Selective inflammatory stimulations enhance release of microglial response factor (MRF)-1 from cultured microglia
Abstract
The mrf-1 gene has been isolated from microglia exposed to cultured cerebellar granule neurons undergoing apoptosis. We have shown that mrf-1 is upregulated in response to neuronal death and degeneration both in vitro and in vivo. However, the exact role of MRF-1 remains unknown. Here we show that MRF-1 is released from cultured rat microglia, and its release is greatly enhanced under inflammatory conditions. When microglia were treated with ATP, the amount of MRF-1 that was released increased 10-fold compared to the basal level of release. Enhanced MRF-1 release was induced within 10 min and peaked within 1 h; after ∼ 4 h, the MRF-1 release had returned to normal. MRF-1 release was stimulated by 2-methyl-thio-ATP (five-fold) and a P2X7 selective agonist, 2′- and 3′-O-(4-benzoylbenzoyl)-ATP (ten-fold). Moreover, the ATP-stimulated MRF-1 release was inhibited by a P2X7 selective antagonist, oxidized ATP (oATP), and also under a Ca2+-free condition. These results indicate that the effects of ATP are dependent on Ca2+ influx through P2X7 receptors. MRF-1 release was enhanced by Ca2+-ionophore A23187 (sixfold), thapsigargin (threefold); however, it was not enhanced by glutamate or lipopolysaccharide. Moreover, a platelet-activating factor enhanced microglial MRF-1 release in a dose-dependent manner. We also showed that a conditioned medium from cerebellar granule neurons undergoing apoptosis markedly increased MRF-1 release from microglia; that effect was significantly inhibited by oATP. These results indicate that selective inflammatory stimulations, including ATP and PAF, enhance MRF-1 release from microglia through a Ca2+-dependent mechanism and suggest that MRF-1 may play a role in cell-cell interactions under inflammatory conditions. GLIA 40:360–371, 2002. © 2002 Wiley-Liss, Inc.
INTRODUCTION
Microglia, brain macrophages, play an important role as immune cells in the central nervous system (CNS) (McGeer et al., 1993; Gehrmann et al., 1995). When injury, infection, or inflammation occurs in the CNS, resident ramified/resting microglia respond within several hours and switch to their activated form, which has amoeboid morphology (Kreutzberg, 1996). Activated microglia engulf degenerating elements and secrete various cytotoxins and cytokines such as interleukin-1β (IL-1β) and tumor necrosis factor-α (TNF-α) for tissue repair and neural regeneration in the CNS (Giulian et al., 1994). The activation of microglia can be modified by cytokines and neurotransmitters acting through receptors for CNS signaling molecules, including adenosine 5′-triphosphate (ATP) (Fields and Stevens, 2000).
The mrf-1 gene encodes a 17 kDa protein consisting of 147 amino acids, which contains a single calcium-binding (EF-hand) motif, and in the CNS is only expressed in microglia. We demonstrated that the gene is upregulated in activated microglia in response to apoptosis of cerebellar granule neurons in culture (Tanaka et al., 1998). It has been shown that mrf-1 is upregulated in response to neuronal death and degeneration in vivo (Tanaka et al., 1998, 2000; Kato et al., 2000) and that MRF-1 then may play a significant role in both developmental programmed cell death and recovery from brain injuries. Some genes that encode the same sequence of amino acid, such as AIF-1 (Utans et al., 1995) and Iba1 (Ohsawa et al., 2000), have been isolated. These three genes may actually be a single gene (Utans et al., 1995; Tanaka et al., 1998; Ohsawa et al., 2000). It has been reported that Iba1 functions as a key molecule in membrane ruffling and phagocytosis of macrophages/microglia (Ohsawa et al., 2000). However, the other functions of these genes in the CNS are not yet clear. We have shown that microglia constitutively express MRF-1 and upregulate its level in response to inflammation (Tanaka et al., 1998, 2000; Kato et al., 2000). Pashenkov et al. (2000) also reported that some fractions of AIF-1 are released into sera and that the levels in the sera increase at the preclinical stage of experimental autoimmune neuritis; however, the mechanism remains unclear. As such, MRF-1/AIF-1/Iba1 may play a significant role in all inflammatory lesions.
Microglia can be activated by ATP, which was released from nerve terminals in close contact or leaked from damaged cells that contain 3–5 mM ATP in the cytosol (Fields and Stevens, 2000). As such, microglia are possibly exposed to high concentrations of ATP. In fact, some reports indicate that high concentrations of ATP stimulation (1–3 mM) induce the release of biologically active substances in cultured microglia: IL-1β (Ferrari et al., 1997b), TNF-α (Hide et al., 2000), nitric oxide (NO) (Ohtani et al., 2000), and IL-6 (Shigemoto-Mogami et al., 2001). It is known that there are two types of purinergic receptors and that microglia express both receptors for ATP (Walz et al., 1993; Haas et al., 1996; Illes et al., 1996; Nörenberg et al., 1997; Visentin et al., 1999). G-protein coupled-type P2 receptors such as P2Y are linked to intracellular Ca2+ release channels, whereas ionotropic P2 receptors such as P2X7 induce an increase in Ca2+ influx into the cytoplasm. In practice, the release of IL-6 is activated via the former receptors, and the release of IL-1β, TNF-α, and NO is mediated by activation of the latter receptors.
Platelet-activating factor (PAF; 1-O-alkyl-2-acetyl-sn-glycero-3-phosphocholine) is an autacoid active in different tissues, including brain tissue. PAF has been found to modulate neuronal function by exerting a pleiotropic effect and it acts via stimulation of specific PAF receptors, which control phosphoinositide turnover, induce Ca2+ release, and induce immediate-early gene expression. Previous studies have reported that PAF is synthesized in CNS pathophysiologies such as brain ischemia (Kunievsky and Yavin, 1994; Francescangeli et al., 1996) or convulsive electric seizure (Kumar et al., 1988). It has also been shown that PAF receptor antagonists suppress postischemic neuronal injury (Panetta et al., 1989; Gilboe et al., 1991; Prehn and Krieglstein, 1993). Mori et al. (1996) have suggested that PAF receptors are predominantly expressed in microglia in the rat brain. It has also been reported that PAF is synthesized in rat cerebellar granule cells by the mild stimulatory effect of calcium ionophore (Yue et al., 1990) or in rat hippocampal neurons following NMDA receptor stimulation (Aihara et al., 2000) in culture. Also, microglia may show a marked chemotactic response to both PAF and ATP (Aihara et al., 2000; Honda et al., 2001). Both ATP and PAF are released from neurons and modulate microglial functions in the injured brain.
In the present study, we demonstrated that an inflammation-related protein, MRF-1, is released from microglia and that this release was dramatically enhanced by selective inflammatory stimulation, including that by ATP and PAF. The response to ATP was observed in ramified microglia in culture as well. Our results also demonstrated that microglial MRF-1 release is enhanced in response to the apoptosis of cerebellar granule neurons and the release is significantly dependent on ATP. These findings suggest that microglia release MRF-1 in vivo in response to neuronal injuries.
MATERIALS AND METHODS
Cell Culture
For microglia isolation, the cerebral cortices were dissected from neonatal rat pups (Sprague-Dawley) and were dissociated with 0.25% trypsin for 10 min at 37°C. After trituration, the cells were grown in a mixture of DME/F-12 Ham (Sigma Chemical, St. Louis, MO) containing 10% heat-inactivated fetal calf serum (FCS; Trace Biosciences, CAN, Australia), 50 U/ml penicillin, 50 μg/ml streptomycin at 36.5°C in a humidified atmosphere of 5% CO2/95% air until confluency (∼ 8–10 days). Microglia were then collected and replated on 35 mm dishes, 12-well plate, or cover glasses (Matsunami, Osaka, Japan) (4 or 2 × 105 cells/dish or well, respectively). They were identified by staining with OX-42 (BMA, Tavistock Square, London, U.K.) (Robinson et al., 1986) or anti-MRF-1 (Tanaka et al., 1998). More than 95% of all cells were microglia. To induce ramified morphology, they were treated with thapsigargin (30 nM) in a serum-free condition for over 16 h (Yagi et al., 1999). Cerebellar cell cultures were prepared from the cerebella of P7 rats. The cells were plated on poly-D-lysine (Sigma)-coated 35 mm dishes (0.3 × 107 cells/dish). The plated cells were cultured in MEM supplemented with 10% heat-inactivated FCS, 50 U/ml penicillin, and 50 μg/ml streptomycin at 36.5°C in a humidified atmosphere of 5% CO2/95% air. For a long-time culture (∼ 10 days) of granule neurons, a high concentration of potassium (final 35 mM) was added to the culture medium at 2 DIV. The experimental procedures conformed to the guidance by the committee of the Research Center of Laboratory Animal, Hokkaido University.
Immunocytochemical Staining
Cultured cells on cover glasses were fixed with 4% paraformaldehyde (PFA)/0.12 M Na+-phosphate buffer, pH 7.2, containing 0.5% glutaraldehyde (GA). If necessary, the cells were treated with a Ca2+, Mg2+-free phosphate-buffered saline (PBS), pH 7.2, containing 0.2% triton X-100 for 5 min. After washing with PBS, the cells were preincubated with PBS containing 10% horse serum for over 1 h and then incubated with anti–MRF-1 antibody (0.1 μg/ml) or OX-42 (1/300 dilution) for 1 h at room temperature. The primary antiserum was detected with biotinylated IgG according to the procedure provided by the manufacturer (Histofine kit; Nichirei, Tokyo, Japan). Staining was made visible by both horseradish peroxidase-conjugated streptavidin and aminoethercarbazol as a substrate. Some of the sister cover glasses were counterstained with Mayer's hematoxylin solution.
[35S]-Methionine (Met) Incorporation Experiments
Microglia were washed with Met-free DME (Sigma) two times and incubated in Met-free 10% FCS/DME containing [35S]-Met (1 μCi/well; Amersham, Arlington Heights, IL) for 20–24 h. The Met-free FCS was obtained by dialysis of heat-inactivated FCS against Met-free DME for 4–5 days. After washing with normal DME medium, the cells were further incubated in the presence or absence of drugs. In some experiments, Ca2+-free 10% FCS/DME was used. The Ca2+-free FCS was obtained by dialysis of heat-inactivated FCS against Ca2+-free DME (Sigma) for 4–5 days. If necessary (Fig. 1 and Table 1), after the media were recovered, the microglia were solubilized with 0.5 ml of a lysis buffer (RIPA; 150 mM NaCl, 1.0% NP-40, 0.5% DOC, 0.1% SDS, 5 mM EDTA, 50 mM Tris-HCl, pH 8.0, 1 mM sodium orthovanadate, 2 mM NaF) (Harlow and Lane, 1988) containing protease inhibitors (protein inhibitor cocktail; Roche Diagnostics, Mannheim, Germany). MRF-1 in the media or the solubilized solution was bound to anti-MRF-1 antibody (1 μg) and then precipitated with protein A-agarose (20 μl; Santa Cruz Biotechnology, Santa Cruz, CA) with moderate shaking for over 5 h at 4°C, respectively. After washing with an RIPA buffer once, the agarose was boiled in the presence of 1 × Laemmli-sample buffer and spun down. The acquired supernatant was loaded on a 15% SDS-polyacrylamide gel (PAG), and proteins were separated by electrophoresis. After drying the gel, it was exposed to an imaging plate (IP) for 5–15 h. The signals of the amount of [35S]-Met incorporated into MRF-1 on the IP were detected by a bioimaging analyzer (BAS2000; Fuji Photo Film, Tokyo, Japan) (Motoji et al., 1995). Each value was obtained from a square or a rectangular box containing an MRF-1–specific band, and values were compared after subtraction of background signals obtained from each equal area on the gel. Percentage of released MRF-1 was determined for each value compared to mean of bases as 100% in each experiment. Total labeled MRF-1 was determined for each dish as the sum of labeled MRF-1 both released and in cell. In both Table 1 and the figure legends, the term n indicates the number of dishes or wells used for getting each value.
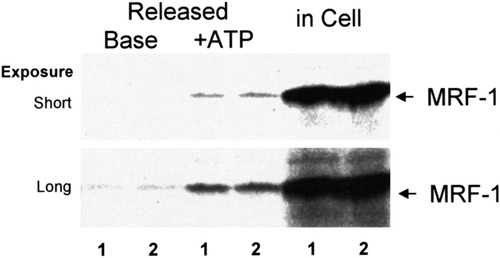
Autoradiogram indicating ATP-stimulated MRF-1 release from cultured microglia. Purified microglia in dishes were cultured in the presence of [35S]-Met for 20 h. After washing, they were consecutively incubated in normal medium (base) and ATP (1 mM)-supplemented medium (+ ATP) for 1 h each and then lysed with an RIPA buffer. MRF-1 in both media and in the cell lysate was immunoprecipitated with anti–MRF-1 antibody and purified by PAGE. [35S]-Met signals of MRF-1 were analyzed by BAS2000 as described in text. Numbers under the autoradiogram indicate that fractions were obtained from each of two dishes.
| Experiment | Cell type | % of released MRF-1 per total labeled MRF-1 | ||
|---|---|---|---|---|
| Base (n) | + ATP (n) | ATP/base | ||
| 1 | Amoeboid | 0.56 ± 0.14 (3) | 4.15 ± 0.62 (3) | 7.4a |
| 2 | Ramified | 1.13 ± 0.28 (6) | 4.02 ± 1.57 (4) | 4.5b |
- * Quantities of [35S]-Met–labeled MRF-1 were analyzed as described in Figure 1 legend (mean ± SEM). ATP was used at 1 mM. In experiment 2, purified microglia were previously treated with thapsigargin (30 nM; 16 h) in a serum-free medium for the induction of a ramified morphology. Microglia were then labeled with [35S]-Met and treated with ATP in a serum-free condition. The total labeled MRF-1 (a sum of labeled MRF-1 both released and in cell) was decreased in association with morphological change, relative amounts of total labeled MRF-1 on amoeboid cells vs. that on ramified cells = 100% ± 5.1% vs. 27.1% ± 2.3%. Tukey HSD test,
- a P = 0.081
- b and 0.087.
Statistical Analysis
Presented as means ± SEM, the data are normally distributed so the parametric one-way analysis of variance (ANOVA) was utilized to assess any differences among the data points. Posthoc analysis was performed with Tukey HSD test (SPSS version 9.0).
Immunoblot Analysis
MRF-1 in media was immunoprecipitated as described before. The protein was separated by PAGE and then transferred to a polyvinylidene difluoride (PVDF) membrane (Millipore, Bedford, MA). After the membrane was treated with Tris-HCl–buffered saline (TBS)-T (0.1% Tween-20) containing 5% BSA (Trace) for over 1 h, it was treated with anti–MRF-1 antibody (0.1 μg/ml) for 1 h. Detection of the MRF-1 protein was performed with an enhanced chemoluminescence detection kit (ECL; Amersham) according to the manufacturer's directions.
Materials
Ca2+-ionophore A23187, ATP, 2′- and 3′-O-(4-benzoylbenzoyl)-ATP (Bz-ATP), α,β-methylene-ATP (Methylene ATP), 2-methyl-thio-ATP (2-MeS ATP), and oxidized ATP (oATP) were purchased from Sigma Chemical. Botulinum toxin A solution (BoNT) was obtained from Wako Pure Chemical Industries (Osaka, Japan). PAF (PAF C-16) was purchased from Cayman Chemical (Ann Arbor, MI).
RESULTS
ATP-Stimulated Release of MRF-1 From Cultured Rat Microglia
At first, we examined the effect of ATP on MRF-1 release in purified microglia. Various quantities of MRF-1 released into media were analyzed with [35S]-Met–labeled microglia. After the microglia were cultured in the presence of [35S]-Met, they were consecutively incubated in both normal (base) and ATP (1 mM)-supplemented medium for 1 h each and were then solubilized with a lysis buffer. Both the media and the cell extract were treated with anti–MRF-1 antibody and immunoprecipitated MRF-1 was purified by PAGE. After autoradiography, signals of [35S]-Met–labeled MRF-1 were analyzed (Fig. 1). When microglia were treated with ATP, the quantity of released MRF-1 increased about sevenfold compared to the amount of basal release (Table 1): basal or ATP-stimulated release of MRF-1 was 0.6% ± 0.1% or 4.2% ± 0.6% of total labeled MRF-1, respectively (n = 3). As shown in Figure 2Ab and Bd, after the microglia were treated with ATP (less than 1 mM) for 1 h, the normal morphology was well preserved. We then examined the distribution of MRF-1 in the microglia. There was a difference in the distribution of MRF-1 among the ATP-treated microglia compared to that of the controls (Fig. 2Bb and e). When fixed microglia were immunostained with anti–MRF-1 antibody without previous treatment with a detergent solution, many, but not all, of the cells were partially stained (Fig. 2Bb). The stained signal of MRF-1 had apparently decreased in the ATP-treated microglia (Fig. 2Be). When fixed microglia are previously treated with a detergent solution, all MRF-1 of the cells become detected with anti–MRF-1 antibody (Tanaka et al., 2000). As shown in Figure 1 and Table 1, more than 95% of the total MRF-1 remained in the cell bodies, and the total distribution of MRF-1 did not change between the control and the ATP-treated microglia (Fig. 2Bc and f). These results obtained by immunostaining suggest the possibility that ATP caused the release of a fraction of MRF-1 localized near the plasma membranes of microglia. The effect of ATP, which greatly enhances release of MRF-1, was dose-dependent and was observed in cases involving concentrations of ATP over 0.6 mM (Fig. 3A). Uridine 5′-triphosphate (UTP; 1 mM) or methylene ATP (1 mM), which binds to P2Y receptors, scarcely enhanced MRF-1 release (about twofold compared to basal release; Fig. 3B). When microglia were treated with 2-MeS ATP (1 mM), which binds to P2X receptors, or Bz-ATP (0.07 mM), which is a P2X7 selective agonist, the MRF-1 that was released into the media increased to approximately 5- or 10-fold that of the basal value, respectively (Fig. 3B). These results indicate that ATP greatly stimulates MRF-1 release via a P2X7 receptor-dependent mechanism. The ATP-stimulated MRF-1 release was further characterized. The enhanced MRF-1 release, induced within 10 min, peaked within 1 h and returned to the basal level within ∼ 4 h after addition of ATP into the culture medium (Fig. 4A–C). The effect of ATP was abolished within 10 min after removal of ATP from the medium (Fig. 4C). Moreover, the effect of ATP was abolished when microglia were treated with ATP in a Ca2+-free medium (Fig. 4D) and was inhibited when microglia were previously treated with oATP (1 mM), a P2X7 selective antagonist (Fig. 4E). It is known that botulinum toxins (BoNTs) inhibit fusion of synaptic vesicles to plasma membrane. ATP increased MRF-1 release, as in the control, even when microglia were previously treated with BoNT (2 × 10−8 M; Fig. 4E) (Mochida, 1995). Compared with UTP, which induces a momentary release from intracellular Ca2+ stores (Toescu et al., 1998), when microglia are treated with high concentrations of ATP (1–3 mM), increased levels of cytoplasmic free Ca2+ ([Ca2+]i), transported through P2X7 receptors, are prolonged and sustained (Visentin et al., 1999; Hide et al, 2000). These results indicate that ATP rapidly stimulates MRF-1 release and that the effect of ATP is dependent on prolonged Ca2+ entry via P2X7 receptors.
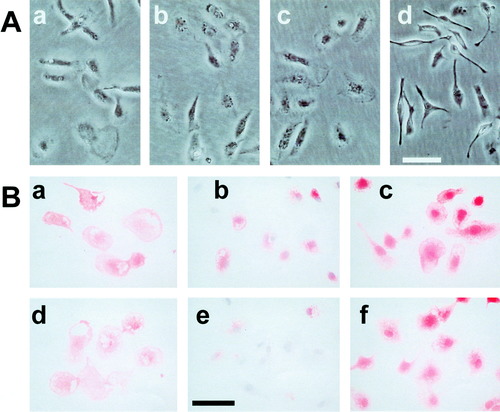
Phase-contrast photomicrographs (A) and immunocytochemical staining (B) in cultured microglia. A: Microglia obtained from a mixed glial culture of the rat cerebral cortices were replated and incubated for several hours. They were then further incubated in the presence of ATP (b, 1 mM), Ca2+-ionophore A23187 (c, 2 μM), PAF (d, 0.1 mM), or its absence (a) for 1 h and photographed with a phase-contrast microscope. B: Purified microglia were incubated in the presence (d–f) or absence (a–c) of ATP (0.6 mM) for 2 h and fixated with PFA/GA in a phosphate buffer. After blocking, they were immunostained with OX-42 (a and d) or anti–MRF-1 antibody (b, c, e, and f). In c and f, cells were previously treated with 0.2% triton X-100; in b and e, cells were counterstained with a hematoxylin solution. Bar = 50 μm.
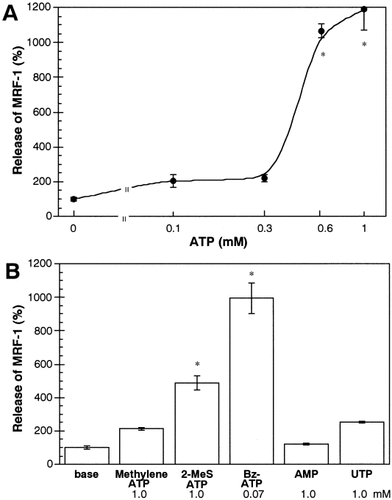
Enhancement of MRF-1 release by ATP and purinergic receptor agonists. Effects of ATP and other nucleotides on MRF-1 release were examined with [35S]-Met–labeled microglia. Cells were incubated in normal medium (base) or drug-supplemented medium for 1 h, and the media were then collected. The quantities of [35S]-Met–labeled MRF-1 in the media were analyzed as described in Figure 1 legend (mean ± SEM; n = 3). Adenosine 5′-monophosphate (AMP) was used as a negative control. *, P values < 0.05 were considered statistically significant against the values of base or 0 mM ATP.
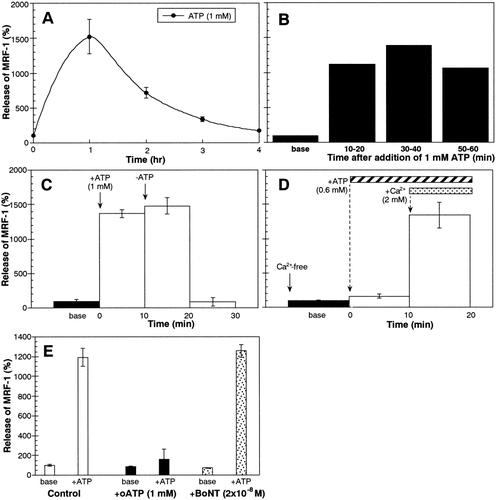
Characterization of ATP-stimulated MRF-1 release. Effect of ATP on MRF-1 release was examined with [35S]-Met–labeled microglia. Cells were incubated in normal medium (base) for over 1 h or drug(s)-supplemented medium for 1 h (A), 10 min (B–D), or 15 min (E) each, and the media were then collected. The quantities of [35S]-Met–labeled MRF-1 in the media were analyzed as described in Figure 1 legend and compared in an equal time period for each experiment (except for B, mean ± SEM; n = 3). In A, ATP was added into media at 0 time. In B, each bar, except for that of the base, was obtained from each of three dishes in which microglia from the same preparation had been plated. In E (middle and right), the values of the base were obtained from the media in which the microglia had previously been treated with oxidized ATP (oATP) or botulinum toxin A solution (BoNT) for 2 h, respectively, and the values were calculated relative to the average of the bases for control.
We previously reported that cultured microglia differentiate from an amoeboid to a ramified form when they are cultured with thapsigargin or Ca2+-ionophore A23187 in a serum-free medium (Yagi et al., 1999). Moreover, we had reported that the ramified cells have some of the physiological characters of resting microglia. We examined whether or not ramified microglia released MRF-1 in response to ATP stimulation. As shown in Table 1, the quantity of total labeled MRF-1 of ramified microglia decreased to 27.1% ± 2.3% compared to that of the control (amoeboid) microglia. However, a significant increase in released MRF-1 was observed in the ramified microglia as well (Table 1). This result suggests that resting microglia may release MRF-1 in response to ATP stimulation in the CNS.
MRF-1 Release Is Enhanced by Ca2+-Ionophore and Thapsigargin (TG) But Not by Glutamate or Lipopolysaccharide (LPS)
Glutamate is also released into the brain as a neurotransmitter. It is known that glutamate also mediates neurotoxicity in certain types of brain injury such as ischemia and modifies [Ca2+]i via NMDA receptors. When purified microglia were treated with glutamate (0.3 mM), MRF-1 levels of release were not significantly increased (Fig. 5). LPS (1.0 μg/ml), which activates microglia, also did not significantly enhanced the level of MRF-1 release from microglia (Fig. 5).
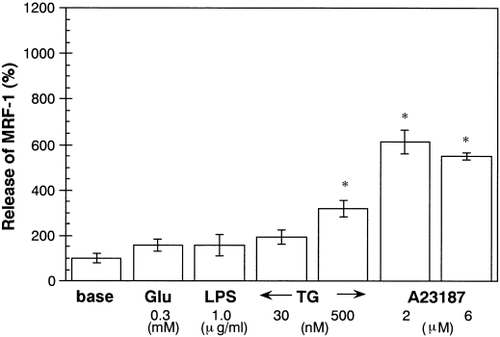
Effects of glutamate (Glu), lipopolysaccharide (LPS), thapsigargin (TG), and Ca2+ ionophore A23187 on MRF-1 release from microglia. Effects of Glu, LPS, TG, and A23187 on MRF-1 release were examined with [35S]-Met–labeled microglia. Cells were incubated in normal medium (base) or drug-supplemented medium for 1 h, and the media were then collected. The quantities of [35S]-Met–labeled MRF-1 in the media were analyzed as described in the Figure 1 legend (mean ± SEM; n = 3). *, P values < 0.05 were considered statistically significant against the values of base.
Our results concerning ATP indicate that prolonged Ca2+ entry may induce an increase in MRF-1 release from microglia. We examined the effects of MRF-1 release of other drugs that induce an increase in [Ca2+]i. It is known that TG, a specific inhibitor of endoplasmic reticulum Ca2+-ATPase, induces an increase in [Ca2+]i as a result of two factors, namely, depletion of the internal store and activation of store-operated Ca2+ influx in microglia (Minelli et al., 2000). When microglia were treated with TG at a high concentration (500 nM), the amount of MRF-1 that was released did increase somewhat (threefold compared to basal release; Fig. 5). Next, we examined an effect of Ca2+-ionophore A23187, which induces an increase in [Ca2+]i, on microglial MRF-1 release. When microglia were treated with A23187 (2 or 6 μM), released MRF-1 increased to about sixfold that of the basal release (Fig. 5). Treatment of microglia with A23187 for several hours did not alter microglial morphology (Fig. 2Ac). These results indicate that a prolonged increase in Ca2+ influx or [Ca2+]i may play a key role in the induction of increased MRF-1 release from microglia.
Enhancement of MRF-1 Release by PAF
Previous studies have suggested that PAF levels may be elevated in CNS pathophysiologies such as brain ischemia, and that PAF receptors are predominantly expressed in microglia. PAF also increases [Ca2+]i via a store-operated Ca2+ influx. We examined whether or not PAF modulated MRF-1 release from microglia. When microglia were treated with PAF, although their morphology altered rapidly and dramatically (Fig. 2Ad), MRF-1 levels released into the media increased in a dose-dependent manner (Fig. 6A), i.e., about 5-fold at 30 μM and over 10-fold at 100 μM compared to that of the basal release. The effect of 100 μM PAF was almost equal to that of 1 mM ATP and was about three times that of TG (500 nM). We detected only a slight increase in MRF-1 release when microglia were treated with 0.3 mM ATP (Fig. 3A). However, when microglia were treated with PAF (30 μM) in the presence of ATP (0.3 mM), the effect of PAF was potentiated (Fig. 6A). This result indicates that PAF may enhance MRF-1 release, dependent on the Ca2+ influx in the presence of extracellular Ca2+. Next, we examined whether or not the effect of PAF was essentially dependent on the store-operated Ca2+ influx. When microglia were treated with PAF (100 μM) in a Ca2+-free medium, MRF-1 release was enhanced about 19-fold compared to the amount of basal release (Fig. 6B). This result indicates that PAF is able to enhance MRF-1 release via a Ca2+ influx-independent mechanism. However, our results suggest that PAF effectively stimulates MRF-1 release from microglia as well.
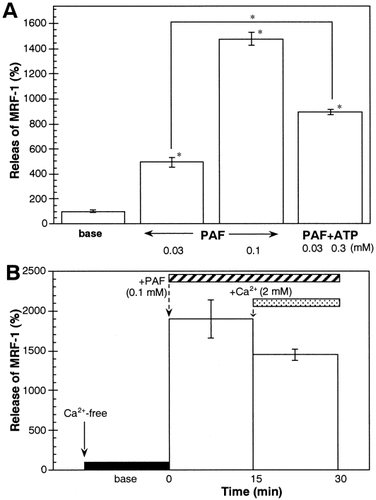
Induction of MRF-1 release by PAF. The effect of PAF on MRF-1 release was examined with [35S]-Met–labeled microglia. Cells were incubated in normal medium (base) for over 1 h or drug(s)-supplemented medium for 30 (A) or 15 min (B), and the media were then collected. The quantities of [35S]-Met–labeled MRF-1 in the media were analyzed as described in Figure 1 legend and compared in an equal time period for each experiment (mean ± SEM; n = 3). *, P values < 0.05 were considered statistically significant against the values of base or between values indicated.
Increase in Microglial MRF-1 Release in Response to Cell Death of Cerebellar Granule Neurons
Finally, we studied whether or not microglia physiologically released MRF-1 in response to neuronal death and/or degeneration. Cerebellar granule neurons maintained in a medium containing a high concentration of K+ (high K+) die due to apoptosis when the medium is exchanged for a serum-free medium containing a normal concentration of K+ (low K+) (D'Mello et al., 1993). We prepared a granule neurons-glial mix culture of cerebellar cells and maintained it in a high-K+ (35 mM) medium for 5 days (Fig. 7A). When the cells were exposed to a low-K+ (5 mM)/serum-free (LK+/-S) medium for 24 h, more than half of all granule neurons died or degenerated (Fig. 7C). In contrast, when they were exposed to a serum-free medium containing a high concentration of K+ (HK+/-S), most of the granule neurons survived for up to at least 24 h (Fig. 7B). After the cells were exposed to these serum-free media, both the LK+/-S and the HK+/-S media were collected and used as conditioned media (CMs) for the following experiments. At first, we examined quantities of MRF-1 in both the CMs. MRF-1 was immunoprecipitated with anti–MEF-1 antibody, purified by PAGE, and visualized by immunobloting. As shown in Figure 8A, the amount of MRF-1 in the LK+/-S CM was much higher than that in the HK+/-S CM. Next, we assayed whether or not the LK+/-S CM induced MRF-1 release from [35S]-Met–labeled microglia. When [35S]-Met–labeled microglia were treated with the LK+/-S CM, but not with the HK+/-S CM, the amount of MRF-1 released into the medium increased about fourfold compared to the basal amount (Fig. 8B). Moreover, the effect of LK+/-S CM was significantly suppressed after microglia were previously treated with oATP (0.3 mM; Fig. 8B). These results indicate that MRF-1 release from microglia increased due to exposure to the cell death of cerebellar granule neurons, thus suggesting that microglia may enhance the release of MRF-1 in response to brain injury in vivo and also that ATP may mediate this enhancement.
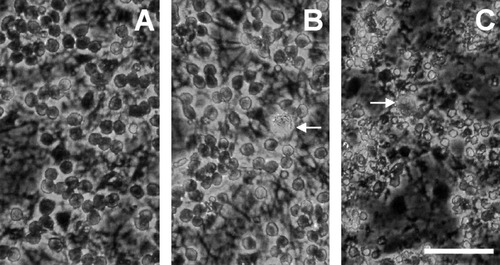
Phase-contrast photomicrographs of a culture of cerebellar cells containing both granule neurons and glia. After cerebellar cells were cultured in a high-K+ (35 mM) medium for 5 days (A), they were exposed to a serum-free medium containing 35 (B) or 5 mM (C) K+ for 24 h and were then photographed with a phase-contrast microscope. In C, some of the dead neurons had detached from the surface of the dish. Arrows indicate microglia. Bar = 50 μm.
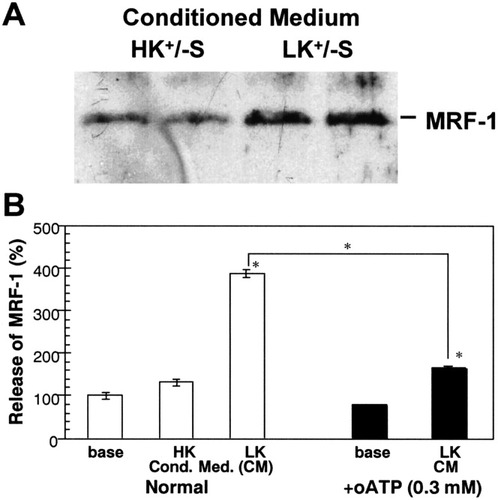
Increase in MRF-1 released from cultured microglia in response to the cell death of cerebellar granule neurons. After cerebellar cells (granule neurons-glial mix culture) were cultured in a high-K+ (35 mM) medium for 5 days, they were exposed to a serum-free medium containing 35 (HK+/-S) or 5 mM (LK+/-S) K+ for 24 h. The media were then collected and filtered as conditioned media. A: MRF-1 released into the conditioned media. MRF-1 in each medium obtained from separate dishes, in which microglia from the same preparation were incubated, was immunoprecipitated with anti–MRF-1 antibody and purified by PAGE. After the proteins were transferred to a PVDF membrane, quantities of MRF-1 were analyzed with anti–MRF-1 antibody by immunobloting. B: Increase in released MRF-1 by a conditioned medium obtained from an apoptotic granule-cell culture. Effects of CM on MRF-1 release were examined with [35S]-Met–labeled microglia. Cells were incubated in normal medium (base) for over 1 h or CM(s)-supplemented 10% heat-inactivated FCS for 30 min, and the media were then collected. The quantities of [35S]-Met–labeled MRF-1 in the media were analyzed as described in Figure 1 legend and compared in an equal time period for each experiment (mean ± SEM; n = 3). In B (right), the values of the base were obtained from the media in which microglia were previously treated with oATP for 2 h, and the values were calculated relative to the average of bases for normal. *, P values < 0.05 were considered statistically significant against the values of base or between values indicated.
DISCUSSION
The present results show that selective inflammatory signals such as ATP or PAF enhance MRF-1 release from microglia. The effect of ATP was dependent on its dose and was suppressed in both a Ca2+-free condition and in the presence of oATP; Bz-ATP was the most potent ATP analog to induce enhanced MRF-1 release. These results indicate that ATP stimulates MRF-1 release through a Ca2+ influx via P2X7 receptors. Moreover, both amoeboid and ramified microglia responded to ATP and their MRF-1 release was potentiated. PAF effectively increased MRF-1 release in a dose-dependent manner as well. We also demonstrated that neuronal death stimulates microglial MRF-1 release and that the release was significantly suppressed by oATP. Our results suggest the possibility that microglia release MRF-1 in response to neuronal injuries via inflammatory signals, including ATP, in vivo.
It is well known that the activation of P2X7 receptors by high concentration of ATP (more than 3 mM) leads to the formation of a large pore, which is permeable to molecules of up to 900 Da (Ferrari et al., 1997a; Hide et al., 2000). MRF-1 is about 17 kDa (Tanaka et al., 1998). Pore formations via P2X7 receptors may not be necessarily important for enhancement of MRF-1 release. The enhanced MRF-1 release from microglia was dependent on the Ca2+ influx. The effect of ATP was abolished under a Ca2+-free condition. When microglia were treated with high concentrations of ATP (≈ 1 mM), the increase in [Ca2+]i was prolonged and sustained (Visentin et al., 1999; Hide et al., 2000). It was already known that both PAF and TG induce prolonged Ca2+ influx via the release of Ca2+ from intracellular stores and also following the activation of the store-operated Ca2+ influx (Toescu et al., 1998; Wang et al., 1999; Minelli et al., 2000). When microglia were treated with PAF (30 μM) in the presence of ATP (0.3 mM), which alone induces only a slight increase in MRF-1 release, the effect of PAF on the enhancement of MRF-1 release was potentiated; the two stimulations worked synergistically, and not in an additive manner, on MRF-1 release. This result suggests that PAF enhances MRF-1 release, at least partially, with dependence on the Ca2+ influx in the presence of Ca2+. We also demonstrated that Ca2+-ionophore A23187 effectively enhances MRF-1 release. Although glutamate did not significantly enhanced MRF-1 release, glutamate presumably does not effectively induce an increase in the [Ca2+]i of microglia (Inoue et al., 1998). These results suggest that prolonged Ca2+ influx and/or increased levels of [Ca2+]i are key factors in the induction of enhanced MRF-1 release. However, PAF enhanced the microglial MRF-1 release even in a Ca2+-free medium, and the effect of PAF was about threefold more pronounced than that of TG. These results suggest that PAF stimulates MRF-1 release via several pathways. MRF-1 may not be a membrane-bound protein (Utans et al., 1995; Tanaka et al., 2000). When microglia are immunostained with anti–MRF-1 antibody, MRF-1 is detected with two different responses to the antibody: a fraction detected without permeabilization with triton X-100 and a fraction that become detected after the permeabilization (Tanaka et al., 2000). The immunostaining results, shown in Figure 2B, indicate that ATP caused an apparent reduction in the former fraction of MRF-1 and suggest the possibility that the ATP-sensitive fraction may be localized near the plasma membranes of microglia. It is already known that BoNTs inhibit the fusion of synaptic vesicles to cytoplasmic membranes. When microglia were previously treated with BoNT, ATP induced enhanced release of MRF-1 such as they were treated with ATP in the control conditions. However, we do not have any information concerning synaptic vesicles of microglia. Further study will be required to understand the mechanism of MRF-1 release, although our findings do suggest that prolonged increase in the Ca2+ influx universally stimulates MRF-1 release from microglia.
We have reported for the first time that MRF-1 is released from microglia and that this release is enhanced by selective inflammatory stimulation, including stimulation by ATP and PAF. Several previous reports have suggested that released AIF-1, which encodes the same sequence of amino acid as MRF-1, plays physiological roles in other tissues. Pashenkov et al. (2000) reported that some fractions of AIF-1 are released into the sera and that these levels increase at the preclinical stage of experimental autoimmune neuritis in rats. An intravenous injection of AIF-1 stimulated glucose-induced insulin release into blood in mice (Chen et al., 1997). Kuschel et al. (2000) reported that AIF-1 was expressed by macrophages in injured skeletal muscle, and addition of AIF-1 to a culture medium suppressed proliferation and differentiation of satellite cells. We examined the effects of MRF-1 on neuronal death in cultures. Polazzi et al. (2001) reported that microglial cells or microglial-conditioned media protect cerebellar granule neurons from apoptosis. When cerebellar granule neurons maintained in a HK+ medium were shifted into an LK+/-S medium for the induction of apoptosis, MRF-1 (0.1–10 μg/ml) was added into the medium. However, MRF-1 did not protect the cerebellar granule neurons from cell death (data not shown). MRF-1 release from microglia was greatly enhanced within 10 min at high concentrations of ATP (0.6–1.0 mM). Several reports have indicated that ATP induces a release of biologically active substances from the microglia through the activation of P2X7 receptors, depending on the ATP concentration. At higher concentrations of ATP (≈ 1 mM), release of IL-1β, NO, or TNF-α was elicited (Ferrari et al., 1997b; Hide et al., 2000; Ohtani et al., 2000). An increase in the IL-1β release by ATP occurred within 10 min, but only when microglia were first treated with LPS prior to stimulation with ATP (Ferrari et al., 1997b). Because the release of NO or TNF-α is dependent on the synthesis of the synthase (iNOS) mRNA or the respective mRNA, respectively, increased release occurs with a delay for over several hours after treatment with ATP (Hide et al., 2000; Ohtani et al., 2000). At lower concentrations of ATP (< 30 μM), ATP rapidly induced the release of plasminogen from rat microglia (Inoue et al., 1998). It has been reported that plasminogen enhanced synaptic transmission via NMDA-glutamate receptors in rat-cultured hippocampal neurons (Inoue et al., 1994). Recently, several studies have found that either TNF-α (Cheng et al., 1994; Munoz-Fernandez and Fresno, 1998) or IL-1β (Carlson et al., 1999) plays a neuroprotective role. Microglia, which had been treated with ATP (1 mM) for several hours, survived and preserved normal morphology for at least 2 days, and MRF-1 added into a medium had no neurotoxic effect on cerebellar granule neurons (data not shown). Considering the acute release of MRF-1 from microglia in response to higher concentrations of ATP or PAF, released MRF-1 may be shown to play a role as an autocrine factor that induces secondary responses in microglia, or as a signal mediator from injured neurons to glia, including astrocytes, in the inflammatory brain. The role played by MRF-1 released in neuronal tissues remains unclear, although our results suggest that MRF-1 is involved in cell-cell interactions under inflammatory conditions.
In this study, we examined the effects of inflammatory signals on the release of an inflammation-related protein, MRF-1, from cultured microglia. Our finding demonstrates that MRF-1 release is enhanced by stimulation of ATP or PAF and suggests that this enhanced release may be induced in vivo in response to brain injuries.
Acknowledgements
Partly supported by the MESC Grant-in-Aid for Scientific Research (to T.K.).