c mice at stereotactic coordinates AP–1.82 mm, ML ± 1.57 mm, DV–1.85 mm in reference to bregma at 5.0 × 1012 GC/ml using a StereoDrive robot stereotaxic system (NeuroStar). Each mouse was allowed 4 weeks to recover before experimentation.
2.6 Behavioral testing
2.6.1 Social interaction
For AAV-TGFβ and Ast-Il10raKO studies, social interaction was determined as previously described (O'Neil et al., 2018). In brief, mice were individually housed 1 week prior to testing. Mice were then injected with i.p. saline or LPS, and motivation to engage in social exploratory behavior was determined at baseline and again 4, 8, and 24 h later. A novel juvenile conspecific was introduced into the test subject's home cage for 5 min. Behavior was recorded and the total duration of time the experimental subject engaged in social investigation of the juvenile (e.g., anogenital sniffing or trailing) was determined by a trained observer who was blind to the experimental groups. Data are presented as repeated measures normalized to each mouse's baseline behavior. Social index was also calculated from the social interaction data as the normalized area under the curve for the 24-h social interaction times (i.e., 100% social interaction at all timepoints = 1.00).
2.6.2 Nest building
In order to measure each mouse's motivation to build a nest from its nesting material, a fresh cotton nestlet was placed in each cage after the 8 h social exploration was performed. At 24 h, the mass of the unused nesting material was measured and subtracted from the initial nestlet mass to determine the proportion of nesting material used.
2.7 RNA isolation
In these studies, RNA was collected from a 1-mm coronal brain section (bregma–1.5 mm), dissected hippocampus, or Percoll-enriched microglia. For tissue, RNA was isolated using the Tri-Reagent protocol (Millipore Sigma) as previously described (O'Neil et al., 2018). For enriched microglia, whole-brain tissue was homogenized in ice-cold PBS using a 10 ml Potter-Elvehjem tissue grinder (Wheaton), and the cell pellet was resuspended in 70% isotonic Percoll (GE Healthcare). A discontinuous Percoll density gradient was layered and centrifuged at 2070 × g for 20 min. Enriched microglia were collected from the interface between the 70% and 50% Percoll layers. These cells were then pelleted and resuspended in QIAGEN Buffer RLT Plus (1:100 β-mercaptoethanol). The RNeasy Plus Mini Kit (QIAGEN) was used to purify and concentrate total RNA. Reverse transcription was performed using the High Capacity cDNA Reverse Transcription Kit (Applied Biosystems) to produce cDNA.
2.8 Quantitative real-time PCR
Quantitative real-time (q)-PCR was performed using the TaqMan Gene Expression Assay (Applied Biosystems) as previously described (O'Neil et al., 2018). In brief, experimental cDNA from coronal sections, dissected hippocampi, or enriched microglia was amplified using qPCR such that a target gene and reference gene (Gapdh) were amplified simultaneously using an oligonucleotide probe with a 5′ fluorescent reporter dye (FAM) and 3′ non-fluorescent quencher (NFQ). When Taq DNA polymerase synthesized a new strand and reached the TaqMan probe, the FAM was cleaved from the NFQ and increased the fluorescent intensity proportional to the amount of amplicon synthesized. Fluorescence was determined using a QuantStudio 3 or 5 Real-Time PCR System (Applied Biosystems). Data were analyzed using the comparative threshold cycle (ΔΔCT) method and results are expressed as fold change from a control group (“GFP Vehicle” or “Control Vehicle”). The Thermo Fisher TaqMan™ probes used in this experiment were Gapdh (Mm99999915_g1), Gfap (Mm01253033_m1), Idi1 (Mm00836417_g1), Il1b (Mm00434228_m1), Msmo1 (Mm00499390_m1), Tgfb1 (Mm01178820_m1), and Tnf (Mm00443258_m1).
2.9 IBA1 and GFAP immunofluorescence
Ionized calcium-binding adapter molecule 1 (IBA1) and GFAP were labeled as previously described (O'Neil et al., 2018). In brief, mice were transcardially perfused with ice-cold PBS (pH 7.4) followed by 4% formaldehyde. Brains were post-fixed for 24 h, cryoprotected in 30% sucrose for 24 h, frozen using dry ice-cooled isopentane, sectioned coronally at 30 μm on a Leica CM1850 cryostat, and stored in cryoprotectant (30% ethylene glycol, 30% polyethylene glycol, 40% 0.2 M phosphate buffer) for immunolabeling. Next, sections were washed in PBS, blocked (5% normal donkey serum, 0.1% Triton X-100, 1% bovine serum albumin in PBS) for 1 h, and incubated with rabbit anti-mouse IBA1 (1:1000; Wako Chemicals) and goat anti-GFAP (1:500; Abcam) primary antibodies overnight at 4°C. Next, sections were washed in PBS and incubated with donkey anti-rabbit Alexa Fluor 647- and donkey anti-goat Alexa Fluor 594-conjugated secondary antibodies for 2 h. Sections were washed with PBS, mounted on slides, and cover-slipped with Fluoromount-G (Invitrogen). Cells were then imaged using an Evos FL Auto 2 (Thermo) imaging system at 20X magnification. IBA1 and GFAP density were determined by threshold area using ImageJ software (NIH). For each mouse, four images were quantified by a trained experimenter, who was blind to the experimental groups, and averaged; these values were then used to calculate the mean and standard error for each experimental group.
2.10 Statistical analysis and data availability
RNA sequencing data were analyzed using the Seurat package in R (Stuart et al., 2019). Significant differential gene expression was identified using a Wilcoxon Rank Sum test with Bonferroni's correction for multiple comparisons. Behavioral, histological, and qPCR data were analyzed using GraphPad Prism software (San Diego, CA). To determine significant main effects and interactions between variables, two-way analysis of variance (ANOVA) was performed. Social exploration was treated as repeated measures across time and analyzed using three-way ANOVA. Comparison between groups was performed using the two-tailed Student's t-test and Bonferroni's correction for multiple comparisons. A value of p < .05 was considered statistically significant. All data are presented as mean ± standard error of the mean (SEM). The scRNA-Seq data discussed in this publication have been deposited in NCBI's Gene Expression Omnibus (Edgar, Domrachev, & Lash, 2002) and are accessible through GEO Series accession number GSE193625.
3 RESULTS
3.1 Age- and LPS-specific transcriptional profiles evident in microglia of the hippocampus
We and others have reported that “primed” microglia of aged mice contribute to an amplified and prolonged neuroinflammatory response to peripheral or central challenge with LPS (Dantzer, 2001; Godbout et al., 2005; Haroon et al., 2012; O'Neil et al., 2018). Moreover, microglial priming was concomitant with impaired regulation of microglia by astrocytes (O'Neil et al., 2018). Therefore, we aimed to further investigate the altered dynamic communication between microglia and astrocytes in the hippocampus with age following activation of the innate immune system. Here, adult (2 m.o.) and aged (18–20 m.o.) male BALB/c mice were injected i.p. with saline or LPS, and cell suspensions were isolated from the hippocampus (Figure 1a). The resulting single-cell RNA was sequenced. The time points of 4 and 24 h were selected to examine the acute and resolving phases of inflammation after peripheral challenge with LPS (Berg et al., 2004; Godbout et al., 2005; Wohleb et al., 2012). Unsupervised UMAP clustering of 33,459 cells isolated from the hippocampus identified nine distinct cell types identified by their expression of signature genes (Figure 1b,c). Notably, there was a relative overrepresentation of astrocytes and microglia from this single-cell preparation compared to the other seven cell types identified. Due to the dimensionality of the experimental design, the data for other cells types did not have not sufficient resolution for meaningful interpretation. For these reasons, the analyses were focused on microglia and astrocytes.
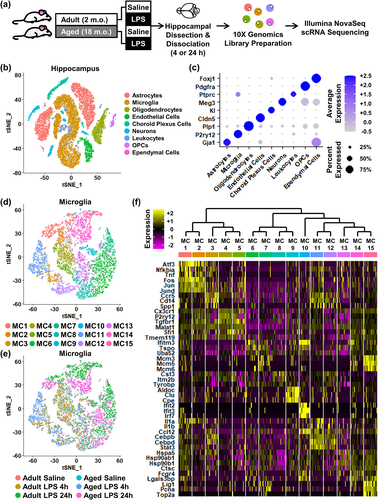
The heterogeneity of microglia was selectively analyzed, and 15 clusters of microglia, termed microglia cluster (MC) 1–15, were identified (Figure 1d). The distribution of these clusters within the six treatment groups are shown (Figure 1e). A representative heat map (relative z-score) of significant differentially expressed genes (DEGs) that identified each MC is shown (Figure 1f). These microglia clustered primarily by age and LPS challenge. For example, MC11 and MC12 robustly overlapped with the Aged LPS 4 h group (lower left quadrant of each t-SNE plot; Figure 1d,e). These data illustrate that microglia in the hippocampus had heterogeneous transcriptional profiles that dynamically shifted based on age and immune activation. How microglia transcriptional profiles were influenced by age and LPS is further delineated in Figures 2 and 3.
3.2 Differential microglial clustering after LPS challenge in the hippocampus of aged mice
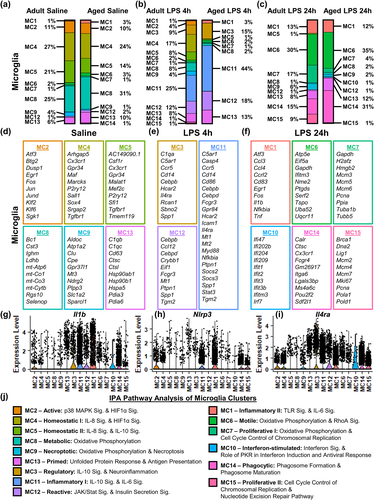
In order to examine the individual clusters of microglia, the relative percentages of each MC by treatment group were determined (Figure 2a–c). In addition, 10 or more DEGs are listed for each cluster based on fold change and significance (Figure 2d–f). There was increased representation of MC8 (31% vs. 25%) and MC13 (10% vs. 6%) in the hippocampus of aged mice compared to adults (Figure 2a). MC8 had increased expression of Cst3, mt-Co1, and Selenop while MC13 had increased expression of C1qb, C1qc, Hsp90ab1, Hsp90b1, and Hspa5 (Figure 2d). Moreover, there was a relative reduction in the representation of MC5 with age (14% vs. 21%). MC5 had increased expression of Csf1r, Cx3cr1, P2ry12, Tgfbr1, and Tmem119 (Figure 2a,d).
Acutely, LPS challenge caused a robust shift in the transcriptional profile of hippocampal microglia at 4 h (Figure 2b). Compared to saline treatment, LPS challenge (4 h) enhanced representation of MC3 (C1qa, Ccr5, Cd14, and Il4ra), MC11 (Casp4, Ccr5, Cebpd, Nfkbia, Socs3, and Stat3), and MC12 (Cebpd, Ccl12, Crybb1, and Ptpn1) (Figure 2b,e). With this acute LPS challenge, the aged hippocampus had increased representation of MC3 (15% vs. 9%), MC11 (44% vs. 25%), and MC12 (18% vs. 12%) compared to adults. In addition, the adult hippocampus maintained MC4, MC5, and MC8. These three clusters, however, had reduced overall representation in the aged hippocampus 4 h after LPS compared to adults (3% vs. 33%).
During the resolving phase (24 h after LPS), there was increased representation of MC1 (Ccl3, Ccl4, Fos, Il1b, Nfkbia, and Tnf), MC6 (Ifitm3, Tspo, and Ptgds), MC7 (Mcm3, Mcm6, and Pcna), MC14 (Cx3cr1, Fcgr4, Itga6, and Lgals3bp), and MC15 (Lig1, Mcm2, Mcm4, Mcm7, Pcna, Top2a) compared to saline controls. (Figure 2c,f). In addition, there was a relative absence of MC4 and MC5 cells in both adult and aged mice. In the aged hippocampus, LPS challenge increased the representation of MC6 (35% vs. 30%) and MC14 (31% vs. 15%) compared to adults. There was also the emergence (1% vs. 0%) of MC10 in the microglia of aged mice. Furthermore, there was a reduction in MC7 (4% vs. 17%) and MC15 (1% vs. 9%) in the aged hippocampus compared to adults.
We next aimed to gain insight into the functionality of these clusters. The top genes in each cluster were evaluated. In addition, the expression of three inflammatory genes was determined across all MCs: Il1b, a key pro-inflammatory cytokine (Figure 2g), Nlrp3, an essential inflammasome component involved in processing immature IL-1β (Figure 2h), and Il4ra, a receptor promoting the transition to alternative macrophage activation (Figure 2i). The differential expression of these genes allowed for further characterization of MCs with similar expression patterns (e.g., MC3, MC11, and MC12).
Next, IPA was used to determine significant canonical pathways (p < .05, |z| ≥ 2) for each cluster (Figure 2j). MC8 and MC13 were increased in the aged hippocampus compared to adults (Figure 2a). MC8 had activation of Oxidative Phosphorylation and expressed several mitochondrial genes, indicating a “metabolic” profile (Figure 2d,j). MC13 represented “primed” microglia because it had increased expression of complement genes, Il1b, and Nlrp3 and activation of the Unfolded Protein Response (i.e., heat shock proteins) and the Antigen Presentation Pathway (Figure 2d,g,h,j). Conversely, MC4, MC5, and MC9 had lower representation in the aged hippocampus compared to adults. Both MC4 and MC5 were termed “homeostatic” based on high expression of Csfr1, Cx3cr1, Tgfbr1, and Tmem119 and low expression of inflammatory genes (Figure 2d,g–j). MC9 was termed “necroptotic” based on IPA analysis. Overall, there was an increased “primed” microglial profile with age at the expense of “homeostatic” microglia.
Acute LPS challenge (4 h) increased MC3, MC11, and MC12 in the aged hippocampus compared to adults (Figure 2b). In general, these MC had increased expression of cytokines, chemokines, and inflammasome-related genes. For instance, MC11 had the highest expression of Il1b (Figure 2g) and Nlrp3 (Figure 2h). IPA confirmed that MC11 had an “inflammatory” profile with activation of Acute Phase Response, IL-6 Signaling, and IL-10 Signaling (Figure 2j). MC12 was termed “reactive” because it had much transcriptional overlap with MC11 but had less Il1b and limited Nlrp3 expression (Figure 2g,h). Moreover, IPA of MC12 showed increased activation of JAK/Stat Signaling and Insulin Secretion Signaling (Figure 2j). MC3 was termed “regulatory” because it had the highest expression of Il4ra (Figure 2i), low Nlrp3 expression (Figure 2h), and activation of IL-10 Signaling indicated by IPA (Figure 2j). Moreover, the adult hippocampus maintained the homeostatic (MC4 and MC5) and metabolic (MC8) microglia populations acutely after LPS. These three clusters, however, had reduced overall representation in the aged hippocampus 4 h after LPS compared to adults (3% vs. 33%). Taken together, there was a robust increase in inflammatory and reactive profiles of aged microglia 4 h after LPS that was paralleled by marked reductions in homeostatic and metabolic profiles.
LPS challenge (24 h) increased the representation of MC1, MC6, MC7, MC14 and MC15 (Figure 2c). MC1 was termed “Inflammatory II" based on the high expression of Il1b (Figure 2f,g), Nfkbia (Figure 2f), Tnf (Figure 2f), and Nlrp3 (Figure 2h) and activation of Toll-like Receptor and IL-6 Signaling by IPA (Figure 2j). In the aged hippocampus, MC6 and MC14 were further increased compared to adults. MC6 was termed “Motile” based on increased RhoA signaling. MC14 was termed “Phagocytic” based on high Fcgr4 expression and activation of the Phagosome Formation and Maturation pathways (Figure 2j). Furthermore, there was a reduction in “Proliferative I" MC7 (4% vs. 17%) and “Proliferative II" MC15 (1% vs. 9%) in the aged hippocampus compared to adults. Microglia in these proliferative clusters expressed high levels of Dna2, minichromosome maintenance (MCM) complex components, Mki67, and Pcna and had activation of Cell Cycle Control of Chromosomal Replication and Nucleotide Excision Repair Pathways (Figure 2f,j). There was also the emergence of MC10 in the hippocampus of aged mice. IPA showed that MC10 had an “Interferon-stimulated” profile (Figure 2j). Thus, microglia had prolonged LPS-induced heterogeneity in both adult and aged mice with significant suppression of homeostatic profiles (MC2 and MC4). Moreover, microglia in the adult hippocampus transition into a proliferative phenotype during the resolving phase of neuroinflammation, and this process was notably attenuated in aged mice (5% vs. 26%).
3.3 Delayed resolution of microglial activation in the aged hippocampus after LPS challenge
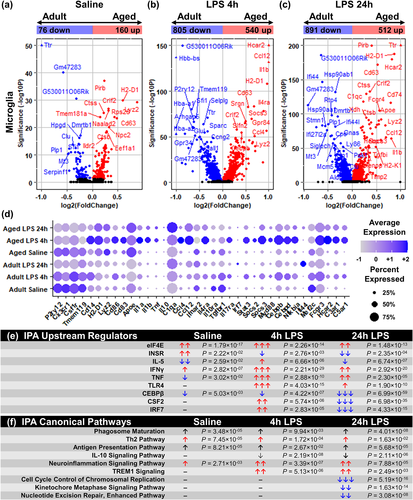
Using this scRNA-Seq data, the effect of age on the total microglial response to LPS challenge was examined. Figure 3a–c shows volcano plots comparing the microglia from the adult and aged hippocampus with saline, LPS 4 h, and LPS 24 h. As a function of age, there were 160 DEGs increased and 76 DEGs decreased with age in hippocampal microglia at baseline (Figure 3a). For example, there was higher RNA expression of lysozyme (Lyz2), major histocompatibility complex class II subunits (H2-D1), cytokine receptor-like factor 2 (Crlf2), and Cd63 and decreased expression of clusterin (Clu), hydroxyprostaglandin dehydrogenase 15 (Hpgd), and metallothionin-3 (Mt3) in hippocampal microglia of aged mice compared to adults. These data are consistent with an increased “primed” microglia profile with age (Norden et al., 2015; Norden & Godbout, 2013).
At 4 h after LPS challenge, there were 1345 DEGs between hippocampal microglia of adult and aged mice with 540 genes increased and 805 genes decreased (Figure 3b). The LPS response at 4 h had higher cytokine- and chemokine-related genes (Ccl4, Ccl12, Il1b, Il4ra) in hippocampal microglia of aged mice compared to adults with corresponding lower expression in homoeostatic genes (P2ry12, Tgfbr1, Tmem119) (Figure 3b).
As a function of age, there were 1403 DEGs identified 24 h after LPS challenge (Figure 3c). There were 512 genes increased and 891 genes decreased in the microglia of the aged hippocampus compared to adults. There was increased expression of complement genes (C1qc) and Il1b. There was also a reduction in LPS-induced heat shock genes (Hsp90aa1 and Hsp90ab1) and interferon-related genes (Ifi44 and Ifr7) in hippocampal microglia from aged mice compared to adults.
Next, several genes from the microglia clustering (Figure 2) and volcano plots (Figure 3a–c) were selected and compared across the experimental groups (Figure 3d). Again, Apoe, H2-D1, and Lyz2 had greater expression in hippocampal microglia from aged mice. Additionally, there was lower expression of genes associated with the regulation of microglial inflammation (Il10ra, Mef2c, and Tgfbr1). Again these data are consistent with a primed microglial profile with aging (Norden et al., 2015; Norden & Godbout, 2013).
Figure 3d also highlights that LPS challenge caused an amplified cytokine (Il1a, Il1b, Tnf, Il10) and chemokine (Ccl4, Ccl12) response in hippocampal microglia in aged mice compared to adults at 4 h. Genes associated with the regulation of inflammation (Cebpd, Il4ra, Myd88, Nfkbia, Socs2, Socs3, and Stat3) were also higher in hippocampal microglia in aged mice compared to adults at 4 h. There was also prolonged expression of cytokines (Il1a, Il1b, Tnf), chemokines (Ccl4, Ccl12), and complement genes (C1qa) 24 h after LPS in hippocampal microglia of aged mice compared to adults. It is important to highlight that these genes were highly concentrated within the “Inflammatory II" microglia (MC1) evident at 24 h after LPS (Figure 2f). Taken together, these data characterize a distinct transcriptional profile present in microglia in the aged hippocampus, but absent in adults, after peripheral LPS challenge.
IPA analysis of the effect of age and LPS on all microglia is represented in Figure 3e,f. Significant upstream regulators and canonical pathways are shown. With age, there was increased signaling associated with eukaryotic translation initiation factor 4E (eIF4E), insulin receptor, and interferon (IFN)-γ with reductions in signaling associated with IL-5, tumor necrosis factor (TNF), and CCAAT/enhancer-binding protein (CEBP)-β signaling (Figure 3e). There was also age-associated activation in Phagosome Maturation, Th2 Pathway signaling, Antigen Presentation Pathway signaling, and Neuroinflammation signaling (Figure 3f).
After LPS injection, there were increased signaling associated with eIF4E, IFNγ, TNF, and toll-like receptor (TLR)-4 at 4 and 24 h in hippocampal microglia from aged mice compared to adults. Moreover, there was age-associated activation of triggering receptor expressed on myeloid cells (TREM)-1 signaling and Neuroinflammation at 4 and 24 h. In addition, IL-10 Signaling was inhibited in microglia from the aged hippocampus compared to adults. Notably Il10 mRNA expression was undetectable in microglia in this scRNA-Seq data set, but IPA indicates that genes associated with IL-10 signaling were decreased in microglia from aged mice after LPS. Finally, Cell Cycle Control of Chromosomal Replication, Kinetochore Metaphase Signaling, and Nucleotide Excision Repair were all inhibited in hippocampal microglia from aged mice compared to adults at 24 h. Collectively, the microglia of the aged hippocampus have an amplified and prolonged response to LPS associated with reduced regulation and turnover.
3.4 Reduced astrocyte-mediated cellular interactions with age
We have previously provided evidence of decreased IL-10 receptor signaling in astrocytes from aged mice after peripheral LPS challenge (Norden, Trojanowski, Walker, et al., 2016). Moreover, this impairment may lead to dysfunctional anti-inflammatory feedback between microglia and astrocytes after LPS (Norden et al., 2014; Norden, Trojanowski, Walker, et al., 2016). To gain insight into this impaired interaction between astrocytes and microglia, Cell Chat analysis of cell–cell communication was examined (Jin et al., 2021). Because comparisons represented single population values for each group, statistical analysis was not included. Microglia within the adult and aged hippocampus have similar levels of incoming and outgoing signals (Figure 4a,b). In addition, outgoing signal strength from microglia was relatively stable across all groups. The one exception was increased outgoing signaling in microglia of aged mice 4 h after LPS (Figure 4b). Furthermore, astrocytes in the aged hippocampus had decreased levels of both incoming and outgoing signals compared to adults across all treatment groups (Figure 4c,d). We interpret these data to suggest that a lack of astrocyte receptor activity leads to reduced astrocyte communication with and regulation of microglia.
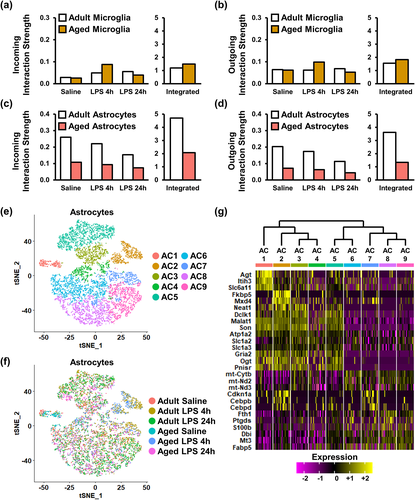
Based on these data, the heterogeneity of astrocytes was analyzed, and nine clusters of astrocytes, termed astrocyte cluster (AC) 1–9, were identified (Figure 4e). The distribution of these nine clusters within the experimental groups is shown (Figure 4f). Figure 4g shows a representative heat map (relative z-score) of significant DEGs that identified each AC1-9. These AC were less diverse compared to the clustering of microglia, and there were similar gene expression patterns within AC1-4 and AC5-9. For instance, AC1-4 had increased Gfap, S100b, and Nfkbia while AC5-9 had increased Gria2, Neat1, and Son. Overall, the RNA profiles of hippocampal astrocytes from aged mice had reduced cell–cell interactions and were influenced by age and LPS.
3.5 Impaired metabolism-related activation of astrocytes after LPS challenge in the aged hippocampus
Continuing with the astrocyte analysis from Figure 4, the percentages of each of the nine clusters are provided as a function of age and LPS (Figure 5a–c). In addition, 10 DEGs, based on fold change and significance, are listed for each cluster (Figure 5d–f). As a function of age, AC1 (Gria1, Slc6a9, Slc6a11, Spon1, and Trpm3) had higher representation in the aged hippocampus (25% vs. 4%) compared to adults (Figure 5a,d). This was concomitant with age-associated reductions in AC5 (11% vs. 19%) and AC6 (16% vs. 27%).
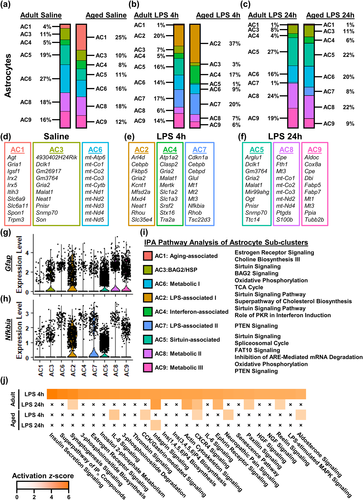
As a function of LPS at 4 h, there was an expansion of AC2 (Fkbp5, Gria2, and Kcnt1) and AC7 (Cebpd, Mt1, Mt2, and Nfkbia) (Figure 5b,e). Moreover, aging increased representation of AC2 (37% vs. 20%), AC4 (17% vs. 4%), and AC7 (20% vs. 17%) compared to adults 4 h after LPS. Alongside these increases, the aged hippocampus had decreased representation of AC5 (1% vs. 16%) and AC9 (6% vs. 14%).
During the resolving phase of neuroinflammation, there was increased representation of AC5, AC8, and AC9 in the hippocampus at 24 h compared to 4 h (Figure 5c). This distribution of clusters was similar to what was detected at baseline. As an effect of age, there was increased representation of AC6 (20% vs. 16%) and AC7 (8% vs. 1%) in the aged hippocampus compared to adults. In addition to these increases, AC9 (Aldoc, Cox8a, Mt1, Mt3, and Ppia) had decreased representation in the aged hippocampus compared to adults (9% vs. 19%).
Similar to the microglia, we aimed to provide insight on these AC. These clusters were more difficult to define compared to microglia. Nonetheless, the evaluation included the significant DEGs, expression of the structural and activity-associated gene Gfap (Figure 5g), expression of the inflammation-associated gene Nfkbia (Figure 5h), and IPA. The AC1 profile was termed “Aging-associated” because it was relatively absent in adults at baseline (25% vs. 4%). IPA shows that “Aging-associated” astrocytes had a transcriptional profile associated with increased Estrogen Receptor Signaling (Figure 5i). This profile, however, was no longer evident after LPS in either age group. The assumption is that this “Aging-associated” profile in aged astrocytes shifted to another transcriptional profile after LPS treatment. AC3 had a profile associated with Sirtuin and BAG2 Signaling (Figure 5i). AC6 was termed “metabolic” based on high expression of mitochondrial genes and activation of Oxidative Phosphorylation and TCA Cycle (Figure 5d,i). Taken together, hippocampal astrocytes were heterogeneous at baseline with primarily regulatory and metabolic transcriptional profiles with the addition of AC1 in the aged hippocampus.
The AC2 profile was uniquely expressed 4 h after LPS and had higher representation in the aged hippocampus compared to adults (Figure 5b). For this reason, this cluster was termed “LPS-associated I". AC2 had robust expression of Gfap (Figure 5g) and the inflammation-related Nfkbia (Figure 5h). IPA of AC2 showed activation of Sirtuin Signaling and Cholesterol Biosynthesis (Figure 5i). Additionally, AC4 and AC7 also had higher representation in the aged hippocampus compared to adults 4 h after LPS (Figure 5b). AC4 was termed “IFN-associated” based on the IPA showing PKR in Interferon Induction (Figure 5i). AC7 was also uniquely increased with LPS challenge at 4 h. AC7 had the highest levels of Nfkbia (Figure 5h) and increased Phosphatase and Tensin Homolog (PTEN) signaling (Figure 5i). Concomitantly, there was reduced representation of AC5 in the hippocampus of aged mice. This AC5 was termed “Sirtuin-associated” based on increased Sirtuin Signaling (Figure 5i) and expression of Gfap (Figure 5g) and Nfkbia (Figure 5h). During the resolving phase of neuroinflammation, many of the astrocyte profiles retuned to baseline level. In the aged hippocampus 24 h after LPS, however, there was a persistence of the LPS-associated AC7 and reduction in Sirtuin-associated AC5 compared to adults. These data are interpreted to indicate there is a more inflammatory and less metabolic astrocyte profile after LPS in the aged hippocampus.
IPA identified 99 significant canonical pathways (z ≥ 4 shown) increased in astrocytes from the adult hippocampus 4 h after LPS (Figure 5j). In aged astrocytes, however, only five of these pathways were activated by LPS at 4 h (Aldosterone Signaling, Cholecystokinin/gastrin-mediated Signaling, C-X-C Motif Chemokine Receptor [CXCR]-4 Signaling, IL-8 Signaling, and Neuropathic Pain Signaling). At 24 h after LPS, four different pathways that were activated in adult astrocytes were activated in the aged hippocampus (Integrin-linked Kinase (ILK) Signaling, Integrin Signaling, Necroptosis Signaling, and Nuclear Factor Erythroid 2-related Factor 2 (NRF2)-mediated Oxidative Stress Response). Collectively, astrocytes were activated by LPS challenge, but this activation was significantly suppressed within the aged hippocampus.
3.6 Reduced astrocytic cholesterol biosynthesis pathways in the aged hippocampus after LPS
Similar to the microglia, the effect of age and LPS was examined on the total astrocyte population. Figure 6a–c shows volcano plots comparing the adult and aged astrocytes with saline, LPS 4 h, and LPS 24 h. As a function of age, there were 64 DEGs increased and 101 DEGs decreased in the astrocytes of the aged hippocampus (Figure 6a). For example, there was higher RNA expression of angiotensinogen (Agt), apolipoprotein E (Apoe), complement component 4B (C4b), GABA transporter Slc6a11, and spondin-1 (Spon1) in the astrocytes from the aged hippocampus compared to adults. There was also lower expression of several mitochondrial genes in the aged astrocytes compared to adults.
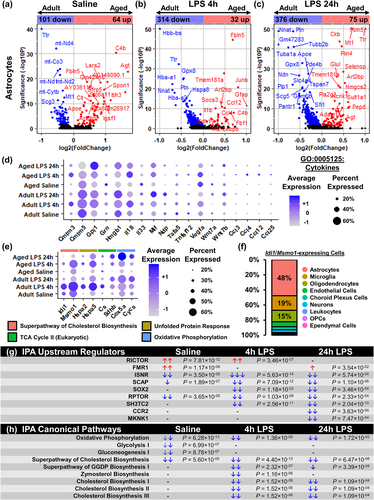
Acutely 4 h after LPS, 32 DEGs were increased and 314 DEGs were decreased with age (Figure 6b). Thus, most of the astrocyte genes activated by LPS in adults were not activated in the aged astrocytes. Inflammatory genes were higher in the aged astrocytes with elevated expression of Ccl4, Gfap, and Il18. The age-associated suppression of gene expression in astrocytes was also evident 24 h after LPS (Figure 6c). Here, there were 75 DEGs that were increased and 376 DEGs that were decreased in the astrocytes of the aged hippocampus compared to adults (Figure 6c).
Next, cytokine genes (GO:0005125) were selected and compared across the experimental groups (Figure 6d). Notably, astrocytes in the hippocampus expressed very few cytokines (e.g., Cmtm5, Gpi1, Hmgb1, and Il18) and chemokines (Ccl4 and Ccl2) after LPS challenge. Moreover, Figure 6e shows differences in expression of genes associated with Cholesterol Biosynthesis, Unfolded Protein Response, TCA Cycle, and Oxidative Phosphorylation across all treatment groups. Again, key genes associated with Cholesterol Biosynthesis (Idi1 and Msmo1) and Unfolded Protein Response (Hspa2 and Hspa5) were lower in hippocampal astrocytes from aged mice, and expression of genes associated with TCA Cycle (Cs and Sdhb) and Oxidative Phosphorylation (Cox5a and Cycs) were increased with both LPS and age. In fact, astrocytes represented 48% of cells expressing Idi1 and/or Msmo1 (Figure 6f). These findings indicate astrocytes contribute to a large portion of cholesterol biosynthesis within the brain. Thus, astrocytes had the majority expression of key genes attributed to cholesterol biosynthesis.
IPA of the effect of age on all astrocytes within each treatment group is represented in Figure 6g,h. Astrocytes from aged mice had reduced expression of genes associated with insulin receptor signaling and regulatory-associated protein of MTOR complex 1 (RPTOR) signaling (Figure 6g). These upstream regulators, and others, were further suppressed by LPS challenge. Moreover, there was age-associated inhibition of several metabolic pathways including Oxidative Phosphorylation, Glycolysis, Gluconeogenesis, and Cholesterol Biosynthesis (Figure 6h). Additionally, LPS challenge suppressed Cholesterol Biosynthesis pathways I, II, and III in hippocampal astrocytes from aged mice. This was concomitant with inhibition of Geranylgeranyldiphosphate (GGDP) Biosynthesis and Zymosterol Biosynthesis. Taken together, astrocytes activated several key pathways in response to LPS that were associated with a metabolic response, rather than a cytokine response. Moreover, cholesterol biosynthesis, in addition to other metabolic pathways, was impaired in the astrocytes of the aged hippocampus.
3.7 Persistence of neuroinflammatory response to LPS challenge after augmentation of astrocytic TGFβ signaling in the aged hippocampus
We next sought to determine if the age-associated exaggerated neuroinflammatory response to LPS challenge could be attenuated. To complete this aim, an AAV2/5 construct was designed to overexpress a mutated, biologically active TGFβ1 protein in astrocytes under the GFAP promoter. Here, aged mice received a bilateral hippocampal injection of either AAV-GFP or AAV-TGFβ (Figure 7a). To validate the experimental design, astrocytic GFP expression was confirmed using immunohistochemistry 4 weeks after injection of the GFP control virus (Figure 7b).
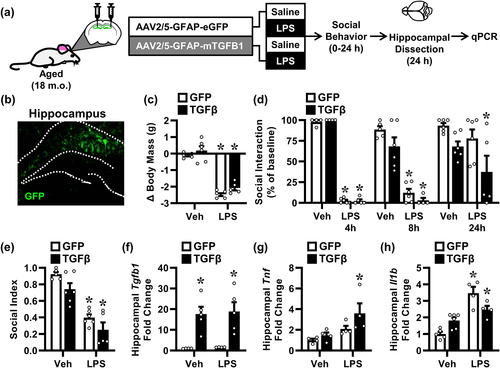
Four weeks after viral administration, aged mice were injected with i.p. saline or LPS. As expected, aged mice injected with LPS lost significant body mass (F[1, 17] = 175.0, p < .0001; Figure 7c). This reduction, however, was independent of the AAV-TGFβ intervention. Moreover, LPS induced robust sickness response with reduced social exploratory behavior (Figure 7d). This reduction in social interaction was evident at both 4 h (F[1, 15] = 3844, p < .0001), 8 h (F[1, 17] = 92.84, p < .0001), and 24 h (F[1, 19] = 4.586, p < .05) after LPS challenge. Nonetheless, there was no effect of the AAV-TGFβ intervention on behavior. Social index was calculated as the normalized area under the curve for this 24-h behavior (Figure 7e). There was a main effect of both LPS (F[1, 18] = 67.80, p < .0001) and AAV-TGFβ intervention (F[1, 18] = 7.10, p < .05). Post hoc analysis revealed that LPS decreased social index for both AAV-GFP (p < .0001) and AAV-TGFβ mice (p < .0001), and this was independent of the AAV-TGFβ intervention. Thus, the hippocampal AAV-TGFβ treatment in aged mice was insufficient to attenuate the prolonged behavioral sickness response to LPS.
Hippocampi were collected 24 h after LPS, and Tgfb1, Il1b, and Tnf mRNA levels were determined. While hippocampal Tgfb1 expression was not effected by LPS, expression was increased by AAV-TGFβ treatment (F[1, 14] = 26.83, p < .001) (Figure 7f). This induction of Tgfb1 expression was absent in AAV-GFP control mice. Thus, as anticipated, AAV-TGFβ treatment enhanced Tgfb1 expression in the hippocampus of both saline- and LPS-treated mice (p < .01 for both). LPS increased expression of Tnf in the hippocampus (F[1, 12] = 9.092, p < .05), but the AAV-TGFβ intervention had no effect this Tnf induction (Figure 7g). LPS also increased Il1b mRNA (F[1, 15] = 45.01, p < .0001) in the hippocampus. This increase was reduced by AAV-TGFβ treatment (p < .05) (Figure 7h). In summary, augmentation of Tgfb1 expression in the hippocampus of aged mice ameliorated part of the age-associated exaggerated response to LPS but was insufficient to attenuate the behavioral sickness response.
3.8 Increased glial activation in Ast-Il10raKO mice after LPS
Our scRNA-Seq data provides evidence that aged astrocytes in the hippocampus had reduced interactions with microglia and synthesized less cholesterol. Moreover, LPS injection caused an amplified inflammatory response in the hippocampus combined with reduced microglial IL-10 signaling in aged mice. Thus, we sought to determine if selective disruption of IL-10 receptor signaling in astrocytes resulted in an amplified microglial response to LPS challenge. To address this, IL-10Rα expression was selectively targeted in astrocytes using an inducible Aldh1l1-Cre/ERT2 construct. For initial validation of selectivity, these Aldh1l1-Cre/ERT2 mice were crossed with R26-tdTomato mice to create Aldh1l1-CreER+::R26-tdTomato mice (Figure 8a). The expression of the tdTomato red fluorescent protein in the hippocampus selectively in astrocytes was confirmed 4 weeks after tamoxifen treatment. Next, these Aldh1l1-Cre/ERT2 mice were crossed with IL-10Rαflox mice to create an astrocyte-specific knockout of the IL-10Rα (Ast-Il10raKO) (Figure 8b). Four weeks after tamoxifen treatment, mice were injected with i.p. saline or LPS, and activation of microglia and astrocytes was determined 24 h later.
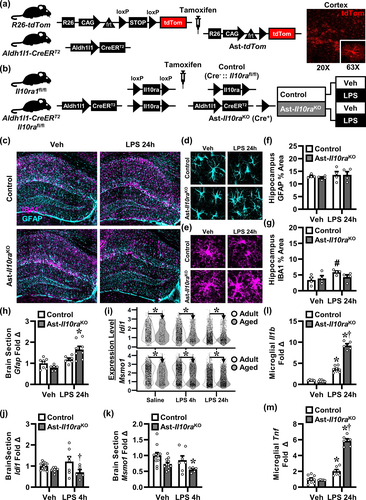
GFAP and IBA1 labeling was used to assess the density of astrocytes and microglia in the hippocampus (Figure 8c). There were not any significant differences in the percent area of GFAP labeling in the hippocampus as a function of LPS or genotype (Figure 8d,f). There was, however, a tendency for increased IBA1 labeling 24 h after LPS challenge (F[1, 12] = 4.056, p = .0670) (Figure 8e,g). The density of IBA1 labeling in the hippocampus of control mice after LPS tended to be increased compared to saline-treated controls (p = .0644) (Figure 8g).
Next, Gfap mRNA expression was determined in a coronal brain section (through cortex and hippocampus). As expected, LPS had a significant effect on Gfap expression (F[1, 24] = 22.98, p < .0001) (Figure 8h). Post hoc analysis of Gfap mRNA expression was highest in the LPS-treated Ast-Il10raKO mice compared to all other groups (p < .0001). In addition, isopentenyl-diphosphate delta isomerase (Idi1) and methylsterol monoxygenase 1 (Msmo1) mRNA levels were also determined in the coronal brain section. These enzymes are associated with cholesterol biosynthesis and are highly expressed in astrocytes (Figure 6e,f). Our scRNA-Seq data confirms Idi1 and Msmo1 expression decreased with age in the hippocampus (Figure 8i). Moreover, Ast-Il10raKO mice also had reduced expression of Idi1 (F[1, 32] = 9.982, p < .01) and Msmo1 (F[1, 30] = 9.197, p < .01) in a coronal brain section compared to controls (Figure 8j,k). Post hoc analysis revealed that Ast-Il10raKO mice had decreased levels of Idi1 after LPS challenge (p < .05) and Msmo1 at baseline (p < .05). These data are interpreted to indicate that IL-10 signaling influences cholesterol biosynthesis in astrocytes.
To assess microglial activation, mRNA levels of Il1b and Tnf in Percoll-enriched microglia were determined 24 h after LPS. There was a main effect of LPS on microglial Il1b mRNA expression (F[1, 21] = 485.8, p < .0001) that was dependent on Ast-Il10raKO (F[1, 21] = 135.8, p < .0001) (Figure 8l). In addition, LPS increased microglial Tnf expression (F[1, 23] = 226.1, p < .0001), and this too was dependent on Ast-Il10raKO (F[1, 23] = 97.03, p < .0001) (Figure 8m). For instance, Il1b and Tnf induction by LPS was potentiated in the Ast-Il10raKO mice compared to controls (P < 0.0001, for both). Taken together, selective loss of IL-10Rα in astrocytes was associated with higher microglial expression of Il1b and Tnf after LPS challenge.
3.9 Amplified sickness behavior in Ast-Il10raKO mice after LPS
In order to confirm these molecular changes led to dysfunctional sickness behavior, body mass, food intake, nest building, and social behavior were assessed over 24 h after LPS challenge (Figure 9a). As expected, LPS injection reduced body mass over 24 h (F[1, 35] = 414.1, p < .0001), and this reduction was exacerbated by Ast-Il10raKO (F[1, 35] = 12.90, p < .001) (Figure 9b). Post hoc analysis confirmed that the Ast-Il10raKO mice lost more weight compared to all other treatment groups, including the LPS-treated control mice (p < .001). LPS challenge also reduced food consumption over 24 h (F[1, 33] = 243.2, p < .0001), and this reduction was exacerbated by Ast-Il10raKO (F[1, 33] = 18.64, p < .001) (Figure 9c). Indeed, post hoc analysis confirmed Ast-Il10raKO mice consumed less food compared to all other groups, including the LPS-treated control mice (p < .001).
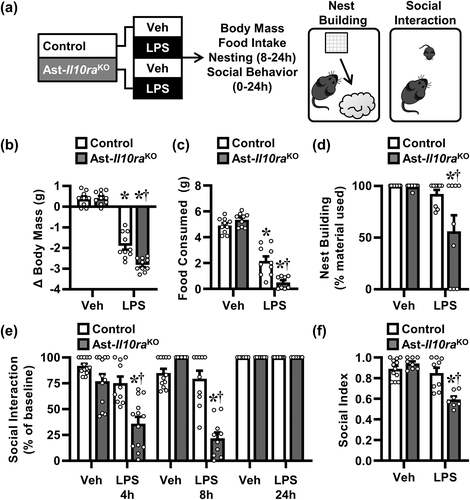
Two behavioral parameters of sickness, nest building and social behavior with a juvenile mouse, were also evaluated. LPS challenge reduced the percentage of material used for nest-building from 8 to 24 h (F[1, 32] = 10.03, p < .01), and this was dependent on Ast-Il10raKO (F[1, 32] = 4.885, p < .05) (Figure 9d). Indeed, LPS-treated Ast-Il10raKO used less nesting material compared to all other groups, including LPS-treated control mice (p < .05). As expected, LPS challenge also caused a time-dependent reduction in social interaction (F[2, 28] = 7.189, p < .01) (Figure 9e). For example, LPS decreased social interaction 4 h (F[1, 44] = 25.22, p < .0001) and 8 h (F[1, 35] = 54.49, p < .0001), and this LPS-induced social withdrawal was exacerbated by Ast-Il10raKO (4 h: F[1, 44] = 4.501, p < .05; 8 h: F[1, 35] = 40.90, p < .0001). Post hoc analysis indicates that LPS-treated Ast-Il10raKO mice spent less time interacting at 4 h (p < .001 for all) and 8 h (p < .0001 for all) compared to all other groups. Social index was calculated as the normalized area under the curve for this 24-h behavior (Figure 9f). LPS challenge reduced social index (F[1, 32] = 30.69, p < .0001), and this reduction was dependent on Ast-Il10raKO (F[1, 39] = 18.95, p < .0001) (Figure 9f). For instance, LPS-treated Ast-Il10raKO mice had a significant reduction in social index compared to all other experimental groups, including LPS-treated controls (p < .001). Thus, the selective loss of IL-10Rα in astrocytes resulted in an amplified sickness response to LPS challenge with enhanced social withdrawal.
4 DISCUSSION
Here, we provide novel data identifying the transcriptional profiles of several clusters of microglia and astrocytes with aging and inflammation. In these studies, there was an expansion of hyper-inflammatory microglia 4 h after peripheral immune challenge in the aged hippocampus compared to adult. Furthermore, microglia in the aged hippocampus failed to enter a proliferative state during the resolving phase of neuroinflammation. Moreover, astrocytes in the aged hippocampus had significantly reduced metabolic activity and regulatory function following LPS challenge compared to adults. Thus, amplified microglial activation in the aged hippocampus was associated with the absence of regulatory signaling from astrocytes. Indeed, novel data shows knockout of the astrocytic IL-10 receptor resulted in an exaggerated and prolonged transcriptional and behavioral response to peripheral LPS, similar to the response detected in aged mice.
One important finding of this study was the identification of the primed microglial profile paralleled by an immunosenescent astrocyte profile in the hippocampus of aged (18 m.o.) mice. For microglia, this primed MC13 was increased approximately 2-fold in the hippocampus of aged mice. This age-related cluster of microglia had higher expression of genes associated with heat shock and complement (C1q) pathways. This cluster also had increased baseline expression of several inflammatory genes, including Il1b. Notably, across all of the aged microglia, there were global increases in major histocompatibility complex class II subunits, CD63, lysozyme, and APOE alongside global decreases in TGFβR, IL-4Rα, and monocyte enhancer factor (MEF)-2C. These data are consistent with previous studies of a primed mRNA profile in the aged brain with enhanced inflammatory genes and reduced anti-inflammatory genes (Norden & Godbout, 2013). Additionally, we and others previously attempted to “rejuvenate” these primed microglia using the colony-stimulating factor 1 receptor antagonist PLX5622 (Elmore et al., 2018; O'Neil et al., 2018). This turnover resulted in microglia that had an intermediate RNA profile with reduced lipofuscin burden (O'Neil et al., 2018). Nonetheless, forced microglial turnover in the aged brain did not reverse their amplified neuroinflammatory response to LPS (O'Neil et al., 2018). Our interpretation of these data was that the aged microenvironment, involving astrocytes, influenced microglial priming. Consistent with this idea, Cell Chat cell–cell communication analysis indicated that astrocytes in the aged hippocampus had less incoming and outgoing signal strength. Moreover, astrocytes in the aged hippocampus appeared immunosenescent by RNA profile and IPA with impairments in cholesterol biosynthesis, oxidative phosphorylation, glycolysis, and gluconeogenesis. A study of aged “ribotagged” mice showed that hypothalamic astrocytes had decreased expression of cholesterol biosynthesis genes concomitant with increased expression of cholesterol transport genes (Boisvert et al., 2018). We extend these findings by showing astrocytes in the aged hippocampus fail to respond to peripheral immune challenge with the production of cholesterol, as detected in adult mice. AC prominent in adults (AC3 and AC5) also had increased Sirtuin Signaling. This is relevant to aging because sirtuins regulate genomic stability, nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) signaling, and glucose homeostasis (Kanfi et al., 2010; Kawahara et al., 2009; Zhong et al., 2010). Overall, both microglia and astrocytes in the aged hippocampus were robustly altered with age, and this set the stage for impaired glial dynamics during the response to LPS challenge.
Another key finding of this study was the hyperactivity of microglia and their failure to resolve the inflammatory process in the hippocampus of aged mice. For example, there was enhanced representation of the “inflammatory” and “reactive” clusters (MC11 and MC12) in the hippocampus of aged mice. For instance, MC11 was overrepresented in the aged hippocampus 4 h after LPS challenge and had increased expression of CEBP-β and -γ, transcription factors that regulate acute-phase cytokine genes, myeloid differentiation primary response 88 (MYD88), a TLR adapter protein, and NF-κB activation. After 24 h, the inflammatory profile was confined to MC1. This cluster of microglia was identifiable by its heightened expression of inflammatory cytokines (Il1b, Tnf) and chemokines (Ccl3, Ccl4, Ccrl2). Moreover, these MC1 genes were expressed at higher levels in aged mice compared to adults. These data are consistent with previous studies showing amplified Il1b expression in the aged brain (Godbout et al., 2005; Henry et al., 2009). We extend these findings with our scRNA-seq data to provide a more specific transcriptional identification of the microglial response to peripheral LPS challenge as a function of age and time. Additionally, there was a two-fold increase in “Phagocytic” MC14 in the hippocampus of aged mice after 24 h after LPS compared to adults. Notably, none of these LPS-induced clusters fit the previously described disease-associated microglia (DAM) or trauma-associated microglia profiles evident with Alzheimer's disease, amyotrophic lateral sclerosis, or traumatic brain injury (Keren-Shaul et al., 2017; Witcher et al., 2021). Several known DAM genes, however, including Axl, Csf1, Ctsl, and Lilrb4a were expressed in the “Inflammatory” MC1. These DAMs are considered “protective” in Alzheimer's disease and amyotrophic lateral sclerosis, and the most overlap in gene expression was in the Il1b-, Tnf-, and Nfkbia-expressing “Inflammatory” MC1 cells. Thus, we interpret these data to indicate that LPS does not induce a DAM profile, and this profile was not present in aged, but otherwise healthy BALB/c mice.
Related to the topic above, some macrophage-specific genes were detected in the MCs. Monocytes, macrophages, and microglia are closely related myeloid cells, and there is overlap in their transcriptional profiles, especially under inflammatory conditions (Derecki et al., 2014; Grassivaro et al., 2020; Prinz et al., 2014). For example, MC14 (which was designated “phagocytic”) had an understandable overrepresentation of macrophage-related genes. However, the MC here all had high expression of microglia-specific markers (P2ry12, Tmem119, and Sall1) and, therefore, was defined as microglia. We do not surmise that these cells were bone marrow-derived and infiltrating from the peripheral blood. In previous work with LPS, we have not observed an increase in myeloid cell recruitment to the brain with age and LPS (Norden, Trojanowski, Villanueva, et al., 2016). Here there were very few leukocytes clusters (determined by expression of Ptprc) present in the scRNA-Seq analyses of the hippocampus (Figure 1d). Based on these data, we conclude that these reported MC profiles are confined to the resident microglia.
There were other important transcriptional profiles of microglia identified here. In addition to the presence of a primed microglial profile in the adult hippocampus, there were unique subsets of the “Proliferative” microglia (MC7 and MC15) in the hippocampus 24 h after LPS in adults. In fact, approximately 26% of microglia in the adult hippocampus enters this proliferative state in the resolving phase of neuroinflammation (Figure 2c). This profile was absent in the aged hippocampus. Moreover, IPA of canonical pathways showed robust inhibition of mitotic signaling pathways in hippocampal microglia in aged mice 24 h after LPS challenge. This is relevant because there is turnover of long-lived tissue macrophages in the periphery under inflammatory conditions (Gentek et al., 2014). Thus, it is reasonable to predict that highly activated microglia turn over after an inflammatory response in the brain. Indeed, in a model of a demyelinated brain, microglia undergoes necroptosis and repopulation to shift from a pro-inflammatory profile to a pro-regenerative state (Lloyd et al., 2019). In this case, this process was driven by IFNα/β receptor subunit 2 (IFNAR2) signaling in microglia. Here, microglia in the aged hippocampus had reduced levels of Ifnar2 at 4 and 24 h after LPS challenge (Figure 3d). This suggests a type I interferon-driven mechanism for microglial turnover is impaired with age in microglia. Taken together, these RNA and IPA data indicate microglial turnover occurs during the resolving phase of neuroinflammation, and this process is impaired with normal aging.
A major finding of these studies was the immunosenescent profile of astrocytes in the aged hippocampus that was evident after LPS challenge. For instance, there were 314 DEGs with higher expression in astrocytes from the adult hippocampus and only 32 DEGs with higher expression in astrocytes from the aged hippocampus 4 h after LPS (Figure 6b). Moreover, several major metabolic and biosynthesis pathways were activated in astrocytes from the adult hippocampus after LPS challenge, but these pathways were inhibited in the aged hippocampus. Previous data indicates astrocytic anti-inflammatory pathways may be inhibited by normal aging, including TGFβ signaling (Norden, Trojanowski, Walker, et al., 2016). As such, we enhanced astrocytic production of TGFβ in the aged hippocampus using an AAV construct. Our AAV-TGFβ data, however, show restoration of Tgfb1 alone in the aged hippocampus does not resolve the age-associated hyper-neuroinflammatory response to peripheral immune challenge. This may be due to the lack of TGFβ augmentation in other brain regions, like the hypothalamus, but it is also likely TGFβ is not the only anti-inflammatory signal required to attenuate this microglial inflammation. Collectively, intact IL-10/TGFβ and cholesterol signaling appear critical in the normal regulation of the neuroinflammatory response to peripheral immune challenge.
Novel evidence is provided that astrocytes in the aged hippocampus had markedly decreased cholesterol biosynthesis and expression of APOE in response to LPS. IPA revealed deficits in Cholesterol Biosynthesis I, II, and III in aged astrocytes. In addition, genes associated with cholesterol biosynthesis, including Idi1 and Msmo1, were expressed at lower levels and less activated by LPS in aged mice. Apoe expression was also lower astrocytes from aged mice compared to adults 24 h after LPS. APOE is responsible for shuttling cholesterol to neurons, oligodendrocytes, and microglia and facilitates microglial cholesterol catabolism via low-density lipoprotein receptors. Cholesterol and TGFβ are both necessary to promote a healthy, homeostatic phenotype in microglia and other brain cells (Bohlen et al., 2017). In fact, cholesterol is a major component of the cell membrane and is required for microglia to survive and proliferate (Curi et al., 2017). Related to the discussion above, reduced cholesterol availability would negatively influence self-renewal of microglia. Thus, cholesterol biosynthesis is another astrocytic feedback pathway that is impaired with age that likely contributes to extended neuroinflammation after peripheral LPS.
A relevant point for discussion is that astrocytes did not have a “pro-inflammatory” profile with LPS challenge in either adult or aged mice. We also did not detect transcriptional profiles matching the previously described A1 or A2 designations of astrocytes (Liddelow & Barres, 2017). Here, very few cytokine genes (GO:0005125) were expressed in astrocytes, and the ones that were expressed were at low levels. While it is clear there are critical interactions between microglia and astrocytes, these interactions are more complicated than having binary classifications.
Another significant finding was that the loss of IL-10 receptor signaling selectively in astrocytes resulted in a prolonged neuroinflammatory response that was similar to what was detected in aged mice (O'Neil et al., 2018). IPA of the scRNA-Seq data confirmed that anti-inflammatory IL-10 signaling was inhibited in the aged brain. Moreover, we have previously shown astrocytes isolated from IL-10RKO mice did not resolve the microglial response to LPS in vitro when IL-10 was added directly to the culture (Norden et al., 2014; Norden, Trojanowski, Walker, et al., 2016). Here, we extend these findings in vivo with an astrocyte-specific transgenic mouse model. Indeed, knockout of astrocytic Il10ra resulted in prolonged microglial Il1b and Tnf mRNA expression 24 h after LPS challenge. Additionally, Gfap expression was potentiated in LPS-injected Ast-Il10raKO mice. Of note, no difference in microglial and astrocytic morphology was detected in these mice. Consistent with other studies, these data indicate that glial morphology was not an accurate measure of inflammation in terms of cytokine production (Norden, Trojanowski, Villanueva, et al., 2016). We also provide novel data that knockout of the astrocytic IL-10 receptor in adult mice reduced transcription of the cholesterol biosynthesis enzymes Idi1 and Msmo1. Thus, intact IL-10 receptor signaling in astrocytes may be required for astrocytic cholesterol biosynthesis. Furthermore, Ast-Il10raKO mice lost more body weight, ate less food, displayed impaired nesting behavior, and had amplified social withdrawal after LPS compared to controls. Notably, this response was parallel to the sickness response in aged mice. These Ast-Il10raKO studies expand on our previous data showing astrocytes in the aged brain have decreased expression of the IL-10 receptor and, therefore, did not produce TGFβ in response to microglial inflammation (Norden, Trojanowski, Walker, et al., 2016). Here, we show that IL-10R knockout selectively on astrocytes (of adult mice) resulted in prolonged microglia-mediated inflammation and extended sickness behavior with LPS challenge. Thus, one can elicit an “aged-like” deficit in microglia in the brain of young mice by knocking out one regulatory pathway (IL-10) in astrocytes. Our data cements that astrocytes are critical in the regulation of microglia under inflammatory conditions and this astrocyte-mediated regulation changes dramatically with normal aging.
Of note, microglial IL-10 receptor signaling is necessary for a return to microglial homeostasis, and mice with microglia-specific Il10ra knockout had neuronal impairment and died in response to a high dose of 2.5 mg/kg LPS (Shemer et al., 2020). This response was dependent on microglial TNF autocrine signaling, and without this TNF signaling, microglial IL-10 receptor knockout had no effect on this response. In this model, however, astrocytic IL-10 receptor signaling was intact. Thus, IL-10 signaling in the resolving phase of neuroinflammation is important for both microglia and astrocytes.
In summary, we provide novel and compelling data that microglia in the aged brain are hyper-reactive due to a lack of anti-inflammatory feedback from immunosenescent astrocytes. This lack of an astrocytic response involves both metabolic and regulatory pathways. Impairment in the astrocytic regulation of microglia with LPS challenge leads to an exaggerated and prolonged neuroinflammatory response in aged and Ast-Il10raKO mice.
ACKNOWLEDGEMENTS
This research was supported by National Institute of Health grant R01-AG051902 to Jonathan P. Godbout. Shane M. O'Neil was supported by an OSU Dean's Distinguished University Fellowship and the National Institute of Dental and Craniofacial Research Training Grant T32-DE014320. The authors thank Dr. Andy Fischer for the use of the 10X Genomics Chromium Controller. The authors also thank Dr. Kathrin Meyer for her expertise in viral vector synthesis and administration. This work was supported in part by an allocation of computing time from the Ohio Supercomputing Center.
AUTHOR CONTRIBUTIONS
S. M. O. and J. P. G. planned experiments and wrote the manuscript. S. M. O., E. E. H., S. J., and L. M. W. performed the experiemnts and analyzed the data. All authors read and approved the final manuscript.
Open Research
DATA AVAILABILITY STATEMENT
The scRNA-Seq data discussed in this publication have been deposited in NCBI's Gene Expression Omnibus and are accessible through GEO Series accession number GSE193625.