Divergent Temporal Trends of Mercury in Arctic Char from Paired Lakes Influenced by Climate-Related Drivers
Abstract
Climate-driven changes including rising air temperatures, enhanced permafrost degradation, and altered precipitation patterns can have profound effects on contaminants, such as mercury (Hg), in High Arctic lakes. Two physically similar lakes, East Lake and West Lake at the Cape Bounty Arctic Watershed Observatory on Melville Island, Nunavut, Canada are being affected by climate change differently. Both lakes have experienced permafrost degradation in their catchments; however, West Lake has also undergone multiple underwater Mass Movement Events (MMEs; beginning in fall 2008), leading to a sustained 50-fold increase in turbidity. This provided the unique opportunity to understand the potential impacts of permafrost degradation and other climate-related effects on Hg concentrations and body condition of landlocked Arctic char (Salvelinus alpinus), an important sentinel species across the Circum-Arctic. Our objectives were to assess temporal trends in char Hg concentrations and to determine potential mechanisms driving the trends. There was a significant decrease in Hg concentrations in East Lake char, averaging 6.5%/year and 3.8%/year for length-adjusted and age-adjusted means, respectively, from 2008 to 2019. Conversely, in West Lake there was a significant increase, averaging 7.9%/year and 8.0%/year for length-adjusted and age-adjusted mean Hg concentrations, respectively, for 2009 to 2017 (the last year with sufficient sample size). The best predictors of length-adjusted Hg concentrations in West Lake were carbon and nitrogen stable isotope ratios, indicating a shift in diet including possible dietary starvation brought on by the profound increase in lake turbidity. Our study provides an example of how increasing lake turbidity, a likely consequence of climate warming in Arctic lakes, may influence fish condition and Hg concentrations. Environ Toxicol Chem 2023;42:2712–2725. © 2023 His Majesty the King in Right of Canada and The Authors. Environmental Toxicology and Chemistry published by Wiley Periodicals LLC on behalf of SETAC. Reproduced with the permission of the Minister of Environment and Climate Change Canada.
INTRODUCTION
Mercury (Hg), in its organic, methylated form (methylmercury [MeHg]) is a neurotoxicant that can bioaccumulate and biomagnify through aquatic food webs (Kidd et al., 2012; Lavoie et al., 2013; Lescord et al., 2015), and has the potential to reach concentrations that can be harmful to humans and fish-eating wildlife. Although Hg emissions have generally declined in North America and Europe (2010–2015; Arctic Monitoring and Assessment Program, 2021) and concentrations are declining in Arctic air (MacSween et al., 2022), atmospheric and fish Hg concentrations are often decoupled, because there are multiple, complex, interacting controls that influence biotic Hg concentrations, many of which are modified by climate change (Grieb et al., 2020; Wang et al., 2019). This is of particular concern in the High Arctic, where accelerated climate change has many implications for Hg in fishes.
Across the high Arctic, elevated air temperatures are causing a thinning of lake ice (Arp et al., 2012) leading to longer ice-free periods (Dibike et al., 2011; Sharma et al., 2019), which increases light availability, and consequently lake primary production (Huisman & Weissing, 1994; Michelutti et al., 2005). This, along with warmer water temperatures, can increase fish growth rates (Reist et al., 2006), which have been linked to a decrease in fish Hg (Stafford et al., 2004; Ward et al., 2010), through a phenomenon known as somatic growth dilution (Karimi et al., 2007). Essentially, faster growing fish gain more biomass relative to Hg than their more slowly growing counterparts, thereby “diluting” the tissue Hg burden (Karimi et al., 2007). Greater primary productivity can lead to a different form of biodilution in fish, “bloom dilution,” wherein the amount of Hg in the water column is distributed across a greater number of algal cells in a more productive lake, which decreases exposure to grazers and subsequent trophic levels (Pickhardt et al., 2002). However, studies of salmonid species in Arctic and sub-Arctic lakes concluded that net effects of diet and age on Hg were greater than any potential offset by biomass dilution (Chételat et al., 2021).
Another climate change effect that can influence Hg in aquatic food webs is enhanced permafrost degradation. Globally there is a significant amount of Hg stored in permafrost and active layer soils (Schuster et al., 2018), and as temperatures rise, there is an increase in the frequency and intensity of thermokarst (thawing and collapse of ice-rich permafrost; Grosse et al., 2013; Lewkowicz & Way, 2019; Vonk et al., 2015), which could lead to an increase in Hg supplied from the catchment through enhanced erosion and transport to downstream lakes. In addition, widespread thermokarst is predicted to increase the flux of organic carbon (Frey & Smith, 2005; Guo et al., 2007; Rydberg et al., 2010) and solutes (e.g., sulfate; Kokelj et al., 2009), which can affect the amount of MeHg in a system via methylation of inorganic Hg (Lehnherr, 2014). Both sulfate (Gilmour et al., 1992) and organic carbon (Ravichandran, 2004) can stimulate methylating bacteria; however, they can also serve to make inorganic Hg and MeHg less bioavailable (Lehnherr, 2014; Ravichandran, 2004). Several studies have documented the effects of thermokarst processes on Hg in water and sediments (Deison et al., 2012; Rydberg et al., 2010; St. Pierre et al., 2018). Schaefer et al. (2020) predicted increased concentrations of Hg in fish in the Yukon River (BC, Canada/AK, USA) based on large-scale permafrost thaw and increasing water concentrations during the 21st century under the Intergovernmental Panel on Climate Change Representative Concentration Pathway (RCP 8.5). However, there are as yet few empirical data on the effect of permafrost degradation on fish Hg concentrations, which is often decoupled from water column Hg concentrations (McKinney et al., 2022).
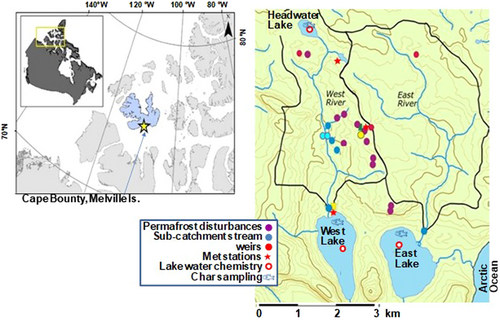
The Cape Bounty Arctic Watershed Observatory (CBAWO), located on the south-central coast of Melville Island (Figure 1), was established in 2003 to investigate climate effects on hydrological processes and permafrost. The CBAWO encompasses two paired watersheds that drain into adjacent lakes (East and West). In 2007, 2011, and 2012, Cape Bounty experienced the three warmest years on record since the instrumental record began in 1949, with June–July–August temperatures averaging 1.9 to 3.2 °C higher than the mean of 1.9 °C for 1949 to 2019 at the nearest long-term weather station (Supporting Information, Figure S1), leading to a deepening of the active layer, thawing of ground ice, and enhanced thermokarst activity (Lamoureux & Lafrenière, 2018; Roberts et al., 2017). In 2007, >100 active layer detachments occurred in the East and West catchments (covering 1.0% and 2.8%, respectively). The permafrost disturbances in West Lake were more severe, and in addition it experienced several “subaqueous slumps” (internal mass movement events [MMEs]) in 2008, 2011, and 2012, leading to dramatic and persistent increases in lake turbidity (Dugan et al., 2012), solutes (Roberts et al., 2017), and Hg concentrations in river water. Because these lakes are otherwise similar, the persistent elevated turbidity in West Lake allows for assessment of potential effects of permafrost disturbances and sediment resuspension on concentrations of Hg and other contaminants in fish.
The top predator and only fish species in both East and West Lakes is Arctic char (Salvelinus alpinus). Arctic char are salmonids that have both nonanadromous and anadromous life histories; the fish in East and West Lakes are landlocked, unable to migrate to the ocean. While anadromous char are the preferred subsistence or “country food” source for Indigenous communities across the circum-Arctic, landlocked char are also utilized by some communities (Lea et al., 2020). Char are generalists and opportunists, exploiting many different prey types. Chief components of their diets typically include zooplankton, benthos (especially chironomidae), prey fish, or smaller conspecifics (Gallagher & Dick, 2010; Gantner et al., 2010; Johnson, 1980). Char are visual predators, so the pronounced increase in turbidity in West Lake could affect their ability to detect prey (Utne-Palm, 2002). Roberts et al. (2017) detected a decrease in fish relative condition (a measure of overall health) following the subaqueous slumps, identifying the increased turbidity as the likely cause. This could have implications for fish Hg concentrations, because lower fish condition can result in higher Hg concentrations in fish (Cizdziel et al., 2003; Suns & Hitchin, 1990).
East and West Lakes are both experiencing many interacting climate-related changes, which in addition to the major stochastic disturbance in West Lake, presents a de facto natural experiment to assess the potential effects of climate change on Arctic char Hg concentrations. As such, there were two main objectives of the present study: (1) to assess the temporal trends in Arctic char Hg concentrations in East and West Lakes, and (2) to determine the potential mechanisms driving any observed trends in each lake. We hypothesized that char Hg concentrations in West Lake would be influenced by increased turbidity and declining fish condition. Conversely, we hypothesized that char Hg in East Lake would be declining, similar to other landlocked char populations (Hudelson et al., 2019) due to other climate-related changes including warmer air temperatures and higher primary production, as well as decreasing atmospheric Hg in the Arctic (MacSween et al., 2022).
METHODS
Study site
The CBAWO is located at 74°50′N, 109°30′W on Melville Island, Nunavut, Canada (Figure 1). This region is classified as Polar Desert with low mean annual precipitation (<160 mm), and cold mean annual temperatures (–17.5°), with monthly means above freezing only in June, July, and August (Favaro & Lamoureux, 2015). The region has warmed by approximately 2 °C since 1949, with a more rapid warming in the mid-2000s, which has led to enhanced permafrost degradation (Favaro & Lamoureux, 2015). The adjacent paired East and West Lakes have similar lake areas (1.6 and 1.4 km2, respectively) and watershed areas (11.6 and 8.0 km2, respectively; additional morphometric data are shown in Table 1). The watersheds are located within a zone of continuous permafrost with an active layer between 50 and 70 cm in depth on prostrate shrub tundra. The summer temperatures in 2007 were particularly warm, exceeding the long-term mean by 1.9 °C (Supporting Information, Figures S1 and S2), which led to a deep surface thaw and >100 active layer detachments within the watersheds. West Lake has experienced multiple MMEs in September 2008, December 2011, and January 2012, which led to a dramatic and persistent increase in water column turbidity greater than 50× from approximately 4 nephelometric turbidity units (NTU) in 2006 to approximately 250 NTU in 2015 (Roberts et al., 2017). Both East and West Lakes have simple food webs culminating in landlocked Arctic char as the singular fish species.
| East | West | Headwater | |
|---|---|---|---|
| Latitude (North) | 74°53′25″ | 74°53′38″ | 74°56′18″ |
| Longitude (West) | 109°32′38″ | 109°35′54″ | 109°37′12″ |
| Meters above sea level | ~5 | ~8 | ~80 |
| Surface area (km2) | 1.4 | 1.6 | 0.14 |
| Watershed area (km2) | 11.6 | 8.0 | 1.5 |
| Maximum depth (m) | 30 | 33 | — |
- a Lake and catchment areas and elevation from The Atlas of Canada (Government of Canada, 2021).
Sample collection and preparation
Arctic char were collected from each lake every year at the end of July between 2008 and 2019 (Supporting Information, Table S1) using gillnets (single mesh size 36–42 mm; 30-m length). Sampling was timed to coincide with the lakes being nearly ice-free, allowing for deployment of gillnets from an inflatable boat. Further details on the fishing procedures are given in the Supporting Information. No char were collected in 2010 when we attempted to capture fish by angling through the ice in early July. Char were also collected in Headwater Lake, a small lake in the West Lake catchment (Figure 1 and Table 1) in 2015 and 2019. This lake, which is 4 km north of West Lake, served as a reference site due to having no nearby permafrost degradation. After collection, fish were measured for total length, fork length, and weight. Fish were then dissected, and their stomachs, liver, gonads, tissue (dorsal muscle), and otoliths were collected, frozen on site in a portable freezer (–20 °C), and transported back to the laboratory for analysis within 2 months of collection.
Water samples were collected for unfiltered and filtered total Hg and MeHg analyses at five different depths (4, 10, 15, 20, and 32 m) throughout the water column from the approximate center of each lake; details on sampling and filtration are provided in the Supporting Information. Briefly, water was collected into an acid-washed 2.5-L Teflon-lined Niskin sampler and then subsampled into certified precleaned 250-mL amber glass bottles with Teflon-lined lids (Environmental Sampling) using the “clean-hands, dirty-hands” technique (US Environmental Protection Agency [USEPA], 1996). All samples were acidified to 0.2% by volume with concentrated, ultrapure HCl (38%) within 12 h of collection. Field blanks were prepared by sending empty glass bottles to the field and back again. These bottles were then filled with ultrapure Milli-Q water, acidified, and analyzed with the other samples. Samples were kept at 4 °C and transported to the Canada Centre for Inland Waters (CCIW) in Burlington, Ontario for analysis.
Water samples for sulfate and total organic carbon (TOC) were collected using the Kemmerer sampler at the same predetermined depths as Hg and manually at 10 to 20-cm subsurface. Waters were collected in amber high-density polyethylene (HDPE) bottles, and were vacuum filtered through 0.22-μm polyvinylidene fluoride filters in the field laboratory within a few hours of collection. Filtered sample aliquots were stored in 25-ml HDPE scintillation vials without headspace and stored on ice/refrigerated until analysis at Queen's University, Kingston, Ontario. Additional surface (0.5–1 m depth) water samples were collected from 2015 to 2019 and analyzed by the National Laboratory for Environmental Testing (NLET; at CCIW, Burlington). Samples were filtered within 4 days of collection for analysis of chlorophyll-a and particulate and dissolved elements.
Limnological conditions of the water column including turbidity were measured with a Richard Brancker Research conductivity, temperature, depth (CTD) instrument equipped with additional Seapoint turbidity and Optek dissolved oxygen sensors (XR-420, SN 10401, factory calibrated annually). The CTD profiles were obtained through ice (late May–early July) and during ice-off (August). In addition to discrete water column measurements, fixed CTD bottom moorings were used to assess over-winter conditions. Seasonal (June–August) mean turbidity was calculated using measurements between 10 and 20 m depth in an effort to represent whole lake conditions consistent with the methods presented in Roberts et al. (2017). Air temperature data were collected by an automated meteorological station (80 masl; WestMet) beginning in 2003 (Beel et al., 2021). Temperatures were recorded with an Onset UA-003 temperature logger (0.1 °C accuracy). Air temperature was used a proxy for ice duration because it was available throughout the study period (Supporting Information, Figures S1 and S2). Weather data from the Mould Bay weather station (200 km west of CBAWO) were also obtained from the Environment and Climate Change Canada website (Environment and Climate Change Canada [ECCC], 2020).
Laboratory analysis
Char dorsal muscle samples were analyzed for total Hg by USEPA (2007) method 7473 using a Direct Mercury Analyser (Milestone Instruments). Certified reference materials (NIST 1566b Oyster Tissue; NIST 2976 Mussel Tissue; DORM-3 [fish muscle protein] and DOLT-4 [dogfish liver]) were analyzed with samples to assess accuracy and precision. The overall average (±SD) agreement with certified values was 100.2% ± 2.1%. Further quality assurance details are provided in the Supporting Information.
For stable isotope analysis, char muscle samples were freeze-dried prior to analysis, and percent moistures were determined. The stable carbon and nitrogen isotope compositions (expressed as δ13C and δ15N values) were determined in nonlipid extracted muscle by the Environmental Isotope Laboratory (University of Waterloo, Waterloo, ON, Canada). Further details on analytical precision of the δ13C and δ15N analysis are provided in the Supporting Information.
Percent lipid was determined gravimetrically on a subset of char muscle samples from all sampling years (East, n = 75; West, n = 59; Headwater, n = 8). Further details are provided in the Supporting Information.
Otoliths were used to determine adult char ages using break-burn or thin-section methods (Swanson et al., 2010). Aging was conducted by John Babaluk (Winnipeg, MB, Canada) and by AAE Tech Services (LaSalle, MB, Canada).
Water samples were analyzed at the Low-Level Mercury Analytical Laboratory (at the CCIW) for total Hg by BrCl oxidation, SnCl2 reduction, gold trap amalgamation, and detection by cold vapor atomic fluorescence spectrophotometry (CVFAS; Bloom & Crecelius, 1983) using a Tekran Instruments model 2500 Hg analyzer. MeHg was analyzed by distillation, aqueous phase ethylation, and detection by CVFAS using a Brooks Rand MERX automated MeHg analyzer (Bloom, 1989; Horvat et al., 1993). The method detection limits for total Hg and MeHg were 0.075 ng/L and between 0.01 and 0.015 ng/L, respectively. Further quality assurance data for Hg and MeHg in water is provided in the Supporting Information. Similar to turbidity, Hg and MeHg results for samples collected at 10 to 20 m depths were used in our analyses to avoid any surface or sediment–water interface effects.
Sulfate concentrations were determined by ion chromatography using a Dionex ICS-3000 (during 2008–2016) or ThermoFisher ICS-5000 ion chromatograph (from 2017 onward) following the same methods as (Roberts et al., 2017). Similar to turbidity and Hg, we only used samples collected at 10 to 20 m depths except for Headwater Lake where sampling depth was 0.5 to 4 m. Water samples collected at 0.5 to 1 m depth from 2015 to 2019 were analyzed for a standard suite of parameters using standard methods (NLET, 1994); we report on silica, and total and dissolved aluminum and iron.
Data analyses
To assess temporal changes in Arctic char Hg concentration over time, analysis of covariance was used to generate estimated marginal means (emmeans) to adjust for (total) length and age separately, because both of these covariates can influence fish Hg (Bodaly et al., 1993; Evans et al., 2005; Swanson & Kidd, 2010). In these analyses, year was used as a categorical variable, and the emmeans were calculated at the overall mean of each covariate (see the Supporting Information). The Hg concentrations and length were log10-transformed to meet assumptions of normality.
Fish relative condition (Kn; a measure of overall fish health) was calculated using the formula Kn = W/Wʹ, where W is the weight of individual fish and Wʹ is the predicted length-specific mean weight for the population (Le Cren, 1951). Relative condition was judged to be appropriate given the differences between the two lakes because it does not assume isometric growth (Blackwell et al., 2000).
Temporal trends of Hg, stable isotope ratios, and condition were assessed using simple linear regression, and comparisons between the two lakes across years were completed using paired t-tests. To determine potential mechanisms driving the temporal changes in char Hg in East and West Lakes, correlations were examined among various climate, chemicophysical, physiological, and food web variables and length- and age-adjusted char Hg (Table 2). Furthermore, general linear models were used where applicable. Due to the challenging site access and the unpredictability of Arctic field work, certain covariate data were not available for every year of the study (Supporting Information, Tables S3–S7). All the covariates included in the correlation analyses had at least 5 years of observations throughout the study period. Finally, changes in air temperature were assessed, as a proxy for growing season (see the Supporting Information). There has been a 1.8 °C regional increase in mean annual air temperature from 1990 to 2019 (Supporting Information, Figure S2 and Table S2B; ECCC, 2020).
| Variable | Length-adjusted char Hg (ng/g) | Age-adjusted char Hg (ng/g) | |||
|---|---|---|---|---|---|
| r | p | r | p | ||
| East Lake | |||||
| Climate | Mean summer air temp prev. year (°C) | 0.76 | <0.01 | 0.66 | 0.05 |
| Mean summer air temp (°C) | 0.38 | 0.27 | 0.25 | 0.5 | |
| Chemicophysical | Turbidity (NTU) | 0.045 | 0.91 | −0.05 | 0.91 |
| Water THg (ng/L) | −0.25 | 0.63 | −0.29 | 0.58 | |
| Water MeHg (ng/L) | −0.06 | 0.91 | −0.46 | 0.3 | |
| SO4 (mg/L) | −0.56 | 0.19 | −0.67 | 0.1 | |
| Physiological | LSMean age-at-size (year) | 0.84 | <0.01 | 0.59 | 0.07 |
| Relative condition | −0.19 | 0.57 | −0.45 | 0.18 | |
| C:N | −0.28 | 0.4 | −0.42 | 0.22 | |
| Food web | δ13C value (‰) | 0.33 | 0.32 | 0.37 | 0.3 |
| δ15N value (‰) | −0.53 | 0.09 | −0.4 | 0.26 | |
| West Lake | |||||
| Climate | Mean summer air temp prev. year (°C) | 0.11 | 0.77 | 0.23 | 0.59 |
| Mean summer air temp (°C) | −0.12 | 0.77 | −0.13 | 0.76 | |
| Chemicophysical | Turbidity (NTU) | 0.24 | 0.57 | 0.24 | 0.56 |
| Water THg (ng/L) | 0.34 | 0.51 | 0.29 | 0.57 | |
| Water MeHg (ng/L) | 0.90 | 0.04 | 0.52 | 0.36 | |
| SO4 (mg/L) | 0.17 | 0.78 | −0.02 | 0.97 | |
| Physiological | LSMean age-at-size (year) | 0.1 | 0.81 | −0.29 | 0.48 |
| Relative condition | −0.43 | 0.25 | −0.46 | 0.24 | |
| C:N | −0.36 | 0.34 | −0.18 | 0.66 | |
| Food web | δ13C (‰) | 0.82 | <0.01 | 0.78 | 0.02 |
| δ15N (‰) | 0.73 | 0.02 | 0.94 | <0.0001 | |
- All data are not available for each year (see the Supporting Information for details). Bolded text represents significant correlations.
- LSMean = least-squares mean; NTU = nephelometric turbidity unit; THg = total mercury.
All statistical analyses were completed using R Studio Ver. 1.0.136 and R Ver. 3.5.1 with core packages, as well as “emmeans” (Lenth et al., 2022). Figures were generated using Sigmaplot Ver. 11.0. Residuals were examined following analyses to assess statistical assumptions (normal residuals, homogeneity of variance).
RESULTS AND DISCUSSION
Collection numbers for adult (>200 g) Arctic char ranged from 7 to 23 in East Lake and from 0 to 21 in West Lake over the period 2008 and 2019 (Supporting Information, Table S1). The variation reflected challenges in deploying nets in the presence of ice and a limited time window for sampling at this remote location. Collection numbers declined in West Lake in 2017 and 2018; only one adult char was collected in 2018.
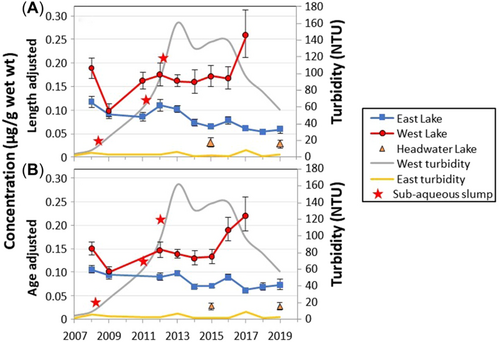
Length-adjusted concentrations of Hg in Arctic char muscle ranged from 0.10 to 0.26 µg/g wet weight in West Lake and from 0.05 to 0.12 µg/g wet weight in East Lake (Figure 2A and Supporting Information, Tables S3 and S4). Char in Headwater Lake had mean (unadjusted) Hg concentrations of 0.03 µg/g wet weight in both 2015 and 2019 (Supporting Information, Table S7). Overall, this range of mean concentrations for the three lakes was similar to mean Hg levels for char in coastal lakes of similar size (Resolute, Small, North) on Cornwallis Island, Nunavat, Canada (Hudelson et al., 2019; Lescord et al., 2015) and to the majority of results for landlocked char in 83 Arctic and sub-Arctic lakes reviewed by Barst et al. (2019). However, to the best of our knowledge, the results for char Hg in West Lake were the first from a lake with persistent highly turbid waters. The Hg concentrations in char muscle from the Cape Bounty lakes were all below the threshold effects level for Hg of 0.3 µg/g wet weight (Barst et al., 2019; Dillon et al., 2010) except for 5 of 105 fish collected from West Lake, which had maximum concentrations of 0.4 µg/g wet weight.
Temporal trends in char Hg, lake water chemistry, and Hg
A significant divergence of Hg concentrations in Arctic char in East and West Lakes was observed over the period 2008 to 2019. In East Lake, there was a significant decline in both length-adjusted (p < 0.001; Figure 2A and Supporting Information, Table S8A) and age-adjusted (p < 0.01; Figure 2B and Supporting Information, Table S8A) char Hg concentrations between 2008 and 2019. The decline averaged 6.5%/year and 3.8%/year for length-adjusted and age-adjusted means, respectively, from 2008 to 2019. In West Lake, however, there was no significant increase in length-adjusted Hg (p = 0.13; Figure 2A and Supporting Information, Table S8A) and age-adjusted Hg (p = 0.09; Figure 2B and Supporting Information, Table S8A) between 2008 and 2017. However, the temporal trend in West Lake from 2009 onward, that is, after the first subaqueous disturbance, which occurred in September 2008 after the 2008 fish were collected, showed a significant increase in both length-adjusted (p = 0.02; Figure 2A and Supporting Information, Table S8A) and age-adjusted (p = 0.02; Figure 2B and Supporting Information, Table S8A) char Hg. The increase averaged 7.9%/year and 8.0%/year for length-adjusted and age-adjusted mean Hg concentrations, respectively, for 2009 to 2017 (the last year with sufficient sample size). It is noteworthy that char in Headwater Lake collected in 2015 and 2019 had mean Hg concentrations very similar to those of East Lake char but far lower than in West Lake char in the prior years (Figure 2A and B). Due to the shallow depth of the West River (Figure 1), it is unlikely that there is exchange of adult char between West and Headwater Lake.
Due to the remote Arctic location of East and West Lakes combined with the unique water chemistry of West Lake, it was initially hypothesized that any temporal changes would be attributable directly or indirectly to climate change. It is not necessarily unusual that we identified opposite temporal trends in char Hg concentrations in two lakes, because climate-related variables have been correlated with increases (Rigét et al., 2010), limited impacts (Hudelson et al., 2019), or decreases in char Hg concentrations (Hudelson et al., 2023). In Lake Hazen (Ellesmere Island, Nunavut, Canada), declining Hg was related to increased snow accumulation in the fall and increased glacial runoff, as well as to declining atmospheric Hg (Hudelson et al., 2023). Over the period 2000 to 2015, long-term monitoring of other landlocked arctic char populations has shown declines of char Hg in Lake Abiskojaure in northern Sweden, while there was a significant increasing trend of char Hg in Lake Ellasjøen in Bjørnøya, Sweden (Morris et al., 2022). Hudelson et al. (2019) examined temporal Hg concentration trends in landlocked char from six small lakes (of similar size to East and West Lake), on Cornwallis Island. They found significant declining Hg in char from three lakes and no trend in three others. The six lakes also had similar water chemistry and no evidence of increasing turbidity. However, East and West Lakes are adjacent, have similar morphometry (Dugan et al., 2012), and had similar low turbidity in 2007 (Figure 2A and B). The diverging trends in fish Hg occurred over 2008 to 2019, a period of extensive watershed and limnological change in the CBAWO.
Drivers of char Hg
To help identify what could be causing these changes, we evaluated the influence of some potential drivers of fish Hg on the temporal trends observed in East and West Lakes. First, lake water chemistry parameters relevant to fish Hg accumulation were assessed at lake depths of 10 to 20 m in an effort to capture average lake conditions (Supporting Information, Tables S5 and S6). Lake turbidity and sulfate (SO42−) in West Lake significantly increased over the period 2006 to 2019 (Supporting Information, Table S8B). In East Lake, only sulfate increased significantly (Supporting Information, Table S8B). The changes in turbidity and SO42− are discussed in more detail by Roberts et al. (2017). In brief, turbidity in West Lake increased from 7.1 in 2006 to 161 NTU in 2013 and remained elevated (Figure 2), whereas it was very low (<10 NTU) in East Lake. Sulfate concentrations increased approximately threefold from 2006 to 2016 in both lakes. The TOC showed no appreciable change in either lake. The turbid waters of West Lake were characterized by concentrations of total (unfiltered) silica, aluminum, and iron, which averaged 14-, 143-, and 114-fold higher than in East Lake during the period 2015 to 2019 (Supporting Information, Tables S5 and S6). Headwater Lake waters also had higher (unfiltered) silica, aluminum, and iron than East Lake, averaging 4.5-, 7.3-, and 4.0-fold higher, respectively (Supporting Information, Table S7). The turbidity in West Lake was thus composed of silicate- and iron-rich mineral material (Cuven et al., 2011) and very fine clays derived from the lake sediments (Cockburn & Lamoureux, 2008), which have remained suspended for many years after they were mobilized by the MMEs in 2008, 2011, and 2012 (Roberts et al., 2017).
The total Hg (THg) and MeHg in lake water showed no trend in East Lake, averaging (±SE) 0.66 ± 0.05 and 0.012 ± 0.001 ng/L, respectively, in unfiltered samples over the study period, with unfiltered and filtered MeHg concentrations frequently below the method detection limits, which were calculated for each sample batch/year and ranged between 0.01 and 0.015 ng/L (Supporting Information, Tables S5 and S8B). In contrast, in West Lake, THg and MeHg in lake water (unfiltered) significantly increased by 40% to 50% from 1.28 ± 0.18 and 0.012 ± 0.002 ng/L in 2012, to 1.98 ± 0.07 and 0.025 ± 0.005 ng/L, in 2017, respectively (Supporting Information, Tables S6 and S8B), concurrent with increases in fish Hg concentrations. Although fish were not collected in 2019 from West Lake, one lake profile was collected in June 2019 suggesting that lake water concentrations of both THg and MeHg had decreased (1.44 ± 0.01 and 0.012 ng/L, respectively), indicating that continued measurements are required to understand the long-term changes in West Lake. The MeHg exports from the catchments of West and East Lake over the period 2007 to 2017 were very similar, averaging 0.045 and 0.042 g/year (Pope, 2018). Total Hg concentrations in surface sediments (0–2 cm) in cores collected at the deep point in each lake were also similar, averaging 50 ng/g dry weight in West Lake and 44 ng/g dry weight in East Lake (Muir and Kirk, unpublished data). Thus, although the trend analysis must be interpreted with caution, taken together, the significant increases in sulfate, THg, and MeHg in lake water of West Lake suggest that enhanced delivery of terrestrially sourced sulfate and inorganic Hg(II), combined with the greatly elevated turbidity, has fueled the production of MeHg within West Lake, which has then been accumulated into the fish food web.
Numerous potential biological and food web–related drivers of fish Hg levels were also explored. There was a significant decrease in char age-at-size (a proxy for lifetime average growth rate, such that faster growing fish are younger at size) between 2008 and 2019 in East Lake (p ≤ 0.01; Supporting Information, Table S8A and Figure 3A), indicating an increase in mean growth rate over time. In contrast, there was no change between 2008 and 2017 in West Lake (p = 0.26; Figure 3A and Supporting Information, Table S8A). Arctic freshwater fish growth can be influenced by many climate-related factors including increases in primary and secondary productivity from longer growing seasons and increases in nutrients from thawing permafrost (Reist et al., 2006). Indeed, the warming in the High Arctic has been the most dramatic within the last two decades (Supporting Information, Figure S2). Although primary productivity has not been quantified directly in East and West Lakes, Roberts et al. (2017) assessed littoral and midlake diatom assemblages before (2004) and after (2014) the permafrost disturbances. They found diatom shifts in East Lake consistent with vertical mixing, nutrient availability, and light, all indicative of climate warming, whereas West Lake was dominated by lower diversity benthic diatoms, reflecting the influence of turbidity.
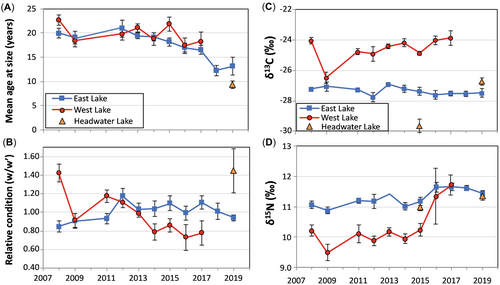
Roberts et al. (2017) also established a link between fish relative condition and turbidity in West Lake based on data from 2008 to 2015. Given that fish condition can influence fish Hg concentrations (see Cizdziel et al., 2003; Suns & Hitchin, 1990), temporal trends in relative condition in both East and West lakes were revisited using data from 2019 and 2017, respectively (Figure 3B). Although there was no significant change in mean char relative condition in East Lake between 2008 and 2019 (p = 0.23; Figure 3B and Supporting Information, Table S8A), there was a significant decline in mean char relative condition in West Lake between 2008 and 2017 (p ≤ 0.01; Figure 3B and Supporting Information, Table S8A). Nearly 70% of the variability in char relative condition in West Lake can be explained by the mean water column turbidity, which increased significantly from 2008 to 2016 (Figure 2 and Supporting Information, Table S8B). The link between relative condition and turbidity is likely because Arctic char are visual predators, and a decrease in light could decrease their search efficiency (Shuter et al., 2012).
Differences among lakes in food webs, defined by δ15N and δ13C values, have also been found to affect fish Hg concentrations (Kidd et al., 2012; Lescord et al., 2015). There was no significant temporal trend in mean char δ13C values (p = 0.12; Figure 3C and Supporting Information, Table S8A) for East Lake between 2008 and 2019, but there was a significant increase in δ15N values (p < 0.01; Figure 3D and Supporting Information, Table S8A) over the same period. In West Lake, there were significant increases in both δ13C values (p < 0.01; Figure 3C and Supporting Information, Table S8A) and δ15N values (p < 0.01; Figure 3D and Supporting Information, Table S8A) from 2009 to 2017. These changes in West Lake suggest that the persistent turbidity brought on by the MMEs affected the food web structure.
In general, the food web structure appeared to be quite different in East and West Lakes, based on isotopic indicators (Figure 4). The mean char δ13C value in East Lake across the study period was −27.36 ± 0.25‰ (SD), which was significantly lower than in West Lake, at −24.61 ± 0.80‰ (SD; paired t-test; T = 8.93, df = 8, p < 0.001). Char from Headwater Lake had a very similar mean δ13C value (−26.7 ± 0.6‰) to East Lake, further underlining the benthic influenced carbon sources in West Lake. The larger spread on the West Lake plot indicates higher temporal variability of δ13C (Figures 3C and 4). The δ13C values suggest that throughout the study period, char in West Lake have likely been more dependent on benthic resources than those in East Lake. Other studies of small high Arctic lakes have concluded that food webs are mainly influenced by benthic carbon sources such as chironomid larvae and emerging adults (Chételat et al., 2010; Gantner et al., 2010; Lescord et al., 2015).
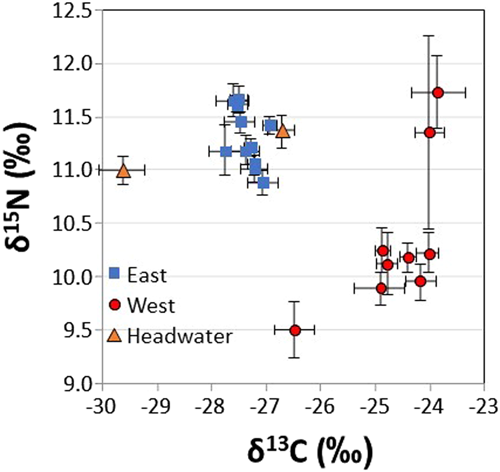
Although there were differences between East and West Lake char δ15N values, we did not compare the results statistically because such a comparison may not be meaningful without baseline δ15N values. However, the increase in δ15N values from 2015 to 2017 in West Lake char coincides with the collection of low sample numbers and could be the result of dietary starvation of the char. Several laboratory feeding studies have shown that feeding of fish below the maintenance level results in increasing δ15N values due to enrichment of 15N from catabolizing of protein (Bowes et al., 2014, Gaye-Siessegger et al., 2007). The evidence for starvation is not definitive. The δ15N values and relative condition were not significantly correlated in West Lake (data not shown). In addition, while percentage of lipids were significantly lower in West Lake char compared with those in East Lake (two-sample t-test, p = 0.002) and mean percentage of lipid in West Lake char declined from 2008 to 2015, samples from 2016 and 2017 had higher percentage of lipid than those from preceding years (Supporting Information, Figure S4).
In East Lake, there was a significant positive correlation between mean summer air temperature from the previous year and both length- and age-adjusted char Hg (p < 0.01 and 0.05, respectively; Table 2). In addition, there was a significant positive correlation between fish age-at-size (a proxy for lifetime average growth rate) and length-adjusted char Hg (Table 2), such that faster growing char had lower Hg concentrations. There was a positive relationship between age-at-size and age-adjusted char Hg as well, but this was not statistically significant (p = 0.07; Table 2).
We hypothesized that there would be a direct relationship between turbidity and/or fish relative condition and char Hg in West Lake. However, neither of these correlates were statistically significant for length- or age-adjusted char Hg (Table 2). Nevertheless, there were significant positive correlations between both length- and age-adjusted char Hg concentration and δ13C and δ15N values (Table 2), which indicates that basal carbon resources and food web structure are likely influencing the char Hg concentration in West Lake. There was also a positive correlation between water MeHg (unfiltered) concentration and char length-adjusted Hg in West Lake but not in East Lake (Table 2). However, there are only 5 years of observations of water MeHg concentrations in West Lake (2012, 2014–2017; Supporting Information, Table S6), which all occurred after the disturbance, so caution should be taken when interpreting this relationship.
In East Lake, a general linear model including the two significant correlates (age-at-size and previous year mean air temperature) explained over 75% of the variability in length-adjusted char Hg concentration (R2 = 0.76, F(2,7) = 15.53, p < 0.01). In West Lake, the two food web indicators, δ13C and δ15N values (Pearson's product-moment; r = 0.68, p = 0.04) were significantly correlated, and thus could not be included in the same model; however, they can explain 66% and 47% of the variability in length-adjusted char Hg concentration, respectively (linear regression; R2 = 0.47–0.66, F(1,7) = 8.13–16.68, p < 0.03).
It is not surprising that fish age-at-size (a proxy for population mean lifetime growth rate) is one of the significant drivers for char Hg in East Lake. The positive relationship between fish age-at-size and Hg concentration is an example of somatic growth dilution, a phenomenon whereby faster growing fish are able to gain more mass per unit food intake than their slower growing counterparts, thereby diluting Hg concentrations in their body tissues (Karimi et al., 2007). Similar results have been found for Arctic char (Swanson & Kidd, 2010; Swanson et al., 2011) and other Arctic fishes (Keva et al., 2017; Sharma et al., 2008; Swanson et al., 2010). Fish growth can be influenced by water temperature (Reist et al., 2006) and changes in nutrients and primary production, which can be impacted by climate (Paerl & Huisman, 2008). It is evident that average growth rates increased in East Lake, because there was a significant decrease in age-at-size concomitant with the decrease in char Hg; however, this cannot be definitively linked to climate.
Interestingly, the other significant climate-related variable, previous year mean summer air temperature, suggests that climate warming could have the opposite effect of the age-at-size relationship, because this relationship was positive (Table 2). It is likely that because age-at-size represents an average lifetime growth rate, it is tracking the effects of the longer term increase in air temperature and possible increases in primary production, whereas previous summer air temperature is tracking the effects of the interannual variability in temperature on char Hg.
Higher char Hg concentrations following warmer summers could be the result of multiple mechanisms. Warmer air temperatures are strongly correlated with earlier ice-off (Supporting Information, Figure S3), which can lead to an increase in lake primary production. As just mentioned, changes in primary production in East Lake could not be quantified directly, but chlorophyll-a, an indicator of algal growth, was very low in both lakes (<0.1 μg/L) in open water samples collected in July and August (Supporting Information, Tables S5 and S6), except for samples collected in 2019, when it was slightly elevated in East Lake (1.8 μg/L). High particle load on filters due to turbidity interfered with chlorophyll analyses in West Lake. Roberts et al. (2017) present evidence suggesting an increase based on shifts of diatom assemblages, which represents a more sensitive measure of primary producers than our limited water chemistry data. Climate-induced increases in primary production in Arctic lakes have been well documented (Prowse et al., 2006). A study by Rigét et al. (2010) found an increase in the Hg concentration of landlocked Arctic char between 1994 and 2008 and suggested that it could be linked to an increase in temperature and primary production. This link has been made previously with temporal trends of Hg in other top predator fish. Carrie et al. (2010) documented increased Hg concentrations in burbot (Lota lota) and attributed this to climate, because of an observed concomitant increase in primary production and algal-scavenging of Hg from the water column. On the other hand, Evans et al. (2013) found increasing Hg concentrations in lake trout and burbot over the early 1990s to 2012 in two major regions of Great Slave Lake, Northwest Territories, Canada, but no evidence for the influence of temperature or productivity. However, more recently, Hudelson et al. (2019) found that dissolved organic carbon (DOC) in coastal lakes on Cornwallis Island was inversely related to Hg in Arctic char, and a study over a gradient of Arctic, sub-Arctic, and midlatitude lakes concluded that low DOC and oligotrophic conditions of Arctic lakes enhanced MeHg bioaccumulation (Chételat et al., 2018). These results for Arctic lakes are opposite to observations of lake DOC and Hg in fish in midlatitude lakes (Watras et al., 1998). Warmer summers could also lead to increased Hg methylation (Paranjape & Hall, 2017) and Hg loading (Rydberg et al., 2010), both of which could contribute to the positive relationship between char Hg concentration and previous year mean summer air temperature.
Given the fact that these two climate-related variables are ostensibly affecting char Hg in East Lake in opposing ways, it is difficult to predict how it will continue to change in the future. In general, it seems increasing fish growth is causing char Hg to trend downward; however, year to year, a warmer season could lead to greater loading of Hg to the system, via scavenging of Hg to the water column, or an increase in methylation. This interplay of different and often confounding drivers of fish Hg concentration has been documented elsewhere. In a recent study on char Hg concentrations from the eastern Canadian Arctic, the authors found that contrary to their hypothesis, faster growing char did not have lower Hg concentrations than their more slowly growing counterparts (Chételat et al., 2021). The authors attributed the absence of a growth dilution effect to the fact that the faster growing fish were eating at a higher trophic position, thereby nullifying any growth dilution effects (Chételat et al., 2021).
It was originally hypothesized that char Hg would increase in West Lake as a function of the increased turbidity and the resultant decrease in fish relative condition. Although a significant decrease in fish relative condition over time directly related to turbidity was observed, neither of these variables was significantly correlated with char Hg concentration in West Lake (Table 2). This does, however, indicate an overall decline in fish health following the series of MMEs. Indeed, the decline in fishing success in West Lake, from 21 adults (>200 g) in 2013 to only one in 2018 (Supporting Information, Table S1) suggests that the population may have undergone a dramatic decline.
It is likely that the pervasive increase in turbidity has affected fish condition in multiple ways. Arctic char are visual predators, and a decrease in light can decrease their search efficiency (Shuter et al., 2012). It is also likely that the increase in turbidity in West Lake altered resources and prey availability in the lake, which could explain why stable isotope food web tracers (δ13C and δ15N values) were significant correlates of char Hg concentrations. Both δ13C values (an indicator of basal carbon resource) and δ15N values (an indicator of trophic position) have become more positive since the first MME in West Lake, concomitantly with char Hg, which could represent several scenarios.
An influx of inorganic suspended solids and the resulting increase in turbidity can have profound effects on ecosystem structure and function. The increase in char δ13C values could indicate a shift to a greater reliance on benthic/littoral resources, because it is well established that benthic/littoral primary producers have more positive δ13C values relative to pelagic primary producers (France, 1995; Hecky & Hesslein, 1995; Post, 2002).
Increased turbidity can affect primary production and ultimately the transfer of energy to higher trophic levels (Lloyd, 1987). The presence of inorganic suspended solids attenuates light, and the capacity for pelagic primary production (Allende et al., 2009). This not only limits grazing opportunities for zooplankton, but can also present a physical barrier. There is evidence that in a turbid lake with high concentrations of suspended sediments (fine clays and silts), the feeding rates of Cladocera are disproportionately decreased compared with the more selective and smaller bodied rotifers (Kirk, 1991).
Consequently, it is likely that char do not have access to zooplankton in turbid West Lake and that feeding on emerging chironomids would also be more difficult due to visual impairment or scarcity. Char often rely on larger bodied zooplankton; however, in West Lake they could have been forced to seek out alternative food sources (e.g., benthos). Roberts et al. (2017) found that there was a shift in diatom assemblage in West Lake before and after disturbance. The diatom community composition in 2004 was approximately 15% of the planktonic Cyclotella spp., which comprised almost 0% of the community when resampled in 2014 (Roberts et al., 2017). We have no zooplankton data from before the disturbance in West Lake; however, zooplankton density was assessed in 2019, and density was five times greater in East Lake than in West Lake (S. Arnott, Queen's University, unpublished data).
There is a well-established link between trophic position (δ15N value) and fish Hg (Kidd et al., 2012), so it is unsurprising that there was a significant positive relationship between char δ15N values and Hg in West Lake. The temporal increase in δ15N values could indicate a greater amount of cannibalism within the population, which has been found to lengthen food chains in similar Arctic lakes (Gantner et al., 2010). Svenning and Borgstrøm (2005) found that all Arctic char have the ability to switch to cannibalism depending on environmental conditions (e.g., prey availability, population density, or the ratio conspecific to alternative prey density). Because there was a major increase in turbidity in West Lake and likely a concomitant shift in available prey, it is logical that cannibalism within the population could increase. However, this assertion cannot be made confidently because δ15N baseline values were not collected throughout the study period (i.e., the char δ15N values are not baseline corrected), so it is possible that the disturbance in West Lake could have altered the isotopic baseline, and that the temporal increase in δ15N values (and its relationship with char Hg) is not a product of increasing trophic position. Future research is needed to determine to what extent the observed trend in bulk δ15N values of West Lake char reflect a shifting isotopic baseline or increased cannibalism.
As previously discussed, over the study period, char length- and age-adjusted Hg concentrations were always higher in West Lake than in East Lake. Although we cannot entirely determine whether Hg concentrations in char muscle have always been higher in West Lake, the results clearly show that the disparity between the two lakes grew over time (Figure 2). Furthermore, long-term studies of Hg in landlocked char in coastal lakes of similar size on Cornwallis Island generally show similar length-adjusted concentrations and a general decline in mean concentrations in recent years (Hudelson et al., 2019). Having more data from before the disturbances would have been invaluable in determining the natural variability and gaining a better understanding of how char Hg concentrations in West Lake could have responded under the same climate stressors as East Lake in the absence of permafrost degradation and MMEs.
As climate change continues, we can expect changes to the proximate drivers of char Hg concentration outlined in our study. Rising water temperatures and increases in primary production could lead to a continued increase in growth rates, and a subsequent decrease in char Hg in East Lake; however, there are thermal optima for fish growth that could be exceeded over time (Reist et al., 2006). Similarly, the frequency and intensity of thermokarst activity is predicted to increase (Grosse et al., 2013; Lewkowicz & Way, 2019), which could lead to disturbances similar to those in West Lake occurring in East Lake or other lakes. Atmospheric Hg in the Arctic is declining slowly (MacSween et al., 2022), and declining Hg in Arctic char in Lake Hazen, Nunavut, and possibly in other High Arctic lakes, appears to be paralleling that trend (Hudelson et al., 2019; Hudelson et al., 2023) The complex and inter-related nature of these drivers highlights how difficult it is to predict future changes in Arctic fish Hg concentrations.
Our study represents a unique natural attempt to better understand the drivers of landlocked char Hg concentrations in the High Arctic. We have demonstrated that turbidity along with increased Hg/MeHg in water is related to the divergence in fish Hg concentrations in the two lakes over the span of a decade. Previous studies have indicated how difficult it is to predict Arctic char Hg under a changing climate (Hudelson et al., 2019), and our study is further evidence of this difficulty. In East Lake, a good analog for many small High Arctic lakes, there was a significant decline in char Hg, which can be explained by char age-at size (lifetime growth rate) tracing longer term trends, and previous mean summer air temperature tracing interannual variability. However, in West Lake, there was a significant increase in char Hg following a subaqueous disturbance and a resultant profound increase in turbidity due to food web changes. The proximate mechanisms driving the char Hg changes in both East and West Lakes were each influenced by the ultimate driver of climate change.
Supporting Information
The Supporting Information is available on the Wiley Online Library at https://doi.org/10.1002/etc.5744.
Acknowledgments
Funding for our research was provided by the Natural Sciences and Engineering Research Council of Canada, ArcticNet, the Canadian International Polar Year, and Environment and Climate Change Canada (ECCC). Logistical support and air transport to Cape Bounty each year was provided by the Polar Continental Shelf Program of Natural Resources Canada. We also thank A. Gleason and G. Lawson (ECCC, Burlington, ON, Canada) for their laboratory assistance and A. Sett (ECCC, Burlington) for field assistance.
Author Contributions Statement
Samantha Burke: Formal analysis; Writing—original draft. Derek C. G. Muir: Conceptualization; Data curation; Funding acquisition; Methodology; Supervision; Writing—review & editing. Jane Kirk: Data curation; Methodology; Validation; Writing—review & editing. Benjamin Barst: Data curation; Investigation; Writing—review & editing. Debbie Iqaluk: Investigation; Methodology; Resources. Xiaowa Wang: Data curation; Methodology; Validation. Mike Pope: Writing—review & editing. Scott Lamoureux: Funding acquisition; Investigation; Methodology; Project administration; Supervision; Writing—review & editing. Melissa Lafreniere: Funding acquisition; Project administration; Supervision; Validation; Writing—review & editing.
Open Research
Data Availability Statement
The data are accessible on request to the corresponding authors ([email protected] and [email protected]) and are also uploaded to the Environment Canada data portal (https://search.open.canada.ca/opendata/?page=1&sort=metadata_modified+desc&subject_en=Science+and+Technology&_ga=2.255985288.2043822430.1685563220-262210633.1653861615&owner_org=ec).




