Challenges and Recommendations in Assessing Potential Endocrine-Disrupting Properties of Metals in Aquatic Organisms
Abstract
New tools and refined frameworks for identifying and regulating endocrine-disrupting chemicals (EDCs) are being developed as our scientific understanding of how they work advances. Although focus has largely been on organic chemicals, the potential for metals to act as EDCs in aquatic systems is receiving increasing attention. Metal interactions with the endocrine system are complicated because some metals are essential to physiological systems, including the endocrine system, and nonessential metals can have similar physiochemical attributes that allow substitution into or interference with these systems. Consequently, elevated metal exposure could potentially cause endocrine disruption (ED) but can also cause indirect effects on the endocrine system via multiple pathways or elicit physiologically appropriate compensatory endocrine-mediated responses (endocrine modulation). These latter two effects can be confused with, but are clearly not, ED. In the present study, we provide several case studies that exemplify the challenges encountered in evaluating the endocrine-disrupting (ED) potential of metals, followed by recommendations on how to meet them. Given that metals have multiple modes of action (MOAs), we recommend that assessments use metal-specific adverse outcome pathway networks to ensure that accurate causal links are made between MOAs and effects on the endocrine system. We recommend more focus on establishing molecular initiating events for chronic metal toxicity because these are poorly understood and would reduce uncertainty regarding the potential for metals to be EDCs. Finally, more generalized MOAs such as oxidative stress could be involved in metal interactions with the endocrine system, and we suggest it may be experimentally efficient to evaluate these MOAs when ED is inferred. These experiments, however, must provide explicit linkage to the ED endpoints of interest. Environ Toxicol Chem 2023;42:2564–2579. © 2023 The Authors. Environmental Toxicology and Chemistry published by Wiley Periodicals LLC on behalf of SETAC.
INTRODUCTION
Metals, both essential and nonessential, occur in the environment at varying concentrations as a function of differences in crustal abundance and various biogeochemical processes that influence metal fate and transport. Anthropogenic activities can mobilize metals in the environment, leading to concentrations that exceed natural background concentrations and, when sufficiently high, can cause effects on aquatic organisms through a diverse array of different modes of action (MOAs). One MOA with increasing regulatory attention is possible perturbation of endocrine function.
The endocrine system forms a fundamental part of the communication network through which cells, tissues, and organs are connected and controlled to optimize and maintain organism function. As such, chemicals that interfere with endocrine function can have wide and pervasive impacts. Endocrine-disrupting chemicals (EDCs) are defined as exogenous substances or mixtures that alter the function(s) of the endocrine system and consequently cause adverse health effects in organisms, their progeny, and/or (sub)populations (World Health Organization [WHO], 2002). The global concern over EDCs has resulted in extensive scientific research and the development of test guidelines and regulations for environmental protection (European Chemicals Agency & European Food Safety Authority [ECHA & EFSA], 2018; European Commission, 2017; Organisation for Economic Co-operation and Development [OECD], 2018; US Environmental Protection Agency [USEPA], 2022). Recent regulatory actions include the adoption of criteria for identifying and classifying EDCs under European Union legislation (Biocidal Products Regulation; Plant Protection Products Regulation; Regulation on Classification, Labelling and Packaging of Substances and Mixtures [CLP]). There are also ongoing discussions to potentially develop new hazard classes and criteria for EDCs in the United Nations Globally Harmonized System of Classification and Labelling of Chemicals.
In Europe, EDCs are evaluated outside of the environmental risk-assessment paradigm, where risks to the environment are characterized by integrating information on exposure and effects. Instead, exposure is largely ignored, the evaluation is based almost entirely on effects (i.e., hazard assessment), and EDCs may in turn be subject to use limitations or even bans from use. Given these potentially far-reaching consequences, it is important that the identification procedure be scientifically robust. Substances not classified as EDCs will still be assessed through other frameworks to ensure that use is regulated below concentrations that pose risk to aquatic life.
According to the identification criteria, which are based on the WHO/International Programme on Chemical Safety definition but adapted for regulation, a substance will be considered as having endocrine-disrupting (ED) properties in the environment if it meets all three of the following criteria. First, it shows an adverse effect on nontarget organisms such as a change in morphology, physiology, growth, development, reproduction, or life span. Second, it has an endocrine MOA (defined as endocrine activity [ECHA & EFSA, 2018]); that is, it alters the function(s) of the endocrine system. Third, the adverse effect is a direct consequence of the endocrine MOA such that there is a biologically plausible link between the adverse effect and the endocrine activity. The second and third criteria, linking an endocrine MOA to an adverse effect, are what really define the European Union regulatory process for ED assessment and are the focus of the present study. In reality, the plausible link between a relevant adverse effect and an endocrine MOA is typically not a simple pathway but a network of possibilities. Of these possibilities, the most plausible pathway resulting in the observed adverse effect needs to be identified based on a weight-of-evidence approach, to avoid potential misclassification of a substance.
Test guidelines and regulation currently focus on the estrogen, androgen, thyroid, and steroidogenic (EATS) modalities in mammals and nonmammalian vertebrates that are reasonably well understood and highly conserved in vertebrates (Norris & Carr, 2020). However, there is increasing research to better characterize non-EATS modalities and the endocrine systems of both vertebrates and invertebrates, and these are likely to receive regulatory attention in the future (Crane et al., 2022; Martyniuk et al., 2022).
The WHO's definition of EDCs implies that the observed adverse effect derives from a direct interaction with the endocrine system that alters hormone synthesis, metabolism, excretion, or interaction with target receptors. Substances with other MOAs that may indirectly elicit changes in the function of the endocrine system are not considered endocrine disruptors. Indeed, the European Union CLP regulation considers that adverse effects that are solely nonspecific secondary consequences of other toxic effects should not be considered for the identification of a substance as an endocrine disruptor, and similar provisions are included in other European Union legislation. Therefore, a critical challenge for regulators is to differentiate between EDCs and those substances with other MOAs that may indirectly influence the endocrine system. A recent example of this challenge was highlighted for carbon disulfide where, despite the availability of a large number of studies, it is unclear whether observed effects on mammalian systems are the result of direct endocrine disruption (ED) or indirect effects leading to a change in endocrine status (Printemps et al., 2022).
The regulatory requirements for EDCs apply to all chemical substances, including metals and inorganic substances. While some of the concepts, challenges, and assessment tools discussed in the present study will also be applicable to organic substances, metals possess several properties which arguably set them apart from organic substances including that some metals are essential for a range of normal physiological functions, including multiple axes of the endocrine system, which may complicate interpretation of observed effects (Stevenson et al., 2019; Wood, 2012). Metals also often have diverse MOAs including oxidative stress, disruption of ionoregulatory balances, inhibition of respiration, effects on bioenergetics, and many others (Brix et al., 2017, 2022; Wood, 2012). These mechanisms can trigger a cascade of physiological effects and compensatory responses in a variety of tissues depending on exposure and distribution pathways that may involve changes to the endocrine system. The diversity of MOAs and responses makes it challenging to attribute definitive cause and effect, which is a necessity for the identification of EDCs from a regulatory perspective. Clearly, there is a pressing need to improve our understanding of how regulatory criteria for EDCs can be applied to metals in a meaningful way.
The objective of the present study is to highlight key issues associated with ED assessment of metals and how they might pragmatically be addressed to advance our understanding on the need for regulatory consideration. We begin by discussing the essential role of metals in endocrine systems and how this may complicate data interpretation when evaluating potential ED properties of metals. We then illustrate examples of different types of metal interactions with the endocrine system to highlight some of the key challenges associated with assessing metals as potential EDCs. Cadmium is used most frequently in our illustrative examples because of the large number of studies that have investigated its interactions with the endocrine system, allowing us to provide relatively complete examples of the challenges in ED assessment for metals. The reader, however, should not infer from this the likelihood that Cd, or any other metal not mentioned in the present study, is or is not an EDC. In the context of our analysis on metals, we set out clear definitions of the terms of ED versus endocrine modulation and effects that may arise indirectly through other mechanisms. Finally, we discuss potential paths forward and tools that could be used or further developed for application in metal ED assessments.
CONCEPTUAL MODEL OF METAL INTERACTIONS WITH THE ENDOCRINE SYSTEM
As is the case with organic compounds, conceptually, metals could interact throughout the life cycle of a hormone from the point of their gene transcription through to influencing hormone binding to a receptor or the function of that receptor. Such interactions will be related to the bioavailable exposure concentration and distribution of metal within the body including interactions with various metal homeostatic mechanisms. However, given some of the differences in physical chemistry (e.g., relating to size, charge, binding affinity, coordination chemistry), interactions of metals and organic compounds with the endocrine system are likely to be different in many cases. An important difference compared with organic compounds is that some metals are essential to the function of many biochemical pathways in organisms, including multiple axes of the endocrine system (Stevenson et al., 2019). Essential metals are directly involved in the normal function of a wide range of hormones including those involved with ion homeostasis, metabolic regulation, reproduction, growth, inflammation, and healing (Stevenson et al., 2019). Most studies to date (both in vivo and in vitro) on the essential role of metals in the endocrine system are related to direct binding of metals to peptide hormones. Iron, copper, and zinc have been shown to play an essential role in the stability and/or function of a variety of peptide hormones, and other metals with similar charge and ionic radius have also been demonstrated to bind to these hormones (Table 1). However, there are also examples of essential metals involved in direct metal binding to amino acid–derived hormones, hormone transport proteins that are important in the trafficking of steroid hormones, and hormone receptors (Hammond et al., 2003; Yamauchi, 2021; Table 1).
| Hormone/receptor | Origin | Target | Biological role | Interaction with metalsa | References |
|---|---|---|---|---|---|
| Amylin | Pancreas | Whole organism | Glucose metabolism | Cu, Ni, Zn | David et al. (2018) |
| Androgen receptor | Gonads | Gonads | Reproduction, sex differentiation | Cd | Martin et al. (2002) |
| Bradykinin | Whole organism | Whole organism | Vasodilation, inflammation | Ag, Co, Cs, Cu, Ni, Zn | Cerda et al. (1999), Naletova et al. (2016) |
| Estrogen receptor | Whole body | Whole body | Reproduction, sexual differentiation, development | As, Cd, Zn | Kitchin and Wallace (2005), Low et al. (2002), Nesatyy et al. (2006), Stoica et al. (2000) |
| Gastrin | Stomach | Stomach | Gastric acid regulation, Fe homeostasis | Fe, Ga, In, Ru, Zn | Baldwin et al. (2015), Pannequin et al. (2002), Xiao et al. ((2014) |
| GHK | Collagen | Whole organism | Cu and Fe homeostasis, wound healing | Cu | Pickart et al. (1980) |
| Glucagon | Pancreas | Whole organism | Glucose metabolism | Zn | Solomou et al. (2015) |
| Glucocorticoid receptor | Whole organism | Whole organism | Transcription | Zn | Low et al. (2002) |
| GnRH | Hypothalamus | Pituitary | Reproduction | Cu | Gajewska et al. (2016), Tran et al. (2019) |
| Growth hormone | Pituitary | Whole organism | Growth, cell production | Zn | Cunningham et al. (1990) |
| Hepcidin | Liver, adipose tissue | Whole organism | Fe regulation | Cd, Fe, Zn | Balesaria et al. (2010), Loreal et al. (2014) |
| Insulin | Pancreas | Whole organism | Energy metabolism, growth, healing | Cd, Co, Zn | Adams et al. (1969), Brader et al. (1997), Coffman and Dunn (1988) |
| Isotocin | Hypothalamus | Brain, pituitary, liver, kidney | Hormone regulation, behavior | Co, Cu, Mn, Ni, Zn | D. Liu et al. (2005), O'Sullivan et al. (2023) |
| Prolactin | Pituitary | Gill | Iono- and osmoregulation | Cu, Zn | Vang et al. (2022) |
| Sex hormone binding globulin | Liver | Whole organism | Hormone transport | Zn | Avvakumov et al. (2000), Hammond et al. (2003) |
| Transthyretin | Liver | Whole organism | Thyroid hormone transport | Cu, Zn | Suzuki et al. (2017), Yamauchi (2021) |
- a Metals in bold have a demonstrated essential role in hormone, hormone release, hormone binding protein, or hormone receptor stabilization and/or function. Other metals listed have been demonstrated to bind to the hormone, hormone binding protein, or hormone receptor.
- This table was adapted from Stevenson et al. (2019), which focused on the role of essential metals interacting with hormones. The table was expanded to include nonessential metals, hormone receptors, and hormone binding proteins.
- GHK = glycyl-L-histidyl-L-lysine; GnRH = gonadotropin releasing hormone.
Given the direct involvement of essential metals in endocrine system function, complications in the interpretation of data used in the ED assessment of metals may occur and indeed, we argue, are more likely to occur than for organic chemicals. Importantly, although the essential nature of some metals provides the primary evolutionary driver for metal interactions with the endocrine system and biological systems in general, similarities in physiochemical properties between essential and nonessential metals means these complications in data interpretation will be encountered for both groups of metals. Further, both other essential and nonessential metals can influence the homeostasis of essential metals, potentially further complicating metal interactions with the endocrine system (Bury et al., 2003). The essential role of some metals in the endocrine system also means that specific binding domains have evolved to support these interactions, potentially providing an opportunity for other essential or nonessential metals with similar ionic radius and charge to also interact in a potentially adverse manner with the endocrine system if they are present at elevated concentrations.
A further important consideration for all metals, because of the essentiality and redox properties of some of them, is that they interact not only with the endocrine system but with many other physiological systems. Consequently, observed effects may potentially result from direct interactions with the endocrine system; indirect interactions with the endocrine system resulting from, for example, damage to tissues involved in hormone synthesis or endocrine-mediated processes; or modulation of the endocrine system induced by metal effects on other physiological systems. The different organizational levels at which these interactions may occur complicate interpretation and create challenges in distinguishing indirect from direct effects.
Considering the different mechanisms by which metals may elicit a change in the endocrine system, we initially develop a high-level conceptual model (Figure 1) and several definitions to help distinguish the different types of interactions (Textbox 1). First, indirect effects are the result of metal effects on physiological systems that do not involve metals directly interacting at the macromolecular level with the processes of hormone synthesis, transport, metabolism, or binding to hormone receptors but rather indirectly affect these processes through interactions with other cellular or tissue processes. These effects may or may not lead to an adverse outcome. Endocrine modulation occurs when metals affect a physiological system, and this disturbance elicits a compensatory endocrine response appropriate for the physiological disturbance which aims to return the animal to homeostasis. In some circumstances, the response may lead to a different adverse outcome as a consequence of that modulatory process affecting another pathway function. We define endocrine disruption as direct interaction at the macromolecular level with the endocrine system through metal binding to DNA, RNA, peptides, or proteins that results in altered hormone synthesis, transport, metabolism, or binding to hormone receptors, leading to an adverse outcome. This definition may be slightly more explicit about the need for a direct interaction between the metal and the endocrine system than that inferred by the WHO (2002), but we believe this is a critical distinction in the case for metals as potential EDCs relative to their indirect effects.
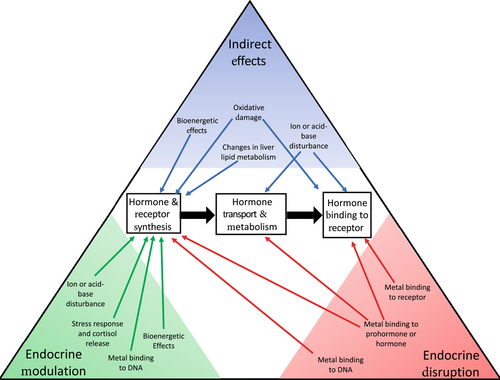
TEXTBOX 1. Potential effects of metals on the endocrine system
Indirect effects—Metal effects on physiological systems that do not involve metals interacting directly at the macromolecular level with the processes of hormone synthesis, transport, metabolism, or binding to hormone receptors but rather that indirectly affect these processes through interactions with other cellular or tissue processes. These effects may or may not lead to an adverse outcome.
Endocrine modulation—Metal effects on physiological systems that induce a compensatory endocrine response appropriate for the physiological disturbance which aims to return the animal to homeostasis but which, in some cases, can lead to a different adverse effect through interactions with other physiological pathways.
Endocrine disruption—Direct interaction of metals with the endocrine system through binding to DNA, RNA, proteins, or peptides that results in altered hormone synthesis, transport, metabolism, or binding to hormone receptors, leading to an adverse outcome.
Our conceptual model (Figure 1) illustrates potential interactions between metals and the endocrine system but is by no means a comprehensive inventory. Similarly, our objective is not to provide a comprehensive review of all metal interactions with the endocrine system or to comprehensively assess the potential of a particular metal to act as an EDC. Rather, we illustrate the types of interactions that can occur with the aim of developing a more generalized framework for how we might most accurately assess whether a metal causes ED.
Much of what is known about the role of metals in endocrine systems is based on mammalian studies and generally within the context of human health. However, the vertebrate endocrine system is highly conserved, and information from mammalian studies is likely to be generally applicable to other vertebrates—birds, reptiles, amphibians, and fish. This has also been shown for organic chemicals that generally cause ED effects across diverse vertebrate phyla (Ankley et al., 2009; Norris & Carr, 2020). In this evaluation, we include some studies, particularly in vitro studies, on taxa other than fish and amphibians (including humans) to provide additional weight of evidence to support data on fish or amphibians. This should not be used to infer conclusions about the ED potential of metals to these other classes of organisms because we have not considered how these studies fit into the broader context of their endocrine systems or their environmental exposure to metals.
The endocrine system of invertebrates differs substantially from that of vertebrates and across different invertebrate phyla. Furthermore, knowledge of invertebrate endocrinology and how it is affected by EDCs is largely confined to insects, crustaceans, and mollusks (Crane et al., 2022), and even studies on these phyla are limited to only a very few species. Our understanding of metal interactions with the endocrine systems of invertebrates is much more limited and an important area of uncertainty but is not an issue unique to metals and has been recognized as a limitation in the ED assessment of chemicals more generally (Crane et al., 2022; Hecker & Hollert, 2011). However, one characteristic of most metals is that some invertebrate taxa are more sensitive to metal exposure in aquatic systems than fish and amphibians. Consequently, environmental quality standards for metals are typically a factor of 3 or more lower than effect concentrations associated with sensitive fish species. In the present study, we constrain our assessment of the literature to studies conducted at concentrations associated with apical endpoints (e.g., growth, development, reproduction) in fish and amphibians. Although ED assessments in the European Union are typically hazard-based rather than risk-based, we think it is important to point out that for metals, ED studies are typically conducted at concentrations that exceed environmental quality standards by at least a factor of 3 and sometimes by an order of magnitude. We also recognize that this issue is not unique to metals.
INDIRECT EFFECTS OF METALS ON THE ENDOCRINE SYSTEM
Metals can cause toxicity via a wide range of mechanisms, and some of these effects have the potential to indirectly affect the endocrine system. Indirect effects are the result of metal effects on physiological systems that do not involve metals directly interacting at the macromolecular level with the processes of hormone synthesis, transport, metabolism, or binding to hormone receptors but rather indirectly affecting these processes through interactions with other cellular or tissue processes. In some cases, indirect effects may result from cellular/tissue damage or disruption at the organ or system level of biological organization. For example, we recently described a series of studies that inferred that Cu was inhibiting reproduction in fish via ED (Brix et al., 2022). Evidence supporting this conclusion involved down-regulated gene expression of multiple hormones (e.g., follicle-stimulating hormone beta and luteinizing hormone beta) in the hypothalamic–pituitary–gonadal (HPG) axis. However, these studies also observed oxidative stress and corresponding histopathological lesions in the gonads that suggest direct oxidative damage to endocrine tissues. Overall, the available weight of evidence in that analysis more strongly supports oxidative stress as the MOA for observed effects on the HPG axis. Many other metals (e.g., Cd, Co, Cr, Fe, Ni, Pb, V), if present at supraphyisiological concentrations, may also induce oxidative stress in a wide range of tissues, providing the potential for indirect effects on endocrine tissues (Frias-Espericueta et al., 2022; Lushchak, 2016; Sevcikova et al., 2011).
In addition to oxidative stress, there are multiple other MOAs for metals that could also lead to indirect effects on the endocrine system. Copper, for example, is known to disturb organism acid-base balance (Wang et al., 1998), and hormone binding to receptors is sometimes pH-dependent (Liu & Patino, 1993). Multiple metals (Cd, Cu, Pb, Zn) are known to affect liver lipid metabolism (Ferain et al., 2021; Huang et al., 2014), and these changes have the potential to impact steroid synthesis in the liver and ovary (Leng et al., 2019). Similarly, multiple metals (Cd, Cu, Ni, Pb) are known to cause bioenergetic effects via multiple pathways that could impact organism growth and reproduction, including associated hormones that regulate these processes (De Boeck et al., 1997). Again, this is not a comprehensive list, and we refer readers to other summaries that provide more detailed reviews on the mechanisms of metal toxicity for effects that may potentially indirectly impact the endocrine system (Wood et al., 2012).
METALS AS ENDOCRINE MODULATORS
Endocrine modulation is the result of metal effects on a physiological system that elicit a compensatory endocrine response appropriate for the physiological disturbance which aims to return the animal to homeostasis (Textbox 1). This differs from indirect effects, which do not elicit an endocrine response but affect the endocrine system via other MOAs. Although endocrine modulation is a compensatory response that is physiologically appropriate for a particular system, it can in some cases still lead to an adverse effect. This occurs when the compensatory response ameliorates a potentially adverse event but because of the interactions between physiological pathways triggers a different, typically less, adverse outcome for the organism. The following examples elaborate on these concepts.
Probably the most ubiquitous example of endocrine modulation in response to metal exposure is the release of cortisol as a general stress response. Many metals elicit a cortisol response in fish (Wood et al., 2012), and cortisol subsequently initiates a variety of compensatory physiological responses in coordination with other hormones including increased gluconeogenesis to provide energy (Mommsen et al., 1999), induction of metallothioneins for metal detoxification (Bury et al., 2008), and changes in ionoregulatory physiology (Mommsen et al., 1999). However, sustained release of cortisol can lead to adverse effects including immunosuppression (Pickering & Pottinger, 1989), reduced growth (De Boeck et al., 2001; Mommsen et al., 1999), and impacts on reproductive steroidogenesis (Carragher & Sumpter, 1990; Carragher et al., 1989).
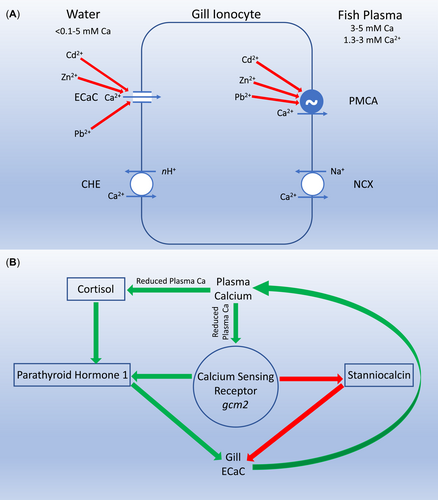
Another, somewhat less direct example involves the response of fish to elevated concentrations of Cd, Pb, or Zn (Figure 2). These metals directly interfere with Ca homeostasis by both competitive interactions at the apical epithelial Ca channel (ECaC/Trpv6) and noncompetitive inhibition at the basolateral plasma membrane Ca-adenosine triphosphatase (Hogstrand et al., 1994; Niyogi & Wood, 2004; Rogers & Wood, 2004). The net effect of these interactions is a marked reduction in Ca uptake at the fish gill and reduction in plasma Ca concentrations (Hogstrand et al., 1995; Rogers et al., 2003). The endocrine system tightly regulates plasma Ca in fish, with parathyroid hormone and cortisol stimulating uptake by increasing ECaC expression (Guerreiro et al., 2007; Lin et al., 2011), while stanniocalcin reduces ECaC expression and corresponding Ca uptake (Tseng et al., 2009). Consequently, when Cd, Pb, or Zn inhibits Ca uptake at the gill (Figure 2A), the corresponding reduction in plasma Ca increases circulating plasma cortisol, which in concert with the Ca sensing receptor increases parathyroid hormone and decreases stanniocalcin. This results in a compensatory increase in ECaC to increase Ca uptake and return plasma Ca to optimal concentrations (Kumai et al., 2015; Kwong et al., 2014; Lin et al., 2014; Figure 2B). In this case, exposure to elevated concentrations of a divalent metal has modulated the endocrine status of the organism, but the endocrine response is compensatory in nature and serves to maintain a normal physiological balance. Therefore, this is clearly not an example of ED.
While the above example is relatively simple and obvious, other examples are not as easily understood due to the complexity of the physiological system and/or challenges in fully characterizing the physiological pathways involved in the effect. In such cases, it may be unclear whether the observed effects should be considered endocrine modulation or ED. To illustrate this, we now consider metal interactions with hepcidin. Hepcidin is a peptide hormone with the iron regulatory isoform produced primarily in the liver (Neves et al., 2017). Hepcidin is the master regulator of Fe metabolism in organisms (Collins et al., 2008; Ganz, 2011), and this is accomplished through multiple pathways but most importantly via the degradation of ferroportin and divalent metal transporter-1 (DMT-1). Ferroportin is responsible for the export of Fe from cells including basolateral membrane of enterocytes in the intestine involved in dietary Fe absorption and macrophages that break down Fe-rich red blood cells (Pan et al., 2020). Studies have also shown that ferroportin can export other metals including Co and Zn but not Cu, Cd, or Mn (Loreal et al., 2014). Divalent metal transporter-1 is involved in Fe uptake at the apical membrane of intestinal enterocytes and can also be found in the apical membrane of ionocytes in the fish gill. While DMT-1 is primarily an Fe2+/H+ exchanger, it has low substrate specificity and can also transport Cd, Co, Cu, Mn, Ni, Pb, and Zn (Cooper & Bury, 2007; Loreal et al., 2014).
Regulation of the hormone hepcidin and consequently Fe is complicated, with multiple feedback loops (Ganz & Nemeth, 2012). Important for this example, hepcidin has an amino-terminal Cu–Ni binding motif, and the hepcidin gene promoter region has three metal responsive elements (MREs). The amino-terminal binding motif is unlikely to be actively involved in regulating hepcidin activity under normal metal homeostatic conditions (Kulprachakarn et al., 2016) but has not been studied under conditions of Cu and/or Ni overload as would occur during exposure to elevated concentrations of these metals. In contrast, the MREs in the hepcidin promoter region are sensitive to Cd, Cu, Fe, and Zn working in conjunction with metal-responsive transcription factor 1 (Balesaria et al., 2010; Loreal et al., 2014). Consequently, when excess concentrations of these metals are present, hepcidin acts as a metal homeostatic regulator by down-regulating ferroportin and DMT-1, leading to a reduction in metal uptake (Figure 3). A further consequence of this response is the subsequent decline of tissue Fe concentrations, leading to anemia. This has been demonstrated to occur in both fish and mice in response to elevated Cu and Zn exposure, respectively (Craig et al., 2009).
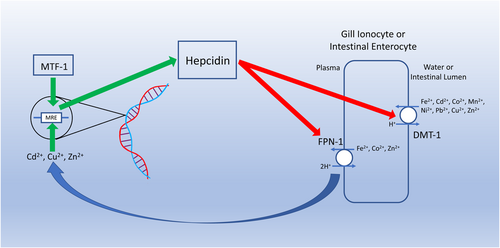
The response of hepcidin to elevated concentrations of Cu or Zn is clearly an example of endocrine modulation, even though it is triggered by metal binding to a gene transcription site and can lead to anemia as a secondary adverse outcome. For these metals, the molecular initiating event (MIE) and subsequent physiological cascade have clearly evolved as part of a system of feedback loops to regulate metal uptake through DMT-1 and ferroportin with the aim of maintaining a homeostatic balance between Fe, Cu, and Zn.
This, however, leaves open the question of Cd, which is a nonessential metal with one unusual exception where it replaces Zn in the active site of carbonic anhydrase in some open-ocean diatoms (Price & Morel, 1990). Cadmium binds to MREs in the hepcidin gene promoter region (Loreal et al., 2014) and, like Cu and Zn, Cd induces anemia in both fish (waterborne exposure) and mice (dietary exposure), with down-regulation of DMT-1 and ferroportin in intestinal enterocytes demonstrated in the latter (Fujiwara et al., 2020; Haux & Larsson, 1984). Cadmium has the same charge and a similar ionic radius to Zn, allowing it to effectively interact, often adversely, with many of the binding domains and proteins in which Zn is involved (Le Faucheur et al., 2021; McGeer et al., 2012). From this perspective, it could be argued that Cd mimicry of Zn elicits an inappropriate up-regulation of hepcidin, leading to an adverse effect in the form of anemia and consequently an example of ED. Alternatively, this could be viewed as an appropriate physiological response by a system that primarily evolved to maintain homeostasis of essential metals but may also have evolved to reduce uptake of some nonessential metals that are encountered in the environment.
EVALUATING THE POTENTIAL FOR METALS TO CAUSE ENDOCRINE DISRUPTION
Having discussed the types of indirect effects metals can have on the endocrine system as well as endocrine modulation by metals, we next consider the potential for metals to cause ED (Textbox 1). Cadmium is perhaps the most studied metal with respect to its potential for disrupting the endocrine system in aquatic organisms. Our objective is not to fully evaluate all the mechanisms and pathways by which Cd exerts toxicity on aquatic organisms or even all of the pathways by which Cd may interact with the endocrine system. Rather, we focus on downstream elements of the HPG axis involved in fish reproduction and use Cd as a case study to demonstrate the type of data and considerations required to evaluate metals as EDCs.
In chronic Cd exposures to fish, reproduction in the form of reduced fecundity has been shown to be a sensitive endpoint (Benoit et al., 1976; Pickering & Gast, 1972; Wang et al., 2014). Cadmium reduces vitellogenin gene expression in the fish liver (for both in vivo and in vitro exposures) as well as the circulating vitellogenin concentration in the plasma, which is essential for oocyte growth (Driessnack et al., 2017; Hwang et al., 2000; Olsson et al., 1995; Vetillard & Bailhache, 2005). This provides a plausible mechanistic link to Cd-induced reductions in fecundity. Transcription of vitellogenin is induced primarily by binding of the estrogen receptor (ERα, ERβ, ERγ) to the vitellogenin promoter (Leanos-Castaneda & Van Der Kraak, 2007; Nelson & Habibi, 2010) but also by binding of 17β-estradiol (E2; Teo et al., 1998). Also, E2 up-regulates the different ER isoforms that variably interact depending on the species to up-regulate each other and themselves (autoregulation; Nelson & Habibi, 2010; Pakdel et al., 1997; Segner et al., 2013). Cadmium exposure in fish reduces ERα, but not ERβ, gene expression in the liver (Driessnack et al., 2017; Vetillard & Bailhache, 2005) at concentrations associated with reduced reproduction. Cadmium exposure has also been shown to reduce plasma E2 concentrations in some studies on some fish species, but not others (Driessnack et al., 2016, 2017; Tilton et al., 2003; Wang et al., 2014).
Several in vitro studies have also demonstrated that Cd binds directly to ERα in rainbow trout (the other ER isoforms have not been studied in this context). Some studies indicate that this occurs in the ligand binding domain, blocking E2 binding by either noncompetitive or competitive inhibition (Nesatyy et al., 2006; Stoica et al., 2000). Other studies suggest that Cd interacts with the DNA binding domain, where it may cause a conformational change in the receptor that prevents E2 binding and influences ER binding to DNA (Le Guevel et al., 2000). If the latter scenario is correct, it could explain not only reduced up-regulation of vitellogenin gene expression but also reduced up-regulation of ER itself.
Cadmium binding to ERα has been experimentally determined using recombinant techniques involving both human and rainbow trout ERα inserted into yeast and several mammalian cell lines. Across these studies, there is some variability in E2–Cd binding dynamics to ERα, with Cd reducing E2 binding at concentrations ranging from approximately 1 to 100 μg L−1 depending on the study (Nesatyy et al., 2006; Silva et al., 2006; Stoica et al., 2000). Differences in Cd binding are likely the result of the specifics of each experimental system but have important implications, not least how exposure concentrations during in vitro experiments with cell lines can be equated to those experienced by fish hepatocytes during in vivo exposure.
Assuming plasma Cd provides the pathway for Cd exposure of hepatocytes, we can consider experimental data on plasma Cd during environmentally realistic in vivo Cd exposures to fish. In rainbow trout exposed to 10 μg L−1 waterborne Cd, Cd concentrations reached 17 μg L−1 in fish plasma at the end of a 3-h exposure (Hollis et al., 1999). A similar approximately 2:1 plasma-to-water ratio was observed in a 30-d exposure in the same species where a 3–μg L−1 Cd exposure resulted in 6 μg L−1 Cd in trout plasma (Chowdhury et al., 2003). Further, in a 30-d exposure to 500 mg kg−1 dry weight dietary Cd, plasma Cd concentrations reached 15–20 μg L−1, demonstrating that the diet can be an important pathway for plasma Cd loading (Chowdhury et al., 2004). Consideration of both waterborne and dietary exposure pathways suggests that plasma Cd concentrations could be within the range where Cd inhibits E2 binding to ERα during in vitro studies, although there is some uncertainty regarding comparability of intracellular and extracellular Cd speciation.
Integrating this information into an adverse outcome pathway (AOP)–type framework provides a useful tool for understanding where there is support for the hypothesis that the mechanism underlying Cd effects on fish fecundity is via ED and, importantly, where uncertainty remains. We adapted a previously developed AOP for ER antagonism (Villeneuve, 2016) for this example with Cd. As shown (Figure 4), while there is clear linkage from reduced vitellogenin transcription through subsequent key events (KEs) to apical and population endpoints, there remains considerable uncertainty regarding the MIE and intermediate KEs leading to reduced vitellogenesis. If ultimately reduced plasma E2 is driving the response and Cd binding to ER is unimportant, then understanding the MIE for E2 reduction becomes critical. This could be an endocrine-mediated effect or an indirect effect via some other mechanism such as Cd-induced oxidative stress in the ovary. Alternatively, if Cd binding to the ER is an important KE or the MIE, then this would be a clear case of ED.
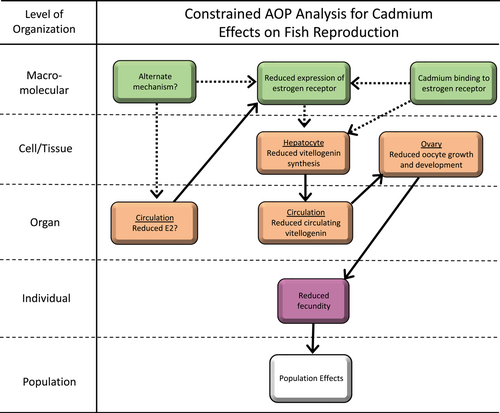
A final uncertainty is that the pathway just described for Cd effects on fish reproduction and the studies supporting this pathway indicate that Cd binding to ER has an antiestrogenic effect, ultimately leading to reduced fish fecundity. Conversely, in some in vitro studies, particularly in human cell lines, Cd binding to ER increases ER activity, suggesting that Cd has an estrogenic, rather than an antiestrogenic, effect. A number of reasons for these contradictory findings have been suggested including differences in experimental cell lines, differences in Cd exposure concentrations or experimental media that might influence Cd bioavailability, and species-specific differences in ER function (Nesatyy et al., 2006; Segner et al., 2013). This has not been well studied in fish, but exposure of juvenile fathead minnows (Pimephales promelas) to 50 μg L−1 Cd during sexual differentiation led to subtle increases in female-to-male ratios, supporting that Cd may have an estrogenic (feminizing) effect at these environmentally unrealistic concentrations (Sellin & Kolok, 2006).
Overall, the evidence for Cd demonstrates the basic uncertainties that are still related to this case and underline the necessity of further focused studies to resolve the uncertainties and conclude what type of interaction of Cd has with the endocrine system.
APPROACHES FOR ASSESSING WHETHER METALS DISRUPT THE ENDOCRINE SYSTEM
The objective of the present study is principally to highlight key challenges associated with ED assessment of metals and to suggest potential paths forward for making these assessments. As demonstrated in the examples provided, the interactions of metals with the endocrine system are complex. This is an evolutionary consequence of essential metals being involved in the normal function of the endocrine system and other physiological systems with which it interacts, as well as the ability of nonessential metals to substitute for essential metals, sometimes with adverse effects.
The existing ED evaluation process in the European Union, and similar processes in other jurisdictions, is aimed at addressing the criteria for determining ED by assembling all relevant and reliable evidence into lines of evidence for both adversity and endocrine activity. These data are grouped according to whether they are in vitro mechanistic information on endocrine-linked activity such as binding to activating receptors or interfering with hormone production, in vivo mechanistic information such as changes in hormone concentrations that interfere with the EATS modalities which informs adversity, and any additional data which are considered sensitive to but not necessarily diagnostic of EATS. The resulting lines of evidence are then used to postulate the MOA to establish if there is a biologically plausible link between the observed adverse effects and endocrine activity. These endocrine activities and adverse effects can be organized into an AOP network.
Clearly, studies to date have been insufficient to fully evaluate the ED potential of metals, so more experiments using methodological approaches similar to previous studies are perhaps unlikely to help resolve this issue. Consistent with suggestions by others regarding EDC assessment for organic chemicals and recent efforts on metals, the development of AOP networks would provide a useful framework for understanding where data are lacking to complete a robust ED assessment for a specific metal (Brix et al., 2022; Browne et al., 2017; Knapen et al., 2020). Unlike the typical AOP that is centered around a particular pathway (i.e., toxicant-independent), we think developing AOP networks centered on a particular metal would be more useful. This is particularly necessary for metals because the interplay between a wide range of MOAs precludes evaluating specific pathways in isolation. Consequently, evaluating a potential ED pathway in isolation of other potential MOA risks incorrectly assigning causality to a particular MOA.
Despite a relatively robust and detailed understanding of the mechanisms underlying acute metal toxicity, our understanding of the mechanisms driving chronic metal toxicity to aquatic organisms is surprisingly limited given the number of studies on this subject (Wood, 2012). Adverse outcome pathway networks for Cu and Ni (Brix et al., 2017, 2022) and detailed reviews of other metals for which AOP networks have not been developed (Wood et al., 2012) suggest a common pattern in our understanding of the mechanisms of chronic metal toxicity to aquatic organisms within the context of interactions with the endocrine system. First, the chronic effects of metals on apical endpoints like survival, development, growth, and reproduction are generally well characterized for many metals. The KEs driving these effects at the tissue and organ levels of biological organization are also often well described. However, at lower levels of biological organization (e.g., cellular), KEs are often limited to changes in gene expression (of uncertain biological significance) and sometimes plasma hormone concentrations to infer ED. In most cases, MIEs are not evaluated; and in some cases, no hypothetical MIE is even postulated.
Given these general deficiencies in our understanding of metal MIEs and initial KEs, we suggest that a high research priority in metal ED assessment is evaluation of hypothesized MIEs where ED has been inferred based on observed changes in a KE. Conceptually, there are two ways to approach this issue, direct investigation of MIE(s) for ED or investigation of other AOPs (i.e., indirect effects or endocrine modulation) and associated MIEs that could also explain observations that infer ED. Our recently developed AOP network for Cu evaluating studies inferring ED of fish reproduction and amphibian metamorphosis indicated that indirect effects were more likely causing these observations but was not conclusive (Brix et al., 2022). Subsequently, Fort et al. (2023) have provided further weight of evidence supporting indirect effects (oxidative stress) as the pathway for effects on amphibians.
These analyses and additional studies increase the weight of evidence that the effects of Cu on these endpoints do not involve direct ED MOAs. However, it is unclear whether an approach that focuses on nonendocrine MOAs provides sufficient weight of evidence for indirect effects when a change in the endocrine system has been measured. We suggest, consistent with an AOP network perspective, that scientifically the most robust approach is to assess all MOAs that are likely contributing to observed disturbances in the endocrine system and to integrate, and weight, the evidence in a balanced manner. However, the problem in the case of metals, though by no means unique to metals, is determining which MOA(s) to prioritize. In many studies that have inferred the potential for metal-induced ED, the KE that led to this inference is often at a sufficiently high level of biological organization (e.g., a reduction in E2 gene expression) that there are numerous potential direct, modulating, and indirect MIEs that could elicit that observation. In other words, the observed evidence is consistent with multiple AOPs, and there is limited weight of evidence for selecting one as the primary causal pathway. In these scenarios, potential MOAs like oxidative stress that may elicit effects on multiple AOPs within a network (and could explain observed effects) may be reasonable to initially evaluate. While this approach will not conclusively eliminate a potential direct endocrine MIE, depending on results, it may shift the weight of evidence toward indirect pathways or alternatively eliminate multiple indirect pathways as likely explanations for observed effects.
Critical to this approach is to design experiments in a way that tests as directly as possible whether a hypothesized MOA is causally linked to observations associated with the endocrine system. Continuing with the example where a reduction in E2 gene expression was observed, if we hypothesize that oxidative stress (indirect effect) is the MOA, then simply measuring oxidative stress and associated histopathological effects in the gonads provides relatively weak weight of evidence for this MOA. Indeed, if these were the only parameters measured, the experiment would have the same design flaw we criticized earlier for considering only one MOA at a relatively high level of biological organization. Linking observations on oxidative stress to reductions in E2 gene expression as a function of both time and exposure concentration would substantially increase the weight of evidence. Inducing a similar level of oxidative stress in the gonads with another chemical that also results in a reduction in E2 gene expression (i.e., positive control) would also provide further weight of evidence for this MOA, and there may be other approaches to strengthen the weight of evidence. Our point is that exploring MOAs that may broadly affect multiple MIEs that could explain an observed disturbance in the endocrine system may be an experimentally efficient study approach, but it is critical that these experiments provide explicit linkages to the observed endocrine disturbance that prompted the experiment. Failure to make these linkages may identify a valid alternative MOA but does not reduce uncertainty about the identity of the actual MOA.
Parallel to this perspective, it is important to recognize that some regulatory test methods such as the amphibian metamorphosis assay and the larval amphibian growth and development assay, which are focused on evaluating direct ED MOAs (OECD, 2018), provide little scope for identifying other MOAs that either are not or are only indirectly related to the endocrine system. For metals, and likely other substances, we encourage researchers to consider a broader set of endpoints when nonendocrine MOAs are hypothesized as an alternative explanation for observed effects. Again, the motivation is that generating comparative within-study data on competing hypothesized MOAs is likely to be more powerful than evaluating each MOA in isolation.
Alternatively or in addition to an MIE- or MOA-focused approach, a screening approach could be applied to evaluate whether metals have the potential to directly interact with hormone receptors, although, given the currently available screening assays, this would focus primarily on the EATS modalities, which are not necessarily the primary targets of potential metal ED activity. While many metals have been evaluated using various whole-animal in vivo ED screening tests, the prevalence of indirect metal effects on physiological systems (Figure 1) can potentially lead to misinterpretation of study results (Brix et al., 2022). In contrast, an initial search of the literature suggests that most metals have not been subjected to a robust battery of in vitro ED screening tests that are focused on MIEs and KEs at the cellular level. A concerted effort to subject metals to a battery of in vitro tests could provide important baseline information on the likelihood that metals are exerting effects through ED and help narrow the scope of future assessments of endocrine MIEs. Of course, this would require careful consideration of the applicability of individual tests which have generally been developed with the intent of screening organics rather than metals.
Many of the in vitro assays focus on specific hormone–hormone receptor interactions, often in systems isolated from the rest of the organism physiology, which presents both advantages and disadvantages with respect to evaluating ED. Beyond the focus on much needed research regarding MIEs and KEs at the cellular level, isolation from all or most of the organism physiology has the advantage of eliminating metals causing indirect effects via toxicity to other physiological systems. The primary disadvantage of this approach is the potential lack of physiological realism with respect to metal exposure. Because metals are naturally occurring substances, organisms have evolved an array of transport chaperones and metal binding proteins that tightly control metal bioavailability in both extra- and intracellular compartments (Finney & O'Halloran, 2003). These regulatory factors are dynamic and respond to changes in metal concentrations. As a result, the relationship between metal exposure concentrations inside and outside the organism is critical to the interpretation of in vitro assays for metals. For example, in the case study we presented for Cd, we assumed that total Cd concentrations measured in fish plasma were representative of hepatocyte exposure to Cd. While a conservative assumption, in reality, Cd binds to a variety of plasma proteins (albumin, transferrin, metallothionein, microglobulins), some of which may be available for transport into hepatocytes, while others are not (McGeer et al., 2012). In vitro assays that do not consider inclusion of these proteins in exposure media may significantly overestimate in vivo metal potency.
A third approach that could be useful in assessing ways in which metals interact with the endocrine system is through greater harnessing of in silico techniques. Computational approaches to predicting metal–protein binding are reasonably well developed and continue to advance (Aptekmann et al., 2022; Lin et al., 2006). However, as we discussed in the example with hepcidin, metal binding domains (MBDs) in proteins and MREs in genes have naturally evolved in response to the essential nature of some metals and the need to regulate nonessential metals. Consequently, the presence of MBDs is unlikely to be a particularly useful screening tool for assessing the ED potential of metals. Indeed, the Swiss-Prot (Uniprot Consortium, 2019) database identifies nearly 100 000 protein sequences (~14% of all protein sequences in the database) as having MBDs (Aptekmann et al., 2022).
The situation with metals, where a small number of metals would be screened against a large number of proteins, is effectively the opposite of the situation with organics, where a large number of compounds are being screened against a small number of receptors currently focused on EATS modalities. Consequently, a more informed metal-specific screening approach may be more useful and tractable for metals compared with organics. For example, in the case study presented on Cd interacting with the ER, it has been hypothesized that Cd substitutes for Zn in the Zn fingers of the DNA binding domain of the ER (Le Guevel et al., 2000), inducing conformational changes that reduce the receptor's ability to autoregulate and/or stimulate transcription of vitellogenin. Thus, screening for Zn fingers in proteins involved in other endocrine pathways may be a useful approach for screening potential endocrine activity for Cd. In contrast, this would not be useful for Cu because this metal will not displace Zn from Zn fingers, but Cu-specific interactions with proteins could perhaps be identified and used in screening applications.
We also suggest that further investigation of oxidative damage to endocrine and nonendocrine tissues may be important to understanding the ED potential of some metals. As we reviewed, several metals have relatively high capacities for generating oxidative tissue damage, and this damage may be a relatively common indirect effect on endocrine function for these metals. The challenge is understanding the etiology of oxidative tissue damage relative to apparent ED. New high-resolution techniques combining transgenic fish with advanced imaging methods that enable measurement of organ-specific oxidative stress in live animals may be a promising path forward in this area (Mourabit et al., 2019). If experiments could be designed to understand the timing of these events, our understanding of the ED potential for multiple metals affecting multiple endocrine pathways might be significantly improved. However, we also caution that conclusive elimination of ED MOAs may still require study of MIEs associated with a specific endocrine-mediating AOP.
Finally, as we initially pointed out and have carried through in our examples in the present study, the state of the science for assessing ED in vertebrates far exceeds our current capabilities to assess ED for invertebrates. The diversity in endocrine systems across invertebrate phyla and generally more limited understanding of endocrine function in any invertebrate species compared with vertebrates limit our ability to assess ED in these taxa. This has important implications for assessing metals given that, for most metals, invertebrates are more sensitive than fish and amphibians, sometimes substantially. For example, our case study with Cd focused on fish reproduction, which is typically the most sensitive apical endpoint for this taxa with effect concentrations (e.g., 10% effect concentrations [EC10s]) in the range of 2.5–5 μg L−1 (USEPA, 2016). In contrast, EC10s for sensitive invertebrates under conditions with comparable metal bioavailability are approximately 0.5 μg L−1. Consequently, water quality standards are also typically approximately 0.5 μg L−1 or slightly lower, to ensure protection of sensitive invertebrates. Although current ED assessments in the European Union are hazard-based (i.e., do not consider concentrations in the environment), this does raise an interesting regulatory question. Namely, how informative is it to designate a substance an EDC based on studies at concentrations that, though from a toxicological perspective relevant for the species evaluated, are unlikely to occur in the environment because water quality standards are much lower to ensure protection of more sensitive taxa? This also highlights the importance of prioritizing research on endocrine AOPs for invertebrates (Crane et al., 2022). Specific to metals, daphnids, amphipods, mollusks, and mayflies are frequently among the most sensitive freshwater invertebrate taxa.
CONCLUSIONS
Our objective in the present study was to highlight key issues related to the ED assessment of metals as they come under increasing regulatory scrutiny for these MOAs. Many of the challenges associated with the ED assessment of metals are the same as those faced in assessing organic compounds, so many of the concepts and assessment tools outlined in the present study will also be applicable to organic compounds. However, there are a few key characteristics of metals that create additional challenges. Specifically, physiological systems have evolved to utilize essential metals for a wide array of functions. Consequently, MBDs are ubiquitous across physiological systems, including the endocrine system, to support essential functions. However, this also provides an opportunity for both nonessential and essential metals to affect a wide array of physiological processes when present at elevated concentrations. This ultimately leads to metals having the potential for an arguably higher number of indirect effects and compensatory endocrine modulation responses that may be misinterpreted as ED compared with most organic chemicals.
To address these challenges, we suggest that there is no single approach that is best suited to all scenarios. In general, a weight-of-evidence approach that relies on experiments conducted at multiple levels of biological organization will be needed, as has generally been required in the ED assessment of organics. As has been suggested by others, development of AOP networks is a useful tool for organizing available data and identifying data gaps for research prioritization in metal ED assessment. Our preliminary analysis of available metal AOP networks, broader metal literature reviews, and the examples in the present study all point to experiments on MIEs for hypothesized metal ED scenarios as a research priority. This recommendation stems from the observation that, in many cases, evidence in support of an ED AOP is at a sufficiently high level of biological organization that it is also consistent with one or more alternative AOPs. Additional studies evaluating other MOAs such as oxidative stress, which may contribute to multiple AOPs within a network, may also be an effective approach to reducing uncertainty. However, these experiments should be explicitly linked to evaluating any ED endpoints that may have prompted the experiment.
Finally, we note that there is an increasing number of studies concluding that metals are EDCs based only on changes in gene expression and/or hormone concentrations without identification of MIEs or evaluating a broader AOP network that considers endocrine modulation and indirect effects. While these studies certainly contribute to our understanding of the ED potential of metals, the assignment of cause without identification of an MIE or consideration of other lines of evidence, we argue, is scientifically and regulatorily unproductive. We suggest that a more constructive approach would be for researchers to place their individual studies within a broader AOP network where their contribution can help to advance understanding of a particular MOA and to identify where any uncertainties remain.
Acknowledgments
The authors acknowledge J. Mertens, C. Cooper, D. Fort, D. Boyle, and F. Van Assche for helpful comments on an earlier draft of the manuscript. Financial support for this research was provided by the Metals Environmental Research Associations, consisting of the International Copper Association, the International Zinc Association, the Cobalt Institute, the Nickel Producers Environmental Research Association, the International Lead Association, the International Molybdenum Association, Rio Tinto, the European Precious Metals Federation, and European Aluminium.
Author Contribution Statement
Kevin V. Brix: Conceptualization; Formal analysis; Investigation; Methodology; Visualization; Writing–original draft; Writing–review & editing. Stijn Baken: Funding acquisition; Project administration; Supervision; Writing–original draft; Writing–review & editing. Craig A. Poland, Ronny Blust, Louise J. Pope: Writing–review & editing. Charles R. Tyler: Conceptualization; Writing–original draft; Writing–review & editing.
Open Research
Data Availability Statement
This is a critical review with no novel data and relies only on information in previously published peer-reviewed papers.




