Catecholamine-induced oligodendrocyte cell death in culture is developmentally regulated and involves free radical generation and differential activation of caspase-3
Abstract
Oligodendrocyte cultures were used to study the toxic effects of catecholamines. Our results showed that catecholamine-induced toxicity was dependent on the dose of dopamine or norepinephrine used and on the developmental stage of the cultures, with oligodendrocyte progenitors being more vulnerable. A role for oxidative stress and apoptosis on the mechanism of action of catecholamines on oligodendrocyte cell death was next assessed. Catecholamines caused a reduction in intracellular glutathione levels, an accumulation in reactive oxygen species and in heme oxygenase-1, the 32 kDa stress-induced protein. All these changes were prevented by N-acetyl-L-cysteine, a thiocompound with antioxidant activity and a precursor of glutathione, and were more pronounced in progenitors than mature cells, which could contribute to their higher susceptibility. Apoptotic cell death, as assessed by activation of caspase-9 and –3 and cleavage of poly(ADP-ribose) polymerase (a substrate of caspase-3), was only observed in oligodendrocyte progenitors. Pretreatment with zVAD, a general caspase inhibitor, prevented activation of caspase-9 and -3, DNA fragmentation, and decreased progenitors cell death. Furthermore, the expression levels of procaspase-3 and the ratio of the proapoptotic protein bax to antiapoptotic protein bcl-xl were several folds higher in immature than mature oligodendrocytes. Taken together, these results strongly suggest that the catecholamine-induced cytotoxicity in oligodendrocytes is developmentally regulated, mediated by oxidative stress, and have characteristics of apoptosis in progenitor cells. GLIA 40:283–299, 2002. © 2002 Wiley-Liss, Inc.
INTRODUCTION
Several studies have shown that certain ischemic insults can preferentially affect white matter. Although the basic mechanisms of white matter lesioning remain unclear, oligodendrocytes are believed to be the main target (Babikian and Ropper, 1987; Pantoni et al., 1996). Oligodendrocytes are the myelin-forming cells in the central nervous system and therefore play a pivotal role in the proper execution of neural functions. Damage to cerebral white matter appears to be more prominent in neonatal than in adult hypoxic-ischemic insults (Rice et al., 1981), which lead to selective damage of developing oligodendrocytes in the periventricular region and adjacent corpus callosum (Jelinski et al., 1999; Levison et al., 2001; Ness et al., 2001). These cells are particularly relevant to periventricular leukomalacia, the principal ischemic lesion in premature infants, resulting in hypomyelination and neurological deficits in children (Volpe, 1994; Jelinski et al., 1999). Other studies have also reported that oligodendrocytes are vulnerable to hypoxic-ischemic insults (Husain and Juurlink, 1995; Mandai et al., 1997).
Calcium homeostasis, neurotransmitter release, and reactive oxygen species have been implicated in ischemic/hypoxia-induced alterations in neuronal function and in subsequent tissue damage (Banasiak et al., 2000). Although accumulating evidence suggests that an increase in extracellular glutamate concentration and overstimulation of glutamatergic receptors appear to play a major role in ischemia-induced neuron (Tapia et al., 1999) and oligodendrocyte damage (Fern and Moller, 2000; Follett et al., 2000), other endogenous neurotransmitters, especially dopamine (DA) and norepinephrine (NE), also seem to be involved. In support of this notion, a massive DA release occurs during ischemic conditions (Globus et al., 1988; Richards et al., 1993; Santos et al., 1996; Toner and Stamford, 1997a, 1997b, 1997c) and strategies that attenuate ischemia-induced DA release, such as destruction of the nigrostriatal pathways, have been shown to be potentially neuroprotective in the striatum (Globus et al., 1987; Buisson et al., 1992). Moreover, direct injection of DA into the striatal area causes neurodegeneration (Hastings et al., 1996; Hattori et al., 1998). However, no studies have investigated whether exposure to DA affects oligodendrocyte survival.
The mechanism of DA neurotoxicity is tightly linked to apoptosis induced by oxidative stress. DA contains an unstable catechol moiety and can therefore oxidize spontaneously in vitro or through an enzyme-catalyzed reaction in vivo to form reactive oxygen species (ROS), free radicals, and quinones (Cohen and Heikkila, 1974; Graham, 1978; Hastings, 1995). Free radicals can damage cellular components such as lipids, proteins, and DNA (Halliwell, 1992). The electron-deficient quinones also react widely with cellular nucleophiles, leading to cytotoxicity. Interestingly, oxidative stress has been associated with apoptosis as well as with the pathogenesis of neurodegenerative disorders, including Parkinson's disease (Halliwell, 1992; Simonian and Coyle, 1996), and neuroinflammatory disorders such as multiple sclerosis (Cross et al., 1997).
An increasing number of proteins have been implicated in oxidative stress and apoptosis. Heme oxygenase-1 (HO-1), also known as the 32 kDa heat shock protein, is exquisitely responsive to many types of stimuli and agents that cause oxidative stress (Maines, 1997). Oligodendrocyte progenitors have been shown to express HO-1 after hypoxic-ischemic insult (Jelinski et al., 1999). Induction of HO-1 has been associated with neuroprotection during hyperthermia (Ewing et al., 1992) and hypoxia (Panahian et al., 1999). On the other hand, activation of cysteine proteases, termed caspases, plays a crucial role in the execution of apoptosis. At least 14 distinct caspases have been cloned and several are expressed in oligodendrocytes (Gu et al., 1999). Among these, caspase-3 is the best studied and has been implicated in ischemia-mediated apoptosis of neurons (Yamashima, 2000) and oligodendrocytes or their progenitors (Han et al., 2000; Shibata et al., 2000; Ness et al., 2001). Another group of proteins involved in regulating apoptosis is the bcl-2 family, which consists of proapoptotic (e.g., bax, bcl-xs, and bad) and antiapoptotic (e.g., bcl-2, bcl-xl, and mcl-1) proteins (Kroemer, 1997). Increased expression of bcl-2 has been associated with inhibition of oligodendrocyte apoptosis by C5b-9 (Soane et al., 1999) and rescue of oligodendrocyte cell line OLN-93 from TNFα-induced cell death (Burgmaier et al., 2000).
In this study, we assessed the potential toxic effect of catecholamines on oligodendrocyte cultures. We present strong evidence that DA and NE cause the death of oligodendrocytes and more markedly of their progenitor cells. We also characterize the type of cell death initiated by catecholamines and define its underlying molecular mechanism. A preliminary report of these results has been published in abstract form (Khorchid and Almazan, 2000).
MATERIALS AND METHODS
Dulbecco's modified Eagle medium (DMEM), Ham's F12 medium, phosphate-buffered saline (PBS), Hank's balanced salt solution (HBSS), 7.5% bovine serum albumin (BSA) fraction V, fetal calf serum (FCS), penicillin, and streptomycin were purchased from Gibco-BRL (Burlington, ON). Other reagents were purchased from the following suppliers: 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl-tetrazolium bromide (MTT) from ICN Canada (Montreal, QC); Immobilon-P membranes from Millipore (Mississauga, ON); ECL Western blotting detection kit from NEN (Oakville, ON); human recombinant platelet-derived growth factor-AA (PDGF-AA) and basic fibroblast growth factor (b-FGF) from PeproTech (Rocky Hill, NJ); in situ cell death detection kit from Roche Diagnostics (Laval, QC); protein assay from Bio-Rad (Mississauga, ON); dihydrorhodamine 123 from Molecular Probes (Eugene, OR); Triton-X-100, poly-D-lysine, poly-L-ornithine, human transferrin, insulin, monoclonal anti-GFAP antibody, rabbit polyclonal anti-actin antibody, and L-buthionine sulfoximine from Sigma-Aldrich (Oakville, ON); mouse anti–HO-1 monoclonal antibody and polyclonal anti–bcl-2 antibody from Stress Gen Biotechnologies (Victoria, BC); monoclonal anti-complement receptor C3b (OX-42) from Serotec (Raleigh, NC); rabbit polyclonal antibody anti–caspase-3 fragment from New England Biolabs (Mississauga, ON); monoclonal anti-PARP antibody from Biomol Research Laboratory (Plymouth, PA); rabbit anti-bax, anti-procaspase-3, and anti–bcl-xl antibodies from Santa Cruz Biotechnology (Santa Cruz, CA); monoclonal anti-2′,3′-cyclic-nucleotide 3′-phosphodiesterase (CNP) antibody from Sternberger Monoclonals (Lutherville, MD). Secondary antibodies used for immunostaining or immunoblotting were obtained from Southern Biotechnology (Birmingham, AL), Jackson Immunoresearch Laboratories (Bio-Can Scientific, Mississauga, ON), or Bio-Rad. Antibody against myelin basic protein (MBP) was a generous gift from Dr. Peter Braun. Tin-mesoporphyrin (SnMP) was obtained from Porphyrin Products (Logan, UT) and Z-VAD-FMK from Enzyme Systems Products (Livermore, CA). All other reagents were obtained from VWR (Mont-Royal, QC) or Fisher (Ottawa, ON).
Primary Cultures
Primary cultures of oligodendrocyte progenitors were prepared from the brains of newborn Sprague-Dawley rats (McCarthy and de Vellis, 1980; Almazan et al., 1993). The meninges and blood vessels were removed from the cerebral hemispheres in Ham's F12 medium. The tissue suspension was passed through a 230 μm nylon mesh and collected by filtration through a 150 μm nylon mesh. The resulting suspension was centrifuged for 7 min at 1,000 rpm and then resuspended in DMEM supplemented with 12.5% heat-inactivated fetal calf serum (complete medium). Cells were plated on poly-L-ornithine–precoated 80 cm2 flasks and incubated at 37°C with 5% CO2 in air. Culture medium was changed after 3 days and every 2 days thereafter. The initial mixed glial cultures, grown for 9 to 11 days, were placed on a rotary shaker at 225 rpm at 37°C for 3 h to remove loosely attached macrophages. Oligodendrocyte progenitors were detached following shaking for 18 h at 260 rpm. The cells were filtered through a 30 μm nylon mesh and plated on bacterial-grade petri dishes for 3 h. Under these conditions, astrocytes and microglia attached to the plastic surface and oligodendrocyte progenitors remained in suspension. The final cell suspension was plated on multiwell dishes precoated with poly-D-lysine at an approximate density of 1.5 × 103/cm2. Cultures were maintained in serum-free medium (SFM) containing 2.5 ng/ml PDGF AA and 2.5 ng/ml bFGF to stimulate proliferation and medium was changed every 2 days. Ninety-five percent of the cells reacted positively with the monoclonal antibody A2B5, a marker for oligodendrocyte progenitors, and less than 5% were galactocerebroside (GalC)-positive oligodendrocytes, glial fibrillary acidic protein-positive astrocytes, or complement type-3–positive microglia. Progenitor cultures were differentiated to oligodendrocytes in SFM without PDGF and bFGF, which was supplemented with 3% calf serum after day 3. Mature oligodendrocyte cultures were more than 90% GalC and MBP-positive cells, while a few progenitors kept dividing and were A2B5-positive. The number of astrocytes or microglia did not increase with differentiation of the cultures as previously reported (Cohen and Almazan 1994).
All experiments were conducted in SFM in the absence or presence of the indicated pharmacological agents. SFM consisted of a DMEM-F12 mixture (1:1), 10 mM HEPES, 0.1% bovine serum albumin (BSA), 25 μg/ml human transferrin, 30 nM triiodothyronine, 20 nM hydrocortisone, 20 nM progesterone, 10 nM biotin, 5 μg/ml insulin, 16 μg/ml putrescine, 30 nM selenium, 50 units/ml penicillin, and 50 μg/ml streptomycin.
Immunofluorescence Staining
For detection of surface antigens, unfixed cells were incubated with monoclonal antibodies A2B5, O1 (anti-GalC) (Sommer and Schachner, 1981), or OX-42 in culture medium. After rinsing with culture medium, the cells were incubated for 20 min with secondary goat antimouse IgM or IgG2a-fluorescein isothiocyanate conjugates. To visualize MBP or GFAP, cells were fixed with 4% paraformaldehyde in PBS for 20 min at room temperature (RT), then with methanol for 5 min at −20°C. Afterward, the cells were washed three times with PBS and blocked for 15 min in PBS containing 0.2% BSA, 5% goat serum, 5% rabbit serum, and 0.2% Triton X-100. Monoclonal anti-MBP or anti-GFAP were diluted in the same solution and applied for 45 min at RT. The secondary goat antimouse IgG2b-TxR or IgG1 was applied for 20 min at RT. Coverslips were mounted with Immuno-mount and examined under a Leitz Diaplan epifluorescent microscope and photographed with Kodak TX 400 ASA film.
MTT Survival Assay
Mitochondrial dehydrogenase activity assayed by cleavage of 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl-tetrazolium bromide was used to determine cell survival according to the protocol described by Mosmann (1983). The reaction detects only living cells and is based on cleavage of the tetrazolium ring by active mitochondria, producing a visible dark-blue formazan product. Oligodendrocyte cultures were incubated with 125 μg/ml MTT at 37°C for 3 h. The medium was then aspirated and the precipitated formazan crystals were solubilized in an acid-isopropanol mixture. After a few minutes at room temperature, samples were read on a microelisa spectrophotometer at 600 nm wavelength.
Determination of Cellular Glutathione Content
Total glutathione was determined using a previously described kinetic assay (Tietze, 1969). The medium was aspirated from six-well culture dishes, and the cells were washed twice with HBSS. Perchloric acid (0.3 M, 500 μl) was added to each well and incubated for 15 min on ice. The perchloric acid solution was transferred to a 1.5 ml microcentrifuge tube and adjusted to pH 7.6 with 3 M potassium bicarbonate (75 μl). After 30-min incubation on ice, the solution was centrifuged at 13,000 rpm for 5 min at approximately 4°C. For determination of glutathione content, 60 μl of the supernatant was added to a 96-well plate containing the following: 60 μl of 2.4 mM 5,5′-dithiobis-2-nitrobenzoic acid, and 60 μl of 40 g/ml glutathione reductase in 0.1 M sodium phosphate buffer (pH 7.6) with 5 mM EDTA. Immediately after adding 60 μl of 0.8 mM NADPH, the initial rate of reaction at room temperature was determined from the change of absorbance at 414 nm using a microelisa spectrophotometer. Total glutathione content (reduced glutathione and glutathione disulfide) was determined by reference to a standard curve and was expressed as nM of glutathione per mg of total cellular protein. To determine the amount of glutathione disulfide present in the samples, aliquots of supernatant from samples or standards were mixed with 2-vinylpyridine to derive reduced glutathione (Griffith, 1980). After 1-h incubation at room temperature, 2-vinylpyridine–treated samples or standards were assayed as described above. Under all experimental conditions presented here, the fraction of glutathione disulfide was always < 1% of the total glutathione. Therefore, the results are presented as reduced glutathione (GSH).
Reactive Oxygen Species Measurement
To analyze the generation of ROS under different conditions, cells were treated with different concentration of DA for 12 h and then incubated with 10 μM dihydrorhodamine 123 (DHR123) for 60 min. After washing three times with HBSS, the fluorescent compound was extracted with 0.2 ml 1-butanol. DHR123 is oxidized by oxygen free radicals to form the fluorescent dye rhodamine 123, and it is an efficient probe for measuring overall oxidative stress in cells. Fluorescence was measured at an excitation of 485 nm and emission of 535 nm with a fluorescence spectrophotometer (F.3010, Hitachi).
Western Blot Analysis
Cells grown in six-well culture plates were harvested, after treatment, in 60 μl of ice-cold lysis buffer that contained 20 mM Tris-HCl (pH 8), 1% Nonidet P-40, 10% glycerol, 137 mM NaCl, 1 mM PMSF, 1 mM aprotinin, 0.1 mM sodium vanadate, and 20 mM NaF. Protein content in the cell lysates was determined with the Bio-Rad protein assay kit. Loading buffer (5X: 2% SDS, 5% glycerol, 5% β-mercaptoethanol, 0.01% bromophenol blue) was added to the lysates before boiling for 5 min. Aliquots containing 20 μg of protein were resolved by SDS polyacrylamide gel electrophoresis (SDS-PAGE) and transferred to Immobilon-P membranes. The membrane blots were blocked for 1 h with 5% dry milk in Tris buffer containing 0.1% Tween 20 and then incubated with primary antibody. Then the blots were incubated with horseradish peroxidase-conjugated secondary antibodies and visualized by chemiluminescence using an ECL Western blotting detection kit. The signals were quantified with a Master Scan Interpretative Densitometer. To normalize for equal loading and protein transfer, the membranes were stripped and incubated with an antibody for β-actin.
Analysis of DNA Fragmentation: TUNEL Labeling
Oligodendrocyte progenitors and mature cells grown on poly-D-lysine–coated glass coverslips in 24-well tissue culture plates were treated with DA for 12 h at 37°C in SFM. For immunocytochemical detection of apoptotic cells, cultures were washed with PBS and then fixed with 4% paraformaldehyde for 20 min at room temperature. Fragmented DNA (high molecular weight or internucleosomal) in the cells was detected by incorporating fluorescein-12-dUTP at 3′-OH ends using terminal deoxynucleotidyl transferase (TdT). TdT-mediated dUTP nick-end labeling (TUNEL) assay was performed following the manufacturer's instructions (Roche Diagnostics, Laval, QC). Anti-fluorescein antibody Fab fragments conjugated with horseradish peroxidase detected incorporated fluorescein. A 3,3′-diaminobenzidine (DAB) substrate kit (Vector Laboratories, Burlingame, CA) was used to detect peroxidase activity. Stained cells were visualized by light microscopy.
Data Analysis
Unless otherwise indicated, results are represented as mean ± SEM of at least three separate experiments performed in duplicate or triplicate. One- or two-way analysis of variance followed by the Tukey test was used to determine statistical significance; P values less than 0.05 were considered significant.
RESULTS
Characterization of Oligodendrocyte Cultures
Different developmental stages of the oligodendrocyte lineage can be identified with the aid of stage-specific antibodies. Oligodendrocyte progenitors displayed ovoid cell bodies and poorly branched processes expressing gangliosides recognized by the monoclonal antibody A2B5 (Fig. 1A). Differentiation of oligodendrocyte progenitors was accompanied with expression of the major glycolipids and proteins of myelin. These include the glycolipid galactocerebroside (GalC), the enzyme 2′,3′-cyclic-nucleotide 3′-phosphodiesterase (CNP), and the three structural myelin proteins, myelin basic protein (MBP), proteolipid protein, and myelin-associated glycoprotein. In our cultures, 12-day differentiated oligodendrocytes appeared as round cell bodies emitting profuse network of processes and were immunolabeled with anti-GalC and anti-MBP antibody (Fig. 1C shows GalC staining).
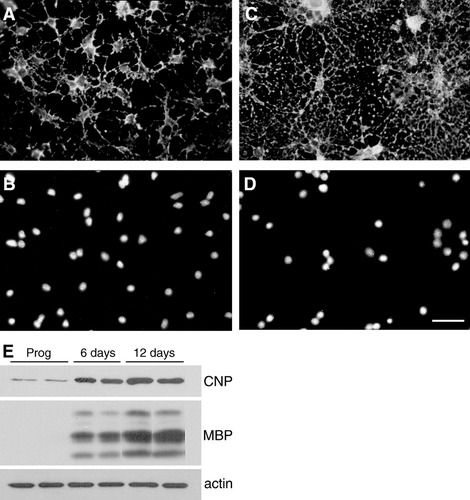
Characterization of oligodendrocyte during development. Oligodendrocyte cultures were characterized by immunofluorescence (A–D) and Western blotting (E). Oligodendrocyte progenitors were labeled with A2B5 antibody (A) and DAPI nuclear staining (B), while mature cells were labeled with anti-GalC antibody (C) and DAPI nuclear staining (D). Cultures in A/B and C/D were photographed with a 40× objective. Scale bar represents ∼ 50 μm. Oligodendrocyte progenitor cultures were allowed to differentiate for 6 and 12 days, harvested, and subjected to immunoblot analysis. Equal amounts of proteins were loaded (15 μg/lane) and protein expression of CNP, MBP, and β-actin was determined (E). Typical blots showing an increase in CNP and all isoforms of MBP in oligodendrocyte differentiated for 6 and 12 days in vitro. In contrast, β-actin levels remain constant during development of oligodendrocytes.
To further characterize our cultures, we examined the expression of CNP and MBP by Western blotting. CNP consists of two isoforms, the major 46 kDa and the minor 48 kDa. We detect only the 46 kDa isoform in our Western blots when exposed for a few seconds, while the 48 kDa requires longer exposure time. MBP consists of seven exons, with alternative splicing of exons 2, 5, and 6 resulting in at least five isoforms (14–21.5 kDa). As expected, oligodendrocyte progenitors expressed very low levels of CNP, whereas oligodendrocyte progenitor cultures differentiated for 6 and 12 days expressed both CNP and MBP (Fig. 1E). There was an increase in CNP and all isoforms of MBP as oligodendrocytes differentiated.
Toxic Effects of DA and NE on Oligodendrocyte Cultures
Both oligodendrocyte progenitors and 12-day differentiated oligodendrocytes were exposed to increasing concentrations of DA or NE. Cell viability was quantified using the MTT assay, which monitors mitochondrial integrity. There was a marked concentration-dependent decrease in cell survival in response to both DA and NE (Fig. 2). In addition, progenitors were more susceptible to the cytotoxic effects of DA and NE than were mature oligodendrocytes. Exposure to 100 μM DA resulted in the death of approximately 36% of oligodendrocyte progenitors and only 2% of differentiated oligodendrocytes (Fig. 2). This differential susceptibility was also evident from the EC50 values for DA, which were 150 and 500 μM for progenitors and mature oligodendrocytes, respectively.
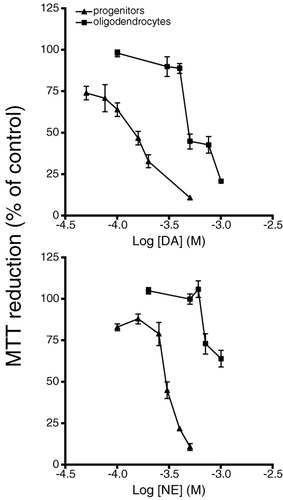
DA and NE toxicity in oligodendrocyte progenitors and differentiated oligodendrocytes. Cultures were exposed to various concentrations of DA (top, filled square) or NE (bottom, filled triangle) in SFM for 24 h. Cell viability was assessed by MTT reduction. Data are the mean ± SEM of 4–6 determinations. Statistical differences from control levels were as follows: in progenitors, NE 100 μM (P < 0.01), NE 150 μM (P < 0.05), NE 250–500 μM and DA 50–500 μM (P < 0.01); in oligodendrocytes, DA 500–1000 μM and NE 700–1000 μM (P < 0.01).
Effects of Catecholamines on Oligodendrocytes Are Mediated by Reactive Oxygen Species
DA induces ROS formation and decreases intracellular glutathione levels
Oligodendrocyte cultures treated for 12 h with DA (150 and 500 μM) showed an increase in ROS formation, as assessed by oxidation of dihydrorhodamine 123 to the cationic fluorescent probe rhodamine 123 (Table 1). Only in oligodendrocyte progenitors did 150 μM DA produce a significant increase in ROS formation (1.5-fold; P < 0.01). On the other hand, both progenitors and mature oligodendrocytes exposed to 500 μM DA increased ROS formation by 1.9- and 1.3-fold, respectively. Therefore, the relative levels of ROS formation were higher in progenitors than mature oligodendrocytes.
| Treatment | ROS (units/mg protein) | GSH (nmol/mg protein) | ||
|---|---|---|---|---|
| Progenitors | Mature | Progenitors | Mature | |
| Control | 13 ± 0.85 | 13 ± 0.33 | 4.42 ± 0.18 | 6.49 ± 0.18 |
| DA 150 μM | 19.5 ± 1.15b | 13.3 ± 0.86 | 2.75 ± 0.03a | 5.77 ± 0.14 |
| DA 500 μM | 24.6 ± 1.72b | 17 ± 0.50b | 2.18 ± 0.15a | 3.97 ± 0.22a |
| NAC 1 mM | 6.05 ± 0.32a | 6.12 ± 0.14 | ||
| NAC + DA 150 μM | 6.09 ± 0.17c | 5.41 ± 0.11 | ||
| NAC + DA 500 μM | 6.09 ± 0.25d | 5.41 ± 0.29e | ||
- * Cultures were exposed to DA for 12 and 6 h to measure ROS formation and intracellular glutathione level, respectively. NAC was added 30 min prior to DA exposure. The values obtained are represented as mean ± SEM.
- a P < 0.001 vs. control.
- b P < 0.01 vs. control.
- c P < 0.001 vs. 150 μM DA.
- d P < 0.001 vs. 500 μM DA.
- e P < 0.01 vs. 500 μM DA.
Glutathione (GSH) is the major cellular antioxidant, which functions to protect cells from oxidative damage caused by ROS. Exposure of oligodendrocyte cultures to DA for 6 h decreased intracellular GSH levels (Table 1). A significant 38% decrease in GSH levels was obtained with 150 and 500 μM DA in oligodendrocyte progenitors and mature cells, respectively. In oligodendrocyte progenitors, a higher decrease of 50% in GSH levels was obtained with 500 μM DA. Therefore, oligodendrocyte progenitors had a more pronounced decrease in intracellular GSH levels than mature cells. N-acetyl-L-cysteine (NAC), an antioxidant and a precursor of GSH, was shown to increase intracellular GSH levels in oligodendrocyte progenitors (Almazan et al., 2000). Similar results were obtained with NAC in our progenitor cultures. In addition, 30-min pretreatment with 1 mM NAC prior to exposure to DA abolished the decrease in glutathione levels in both progenitors and mature cells. Taken together, these results suggest that the increase in ROS formation or oxidative stress and depletion of intracellular GSH is associated with DA-induced cytotoxicity.
Effect of buthionine sulfoximine on DA- and NE-mediated cytotoxicity
To examine whether intracellular glutathione levels play an important role in protection against catecholamines, cultures were treated with buthionine sulfoximine (BSO). This is a potent inhibitor of γ-glutamyl-cysteine synthetase, the rate-limiting enzyme in GSH synthesis. As previously reported by our laboratory, BSO (10 μM) treatment reduced intracellular GSH levels to 20% of controls (Almazan et al., 2000) but had no effect on oligodendrocyte cell survival. However, 30-min pretreatment with BSO prior to DA and NE exposure rendered cells significantly more susceptible to the toxic effects of catecholamines than non–BSO-treated cells (Fig. 3 and Table 2).
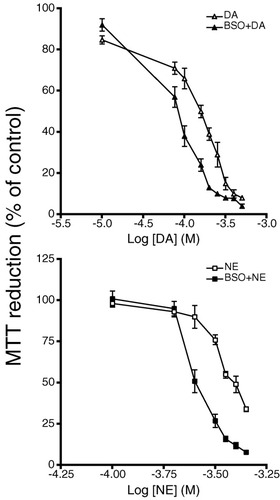
Effects of buthionine sulfoximine on DA- and NE-induced cytotoxicity in progenitors. Cultures were treated with 10 μM BSO for 30 min prior to 24-h exposure to various concentrations of DA (top, triangle) or NE (bottom, square). Open symbols represent DA or NE treatment while filled symbols represent the presence of BSO. Cell viability was assessed by MTT reduction. Data are the mean ± SEM of six determinations. Statistical differences observed with DA compared to BSO + DA, 75 μM (P < 0.05), 100–200 μM (P < 0.001), and 250 μM (P < 0.01); NE compared to BSO + NE, 250–400 μM (P < 0.001) and 450 μM (P < 0.01).
| Treatment | − BSO | + BSO |
|---|---|---|
| Control | 100 ± 3 | 95 ± 3 |
| DA 300 μM | 91 ± 6 | 56 ± 12ab |
| DA 500 μM | 40 ± 7a | 15 ± 3a |
| NE 500 μM | 102 ± 3 | 90 ± 8 |
| NE 700 μM | 93 ± 5 | 36 ± 6ac |
- * Differentiated oligodendrocytes were treated with 10 μM BSO for 30 min prior to DA and NE exposure for 24 h. Cell viability was measured by MTT reduction. Data are the mean ± SEM of six determinations and are expressed as percentage of control of non–BSO-treated oligodendrocytes.
- a P < 0.001 vs. control.
- b P < 0.01 vs. 300 μM DA.
- c P < 0.001 vs. 700 μM NE.
Effects of DA and NE on HO-1 expression
Expression of HO-1, a protein induced by oxidative stress, was assessed in oligodendrocyte cultures by Western blot analysis. A time-dependent increase in HO-1 expression after DA exposure was observed in oligodendrocyte progenitors (Fig. 4A). The effect of DA was also concentration-dependent (Fig. 4B). The effect of NE on HO-1 expression was similarly both concentration- and time-dependent (data not shown). The increase in HO-1 in response to DA and NE was more marked in progenitors than in mature cells. Exposure to 150 μM DA for 24 h resulted in a 75-fold increase in HO-1 levels in progenitors and only a 7-fold increase in mature oligodendrocytes. The level of HO-1 was very low in untreated control progenitor cultures since none was detected in Western blots, whereas the control levels in mature oligodendrocytes was detectable.
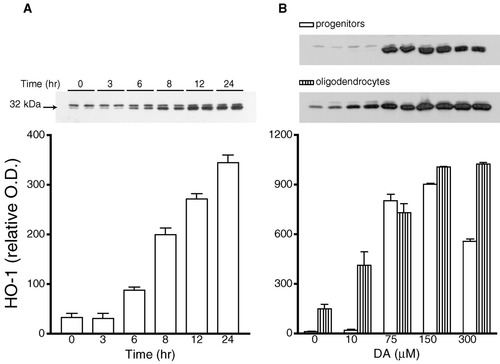
DA stimulation of HO-1 expression in oligodendrocyte progenitors: time- and concentration-dependency. A: Cultures were exposed to 150 μM DA in SFM for different time periods, harvested, and subjected to immunoblot analysis. Top: Representative autoradiogram immunoblotted with HO-1 antibody showing duplicate samples for oligodendrocyte progenitors at 0, 3, 6, 8, 12, and 24 h after DA exposure. Bottom: Autoradiograms of HO-1 expression were quantified by densitometry and values are represented in arbitrary optical density units (O.D.) as the mean ± SEM of six determinations. Statistical differences observed from control values were 6, 8, 12, and 24 h (P < 0.01). B: Oligodendrocyte progenitors (open bars) and mature cells (striped bars) were exposed to various concentrations of DA in SFM for 24 h, harvested, and subjected to immunoblot analysis. Top: Representative autoradiograms of typical experiments shown in duplicate for progenitors and mature cells. Bottom: Autoradiograms of HO-1 expression were quantified by densitometry and represented in the graphs as the mean ± SEM of triplicate samples from an experiment. Statistical differences from control levels were as follows: in progenitors, DA 75-300 μM (P < 0.01); in oligodendrocytes, DA 10 μM (P < 0.05) and DA 75–150 μM (P < 0.01).
Effect of antioxidants on DA- and NE-mediated cytotoxicity and HO-1 expression
Two antioxidants, NAC and ascorbic acid (AA), were used to determine whether oxidative stress is involved in catecholamine-mediated effects on oligodendrocytes. NAC (1 mM) prevented cell death caused by catecholamines in oligodendrocyte progenitor and mature cultures, as assessed by MTT reduction (Fig. 5). AA (100 μM) blocked the effect of both catecholamines in mature cells and that of NE in progenitors as well as significantly reduced the effect of DA in oligodendrocyte progenitor cell death. Similarly, these antioxidants prevented the catecholamine-induced expression of HO-1 in oligodendrocyte cultures (Fig. 6). These results indicate that exposure of oligodendrocyte cultures to catecholamines leads to an increase in oxidative stress, which increases HO-1 expression as well as cell death.
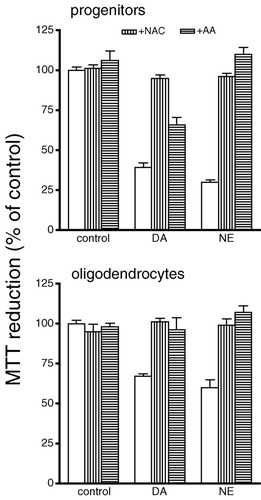
Effect of antioxidants on DA- and NE-induced cytotoxicity in oligodendrocyte cultures. Cultures were treated with 1 mM NAC (vertically striped bars) or 100 μM AA (horizontally striped bars) for 30 min prior to 24-h exposure to DA or NE. The concentrations used were 150 μM DA or 300 μM NE for progenitor cultures and 500 μM DA or 700 μM NE for mature cultures. Cell viability was measured by MTT reduction. Data are the mean ± SEM of eight determinations. Statistical differences observed: in progenitors, DA/NE compared to NAC + DA/NE (P < 0.001), DA/NE compared to AA + DA/NE (P < 0.001); in oligodendrocytes, DA/NE compared to NAC + DA/NE (P < 0.001), DA compared to AA + DA (P < 0.01), NE compared to AA + NE (P < 0.001).
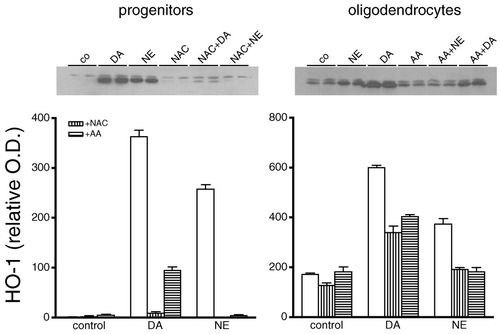
Effect of antioxidants on DA- and NE-induced HO-1 expression in oligodendrocyte cultures. Cultures were treated with 1 mM NAC (vertically striped bars) or 100 μM AA (horizontally striped bars) for 30 min prior to 24-h exposure to DA or NE, harvested, and subjected to immunoblot analysis. The concentrations used were 150 μM DA or 300 μM NE for progenitor cultures and 150 μM DA or 500 μM NE for mature cultures. Top: Representative autoradiograms immunoblotted with HO-1 antibody showing duplicate samples for progenitor and oligodendrocytes. A different antioxidant was used for each developmental stage to show a representation of all data quantified below. Bottom: Autoradiograms of HO-1 expression were quantified by densitometry and values are represented in the graph in arbitrary optical density units (O.D.) as the mean ± SEM of three experiments performed in duplicate. Statistical differences observed in progenitors and oligodendrocytes were DA/NE compared to NAC + DA/NE (P < 0.001), DA/NE compared to AA + DA/NE (P < 0.001).
Effects of tin-mesoporphyrin (SnMP), an inhibitor of HO-1, on DA-mediated cytotoxicity
In an effort to establish a link between HO-1 induction and DA-mediated cytotoxicity, SnMP was used to inhibit HO-1 activity. Pretreatment of oligodendrocyte progenitor cultures for 30 min with SnMP (50 μM) increased cell death caused by DA (150 μM), as assessed by MTT reduction (Table 3). These results suggest that DA-induced HO-1 expression in oligodendrocyte progenitors serves to protect these cells from DA-mediated cytotoxicity.
- * Cultures were treated with SnMP (50 μM) for 30 min prior to 24-h exposure to 150 μM DA. Cell viability was measured by MTT reduction. Data are the mean ± SEM and are expressed as percentage of control.
- a P < 0.001 vs. control.
- b P < 0.05 vs. DA.
Catecholamines-Induced Oligodendrocyte Death Includes an Apoptotic Mechanism
Effect of DA on caspase activation and poly(ADP-ribose) polymerase (PARP) cleavage
We next examined whether catecholamine-induced oligodendrocyte death implicates an apoptotic mechanism. Biochemical features of apoptosis-mediated cell death include the activation of one or more cysteine proteases of the caspase family, the upregulation of proapoptotic proteins of the bcl-2 family such as bax and DNA fragmentation. One measure of caspase activation is proteolytic cleavage of the procaspase into smaller products. Caspase-3 is synthesized as a 32 kDa precursor that is cleaved during activation to yield 18 and 12 kDa subunits. Cell lysates from oligodendrocyte cultures treated with 150 μM DA for different time periods were processed for Western blot analysis using antibodies that recognize procaspase-3 (32 kDa) or the 18 kDa caspase-3 cleavage product or proapoptotic protein bax (21 kDa). DA treatment of oligodendrocyte progenitors resulted in a time-dependent decrease in procaspase-3 with a concomitant increase in the cleavage product. In progenitor cultures, the expression levels of bax were not significantly altered until 24 h after DA treatment (∼ 40% increase over controls; Fig. 7A). However, we could not detect any of the above changes in mature oligodendrocytes following their exposure to DA (results not shown). In addition, NAC prevented DA- and NE-mediated caspase-3 activation in oligodendrocyte progenitors
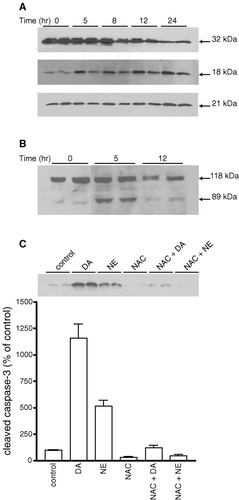
A and B: Effect of DA treatment on the expression of procaspase-3, bax, and cleaved caspase-3 and PARP in oligodendrocyte progenitors. Cultures were exposed to 150 μM DA in SFM for different time periods, harvested, and subjected to immunoblot analysis. A: Representative immunoblot for the 32 kDa procaspase-3 (top) showing duplicate samples for oligodendrocyte progenitors at 0, 5, 8, 12, and 24 h after DA exposure. The exact duplicate autoradiogram immunoblotted for the 18 kDa cleaved caspase-3 (middle) or the 21 kDa bax (bottom). B: Representative immunoblot for PARP (118 kDa) showing duplicate samples for oligodendrocyte progenitors at 0, 5, and 12 h after DA exposure. Note the proteolytic cleavage pattern of PARP and the formation of the 89 kDa PARP fragment. C: Effects of NAC on DA- and NE-induced caspase-3 activation in oligodendrocyte progenitors. Cultures were pretreated with NAC (1 mM) for 30 min prior to 24-h exposure to 150 μM DA or 300 μM NE. Cells were harvested and subjected to immunoblot analysis. Autoradiograms of immunoblots showing cleaved caspase-3 expression were quantified by densitometry. Data are represented in the graph as the mean ± SEM of triplicate samples from a representative experiment; replicate experiment indicated similar findings. Statistical differences observed with control compared to DA (P < 0.001), control compared to NE (P < 0.01), DA compared to NAC + DA (P < 0.001), NE compared to NAC + NE (P < 0.01).
Another measure of caspase-3 activation is the cleavage of specific substrates. Poly(ADP-ribose) polymerase (PARP) is a 118 kDa polypeptide that is cleaved by caspase-3 to yield an 89 kDa product. Exposure of oligodendrocyte progenitors to 150 μM DA for 5 and 12 h led to a decrease in PARP (118 kDa) and a clear increase in the 89 kDa product at 5 h (Fig. 7B), again indicating that caspase-3 is activated during DA-induced cell death of progenitors. Activation of caspase-3 by both DA and NE was completely prevented by NAC (Fig. 7C), thus demonstrating that ROS production is required for the initiation of apoptosis.
Effect of DA on DNA fragmentation: blockade by NAC and zVAD
DNA fragmentation was demonstrated using the TUNEL assay. This technique allowed the apoptotic cell bodies to be visualized and quantified. Progenitors and differentiated oligodendrocytes exposed to DA for 12 h showed an increase in TUNEL-positive cells as compared to control (Fig. 8). A concentration of 75 μM DA increased the proportion of TUNEL-positive oligodendrocyte progenitors from 8.3% ± 3.6% to 32.8% ± 8.9% (Table 4). Similarly, 500 μM DA significantly increased the percentage of TUNEL-positive cells in mature oligodendrocyte cultures compared with the control (sixfold; P < 0.001). Treatment of oligodendrocyte progenitor and mature cultures with NAC (1 mM) prior to exposure to DA decreased the percentage of TUNEL-positive cells back to control levels. In contrast, treatment with zVAD, the general caspase inhibitor, prevented the DA-mediated increase in TUNEL-positive cells only in oligodendrocyte progenitors. These results suggest that both progenitors and mature oligodendrocytes are undergoing cell death upon exposure to DA and that oxidative stress is implicated in this process. In addition, the results further support the role of caspase activation in DA-mediated apoptosis of oligodendrocyte progenitors.
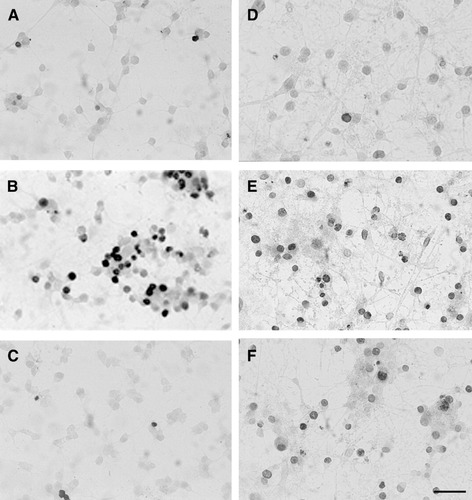
Immunocytochemical staining of oligodendrocyte cultures using TUNEL assay. Bright-field photomicrographs of oligodendrocyte progenitors (A–C) and mature oligodendrocytes (D–F). In control cultures, a few TUNEL-positive cells were noted (A, D). The number of TUNEL-positive cells increased upon exposure of oligodendrocyte progenitors and mature cultures to 75 and 500 μM DA, respectively (B, E). zVAD (50 μM) attenuates TUNEL labeling in progenitors but not mature oligodendrocytes that have been exposed to DA (C, F). Scale bar represents ∼ 50 μm.
| Treatment | Progenitors | 12 days differentiated |
|---|---|---|
| Control | 8.3 ± 3.6 | 7.6 ± 1.2 |
| DA | 32.8 ± 8.9a | 45.0 ± 3.2a |
| NAC | 8.7 ± 3.1 | 6.9 ± 0.6 |
| NAC + DA | 6.1 ± 2.9b | 7.5 ± 0.8b |
| zVAD | 5.5 ± 1.6 | 6.8 ± 0.5 |
| zVAD + DA | 6.4 ± 3.2b | 42.2 ± 4.8a |
- * Cultures were treated with NAC (1 mM) or zVAD (50 μM) for 30 min prior to 12-h exposure to DA. The concentrations used were 75 μM DA for progenitor cultures and 500 μM DA for oligodendrocyte cultures. DNA fragmentation was assessed by the TUNEL assay. TUNEL-labeled cells were quantified for each treatment from six coverslips. The values obtained are expressed as percentage of TUNEL-positive cells from total cells counted and are represented as mean ± SEM.
- a P < 0.001 vs. control.
- b P < 0.001 vs. DA.
Effect of zVAD on caspase-3 and -9 activation and cell death in oligodendrocyte progenitor cultures
Our results demonstrated the DA-mediated activation of caspase-3 and apoptotic cell death in oligodendrocyte progenitors. Hence, zVAD was used to determine whether the cells could be rescued by blocking caspase-3 activation. In oligodendrocyte progenitors, DA also activated caspase-9, an immediate effector of caspase-3. Pretreatment with zVAD (50 μM) inhibited DA-mediated caspase-3 and -9 activation (Fig. 9A). This inhibition significantly reduced DA-induced cell death but did not fully protect oligodendrocyte progenitors (Fig. 9B).
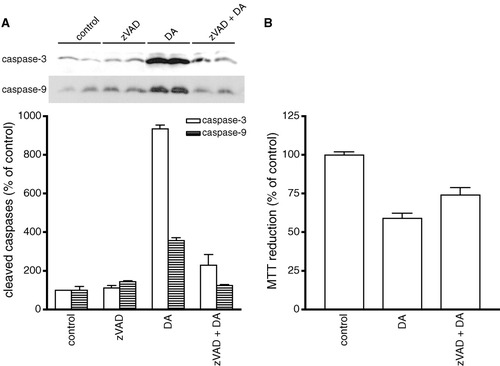
Effects of zVAD on DA-induced caspase-3 and -9 activation and cytotoxicity in oligodendrocyte progenitors. Cultures were pretreated with 50 μM zVAD for 30 min prior to 24-h exposure to 150 μM DA. A: Cells were harvested and subjected to immunoblot analysis of cleaved caspase-3 (open bars) and caspase-9 (striped bars) expression and quantification by densitometry. Data are represented in the graph as the mean ± SEM of triplicate samples from a representative experiment; replicate experiment indicated similar findings. Statistical differences observed with control compared to DA (P < 0.001), DA compared to zVAD + DA for caspase-3 and -9 (P < 0.001 and < 0.01, respectively). B: Cell viability was measured by MTT reduction. Data are the mean ± SEM of eight determinations. Statistical differences observed with control compared to DA (P < 0.001), DA compared to zVAD + DA (P < 0.05).
Developmental changes in the expression of bcl-2 family members and procaspase-3
To address the hypothesis that a change in sensitivity to catecholamines could be caused by a developmental change in the apoptotic effector pathway, we examined the expression levels of bcl-xl, bax, bcl-2, and procaspase-3 in cultured oligodendrocytes. Bcl-2 was identified as a 26 kDa polypeptide by Western blot analysis, bax as a 21 kDa species, bcl-xl as a doublet with apparent molecular masses of 29–31 kDa, and procaspase-3 as a 32 kDa protein. In oligodendrocyte progenitors allowed to differentiate for 6 and 12 days, bcl-xl expression increased moderately between 6 and 12 days in culture, bax, bcl-2, and procaspase-3 expression decreased significantly by 12 days (Fig. 10). Quantification of these bands revealed that the bax/bcl-xl ratio decreased approximately fivefold while bax/bcl-2 ratio remained the same in the transition from progenitors to differentiated cells.
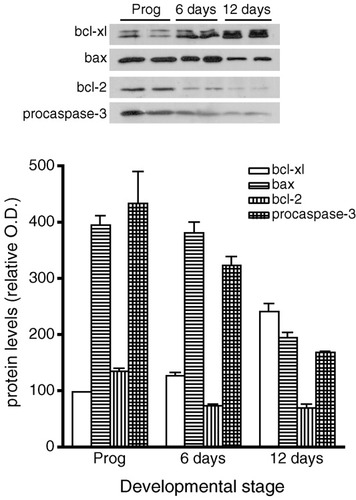
Changes in the expression of bcl-2 family members during oligodendrocyte development. Oligodendrocyte progenitor cultures were allowed to differentiate for 6 and 12 days, harvested, and subjected to immunoblot analysis. Equal amounts of proteins were loaded (20 μg/lane). Top: Representative immunoblots for bcl-xl (29–31 kDa), bax (21 kDa), bcl-2 (26 kDa), and procaspase-3 (32 kDa) showing duplicate samples at each developmental stage. Bottom: Autoradiograms were quantified by densitometry and values for bcl-xl (open bars), bax (horizontally striped bars), bcl-2 (vertically striped bars), and procaspase-3 (hatched bars) are represented in arbitrary O.D. units as the mean ± SEM of three experiments performed in duplicate.
DISCUSSION
This study demonstrates that catecholamines are cytotoxic to cultured oligodendrocyte. Furthermore, exposure of progenitor and mature cultures to DA is accompanied with an increase in ROS formation and a decrease in intracellular glutathione levels. Catecholamines also increase HO-1 expression in oligodendrocyte cultures. Results with antioxidants NAC and AA implicate that oxidative stress is the mechanism by which catecholamines mediate oligodendrocyte cell death and increase HO-1 expression. DA exposure increased the numbers of TUNEL-positive oligodendrocyte progenitors and mature cells. However, only in oligodendrocyte progenitors is DA-mediated cell death linked to activation of caspase-9 and –3 as well as cleavage of PARP. Furthermore, the higher levels of both procaspase-3 expression and the ratio of the proapoptotic protein bax to antiapoptotic protein bcl-xl in immature oligodendrocytes compared to mature cells may contribute to the differential responses.
Generation of ROS by oxidation of DA is considered to result in neuronal loss in Parkinson's disease (PD). This is supported by postmortem studies of PD brain showing that in the substantia nigra there are increased indexes of oxidative stress, including increased levels of iron, increased lipid peroxidation, decreased levels of mitochondrial complex I, and decreased level of glutathione (Hirsch et al., 1991; Jenner, 1993). The present results indicate that in oligodendrocytes cultures DA induced an increase in ROS formation with concomitant decrease in intracellular glutathione levels. This increase in oxidative stress is associated with DA-induced cell death in oligodendrocyte progenitors and mature cells, since it can be attenuated with the antioxidants, NAC and AA. A previous study has shown that NE-mediated cell death of mature oligodendrocytes could also be prevented by catalase (Noble et al., 1994). Furthermore, oxidation in vitro of DOPA, a precursor of DA, forms 2,4,5-trihydroxyphenylalanine (TOPA) and TOPA quinone (Newcomer et al., 1993). Interestingly, both of these compounds act as selective non-NMDA agonists and activate excitotoxicity in neurons (Rosenberg et al., 1991; Newcomer et al., 1995). However, results obtained in our laboratory (data not shown) indicate that DA cytotoxicity in oligodendrocytes is not affected by CNQX, a non-NMDA antagonist, suggesting that a glutamatergic response is not involved.
Our results also demonstrate that oligodendrocyte progenitors are more vulnerable to catecholamine-induced toxicity than mature cells. The greater susceptibility of progenitors to DA and NE may be related to the fact that glutathione levels in mature oligodendrocytes are significantly higher than in progenitor cells, as has been previously reported (Juurlink et al., 1998; Almazan et al., 2000). This is further supported by our observations that the relative levels of DA-induced ROS formation were higher in progenitors than mature cells, and depletion of intracellular glutathione by inhibition of the rate-limiting enzyme, γ-glutamyl-cysteine synthetase, with BSO sensitized both progenitors and mature oligodendrocytes to catecholamine toxicity. In line with our study, the sensitivity of oligodendrocytes to free radical toxicity generated by depletion of glutathione or by a xanthine-xanthine oxidase system (Back et al., 1998), by cadmium exposure (Almazan et al., 2000), as well as by ischemic injury (Fern and Moller, 2000) is dependent on the maturation stage of the cultures.
HO-1 is a stress-related protein and its altered expression is a marker of stress response activation. HO-1 catalyzes the rapid degradation of the pro-oxidant heme to bile pigments with free radical scavenging capabilities. HO-1 expression in neurons, astrocytes, and macrophages has been observed in a wide range of experimental diseases of rodent brain, such as traumatic brain injury (Fukuda et al., 1996), ischemia (Nimura et al., 1996), and experimental allergic encephalomyelitis (Emerson and LeVine, 2000). The expression of HO-1 was also observed in Alzheimer's (Schipper et al., 1995) and Parkinson's disease (Schipper et al., 1998). In this study, catecholamines increased HO-1 expression in oligodendrocytes. The ability of antioxidants to block this increase suggests that oxidation of catecholamines is essential for HO-1 expression. HO-1 overexpression in response to hyperoxic oxidant injury was associated with a marked decrease in cell growth and DNA synthesis as well as increased cell survival (Lee et al., 1996). Furthermore, overexpression of the HO-1 gene in mice provided neuroprotection against glutamate-induced cell death (Chen et al., 2000). Our results demonstrate that inhibition of HO-1 with SnMP resulted in a further increase in DA-mediated cytotoxicity. Consequently, in oligodendrocytes, this stress response may represent an endogenous mechanism designed to minimize and limit catecholamine free radical-mediated damage.
The mechanisms by which HO-1 could provide protection may involve any of its following functions. It can act as a molecular chaperone in refolding of denatured polypeptides and in reduction of stress-induced denaturation and aggregation of proteins (Sharp et al., 1999). HO-1 can also degrade the heme moiety of cytochrome c (Kutty and Maines, 1982), hence inactivating the cytochrome c activity that is linked to cell death. The products of tetrapyrole cleavage, biliverdin and carbon monoxide, could also provide protection. Biliverdin and bilirubin are known to be potent antioxidants (Stocker et al., 1987; Llesuy and Tomaro, 1994) and exogenously administered carbon monoxide has recently been shown to confer protection against oxidative stress (Otterbein et al., 1999).
Exposure of oligodendrocyte progenitor and mature cultures to DA increased DNA fragmentation as indicated by TUNEL staining, suggesting that DA toxicity may involve apoptosis. In addition, NAC prevented this DA-induced increase in TUNEL-positive staining of oligodendrocyte progenitors and mature oligodendrocytes. Several reports suggest that formation of ROS through the oxidation of DA is responsible for initiating apoptosis in a variety of cell types (Luo and Roth, 2000). The onset of apoptosis may be related to an imbalance between the oxidation of DA and its neutralization by the antioxidant system. Oligodendrocytes and their progenitors contain low levels of glutathione peroxidase, glutathione reductase, and catalase activity, as well as high levels of free iron, and all of these factors can facilitate free radical formation (Adamo et al., 1986; Husain and Juurlink, 1995; Connor and Menzies, 1996; Thorburne and Juurlink, 1996; Juurlink et al., 1998). Moreover, auto-oxidation of catecholamines is associated with the stoichiometric production of H2O2. This compound can also cause free radical damage in progenitor and mature oligodendrocytes (Richter-Landsberg and Vollgraf, 1998; Fragoso and Almazan, 2000) as well as the onset of apoptosis.
Since TUNEL alone has been questioned as a marker of apoptosis, other criteria were next examined. Proteases, especially caspases, have been extensively studied for their role in the execution of apoptosis. Caspase-3 is an essential component of the apoptotic pathway (Nicholson et al., 1995). Our results show that catecholamines stimulate caspase-3 activation in progenitors but not in mature oligodendrocytes, and the relative levels of procaspase-3 were higher in progenitors. Further support for caspase activation in progenitor cultures treated with DA was provided by the selective cleavage of poly(ADP-ribose) polymerase (PARP, 116 kDa protein) to generate an 85k Da fragment that was detectable 5 h after treatment. In addition, the general caspase inhibitor, zVAD, prevented the DA-mediated increase in TUNEL-stained cells only in oligodendrocyte progenitor cultures. Taken together, these results suggest that in mature oligodendrocyte cultures, other mechanisms may be implicated in DA-mediated cell death. At this stage, apoptosis cannot be entirely excluded since other reports have shown that complete chromatin condensation does not require a caspase-dependent step (Zamzami and Kroemer, 1999). Similarly, apoptosis-inducing factor (AIF) initiates chromatin condensation and DNA fragmentation but does not require caspase activation (Susin et al., 1999). Therefore, other apoptotic pathways or zVAD-independent caspases may be involved in initiating apoptosis in mature oligodendrocytes. Furthermore, the involvement of proteases, including calpains and cathepsins (Yamashima, 2000), that are not members of the caspase family in programmed cell death has been demonstrated (Johnson, 2000).
In oligodendrocyte progenitors, zVAD completely blocked DA-mediated activation of caspase-3 and -9, an immediate effector of caspase-3, but only partially rescued cells from DA-mediated cytotoxicity. Since complete rescue was not achieved, a component of cell death in oligodendrocyte progenitors may be necrotic in nature or caspase-independent. The intensity of oxidative stress may determine the cell death pathway since exposure to high concentrations of H2O2 has been shown to lead to necrosis while lower concentrations induced apoptosis (Gardner et al., 1997). Most interesting are the recent observations that hypoxia-ischemia can produce both necrotic and apoptotic death of oligodendrocyte progenitors in perinatal rats (Ness et al., 2001). Since ischemic conditions can cause a large increase in DA release, it can be suggested that catecholamines may participate in oligodendrocyte cell death in vivo as shown in vitro. One could also speculate that death of oligodendrocytes may involve an alternative mechanism such as autophagy, which is implicated in DA-mediated striatal degeneration (Petersen et al., 2001). Autophagic death can be induced by a mutant form of α-synuclein (Stefanis et al., 2001), a protein that accumulates in structures termed Lewy bodies in PD and most significantly in oligodendrocytes of multiple system atrophy (Tu et al., 1998). Aggregates of mutant and wild-type α-synuclein were also shown to induce apoptosis in human neuroblastoma cells (El-Agnaf et al., 1998).
In many cell types, bax, bcl-2, and bcl-xl play a dominant role in regulating apoptosis (Banasiak et al., 2000). The antiapoptotic action of bcl-2 or bcl-xl is believed to involve its binding with Apaf1, the Ced4 homologue, and cytochrome c to prevent activation of caspase-9. Alternatively, bcl-2 or bcl-xl can complex with bax to form a heterodimer, thus blocking the proapoptotic action of bax. Formation of this heterodimer is dependent on the relative amounts of bax, bcl-2, and bcl-xl and determines cell fate (Oltvai et al., 1993; Zha and Reed, 1997; Otter et al., 1998; Perlman et al., 1999). Therefore, an elevated bax/bcl-2 or bax/bcl-xl ratio leads to cell death by apoptosis. Mature oligodendrocytes were shown to have lower levels of bax mRNA than progenitors (Madison and Pfeiffer, 1996), and we have confirmed that the protein levels are also significantly higher in immature cells. Interestingly, we show that during oligodendrocyte development, there is a decrease in the bax/bcl-xl protein ratio, which might explain why mature oligodendrocytes are more resistant to the toxic effects of catecholamines. Such developmental regulation of apoptosis, which appears to occur at the level of regulation of members of the bcl-2 gene family, has been reported to occur in dorsal root ganglion neurons (Vogelbaum et al., 1998).
In summary, results presented here provide evidence that oligodendrocyte progenitors are more sensitive to catecholamine-induced toxicity than mature cells. Several factors have been found that might explain the vulnerability of progenitors, including lower levels of GSH and higher levels of pro–caspase-3, and proapoptotic protein bax. Furthermore, although catecholamine-induced toxicity in oligodendrocytes and progenitor cells involved free radical generation, different mechanisms of cell death appear to operate according to the developmental stage. In progenitors, clear apoptotic features were found, including DNA fragmentation, activation of caspase-9 and -3, and cleavage of the downstream substrate PARP. Further experiments are required to delineate the mechanisms involved and to understand how cell death regulatory proteins such as members of the bcl-2 family interact to modulate death of oligodendrocyte.
Acknowledgements
The authors thank Dr. W.E. Mushynski for editing the article. Supported by grants from the Multiple Sclerosis Society of Canada (to G.A. and A.K.) and Canadian Institutes of Health and Research (to G.A.).