An avian bioassay for environmental estrogens: The growth response of zebra finch (Taeniopygia guttata) chick oviduct to oral estrogens
Abstract
The rat uterotrophic assay is a recommended tier 1 screening assay for environmental estrogens, but no comparable assay exists for altricial birds. We orally dosed zebra finch chicks daily during their linear growth phase (days 5–11) with estradiol benzoate (EB), genistein, methoxychlor, or octylphenol, all dissolved or suspended in canola oil, or canola oil alone, as a vehicle control. On day 12, oviducts were removed, weighed and examined histologically. All doses of EB (0.1–1,000 nmol/g body wt), genistein at 100 nmol/g, and methoxychlor and octylphenol at 1,000 nmol/g, markedly increased oviduct weight, with the highest dose of EB inducing a 60-fold increase over controls. Oviducts were differentiated in a dose-depedent manner to the point of having tubular glands and a pseudostratified, ciliated epithelium at the higher doses of EB. Our earlier results show that EB at 100 and 1,000 nmol/g impairs reproductive performance of zebra finches. Thus, the zebra finch oviduct bioassay measures estrogenicity over a wide dose range and, for EB exposure, can predict impairment in adult reproductive performance. The responsiveness of chick oviducts to estrogen stimulation may serve as a useful marker of estrogen exposure in wild populations of songbirds.
INTRODUCTION
The oviduct has a long and important history as a target organ for the bioassay of estrogen. The role that ovarian hormones play in supporting the oviduct after ovariectomy was demonstrated by Adler in 1911 [1]. The growth response of the oviduct to estrogen treatment was later exploited in adult chickens (Gallus domesticus) as an assay for placental hormone [2], and in chicks as an assay for a variety of synthetic estrogens [3]. The chick oviduct was also extensively used in the 1960s and 1970s as a model for investigating mechanisms of how steroid hormones induce synthesis of albumen proteins [4]. But despite the considerable research literature on oviducts of G. domesticus and the recommended adoption by the Organization for Economic Cooperation and Development of the rat uterotrophic assay as a tier 1 screening assay for estrogenic compounds (task force on endocrine disruptors testing and assessment of the test guidelines program; joint meeting of the Chemical Committee and the Working Party on Chemical, Pesticides and Biotechnology, Paris, France), estrogen-induced oviduct growth has been studied in only a few avian, mostly precocial, species (e.g., Japanese quail [Coturnix japonica] [5], helmeted guineafowl [Numida meleagris] [6], and mallard [Anas platyrhynchos] [7]), and a small number of altricial species (e.g., ringed turtle-dove [Streptopelia risoria] [8], house sparrow [Passer domesticus] [9], European starling [Sturnus vulgaris] [10] and zebra finch [Taenopygia guttata] [11]). Most of these studies have involved only adult birds; little is known about the estrogen sensitivity of oviduct growth in altricial songbird chicks [9], in which estrogen-sensitive sexual differentiation occurs after, as well as before, hatch [12].
The growth response of the female zebra finch song system is an important model for studying posthatch sexual differentiation of the brain [13]. The female zebra finch normally cannot sing. But injecting female zebra finch chicks with estrogen causes partial development of malelike song control nuclei in the brain (i.e., area × of the parolfactory lobe, the robust nucleus of the archistriatum, and the so-called higher vocal control center), and such masculinized females can sing when stimulated with testosterone as adults [14]. However, whether high circulating levels of estradiol are responsible for the development of male-typical song control nuclei in normal differentiation of the male finch is seriously doubted [15].
We recently showed that oral exposure to estrogen also masculinizes female zebra finch chick brain in a doselike fashion [16]. We also found that oral exposure to estrogen severely disrupts adult reproductive performance in sex-specific ways. Males showed reduced fertility as adults and females showed impaired egg production and an increased incidence of broken eggs. The combination of these effects leads to complete reproductive failure when estrogen-treated males and females are mated with each other [17]. Because oral exposure is a natural route of exposure for altricial nestlings fed by parents in the wild, the possibility exists that exposure to environmental estrogens could masculinize female brains and impair reproductive performance.
Although most interest in early estradiol treatment of songbirds has focused on changes in the brain and behavior and only recently on adult reproductive performance, we asked whether an oviduct estrogen growth response similar to that occurring in rats, mice, and G. domesticus also may occur in female zebra finch chicks. If so, the zebra finch could provide a useful model for characterizing the estrogenicity in birds of putative environmental estrogens and their potential ability to alter normal development of brain and behavior and impair adult reproductive performance.
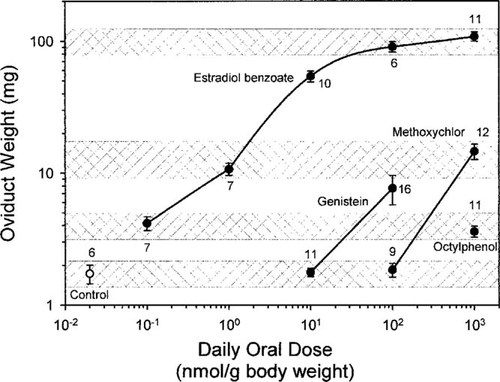
Oviduct weight (log plot; mean ± standard error) of 12-d-old zebra finch chicks dosed daily from days 5 through 11 with canola oil-only control (Control), or estradiol benzoate, methoxychlor, or octylphenol at doses indicated. Number of animals treated is indicated next to each data point. Means within the same cross-hatched area are significantly different from all others except those within the same cross-hatched area (p < 0.01, Fisher's corrected t test, except estradiol benzoate at 1 nmol/g vs estradiol benzoate at 100 nmol/g differ at p < 0.05).
MATERIALS AND METHODS
Zebra finches were produced from the Department of Animal Science's random-bred colony. The colony was founded from local avicultural stock of wild-type phenotype in 1997. Finches were offered Finch Super mixed birdseed (Volkman Seed, Ceres, CA, USA) and water ad libitum. Birds were additionally given ground boiled egg and shell twice per week (∼15 g per breeding pair plus offspring). Water in a shallow (∼4-cm-deep) dish was provided for bathing 1 d per week. Wooden dowels were provided as perches. Photoperiod was 16:8 h light:dark; room temperature was approximately 21°C.
Experimental birds were reared by their parents in individual pair breeding cages (46 × 46 × 46 cm) until sacrifice at 12 d of age. Pairs were induced to breed by providing sheet metal nest boxes (15 × 15 × 13 cm) and shredded burlap for nest construction. Nests were checked daily for the presence of eggs and chicks. Chicks were initially identified by application of food coloring to down feathers on the day of hatch. Number of females in each treatment group is indicated in Figure 1. Chicks were weighed daily and dosed orally, according to body weight, once per day, on days of age 5 through 11 with estradiol benzoate (EB) dissolved in canola oil (or suspended at the higher doses, 100 and 1,000 nmol/g) at 0.1, 1, 10, 100 or 1,000 nmol/g body weight, methoxychlor (Sigma Chemical, St. Louis, MO, USA) at 100 or 1,000 nmol/g, genistein (Sigma) at 10 or 100 nmol/g, or 4-octyl phenol (Aldrich Chemical, Milwaukee, WI, USA) at 1,000 nmol/g. Canola oil alone also was used as a vehicle control. Number (n) of pairs producing chicks used in each dose group were as follows: n = 5 for canola oil controls; n = 5, 5, 9, 3, and 7 for EB at 0.1, 1, 10, 100, and 1,000 nmol/g, respectively; n = 6 and 9 for methoxychlor at 100 and 1,000 nmol/g, respectively; n = and 11 for genistein at 10 and 100 nmol/g, respectively; and n = 6 for octylphenol at 1,000 nmol/g. Twenty-seven of these 32 pairs produced chicks that were used in two or three and, rarely, four different treatment groups. Inspection of the data showed no evidence of family differences in estrogen sensitivity. Substances were delivered by oral gavage at 2 μl/g body weight, with Wiretrol II positive-displacement micropipettes (Drummond Scientific, Broomall, PA, USA). Chicks were killed at 12 d of age by an intraperitoneally injected overdose of sodium pentobarbital. Oviducts were dissected at the juncture with the cloaca, removed, immediately weighed wet, and stored in buffered formalin. Oviduct weights were analyzed with one-way analysis of variance (GB-Stat, Dynamics Microsystems, Silver Springs, MA, USA) and comparisons among treatment group means were tested with the least square difference method. A separate one-way analysis of variance of body weight revealed no significant difference from canolatreated controls (SASr̀, Cary, NC, USA; Tukey's test for multiple comparisons). Significance denotes p < 0.05, unless otherwise stated. A subset of oviducts from treatment groups EB at 0, 0.1, 1, 10, 100 nmol/g, genistein at 100 nmol/g, and methoxychlor at 1,000 nmol/g (n = 3–4 per group) were embedded whole in paraffin wax and step-sectioned. Four-micron sections were stained with hematoxylin and eosin and examined by light microscopy. The magnum from each oviduct was categorized, without prior knowledge of treatment, into one of four categories (A, B, C, or D) in increasing order of mucosal thickness and number of mucosal tubular glands. Only left oviducts were included in the analysis and the histologic examination. Protocols were approved by the University of California-Davis Institutional Animal Use and Care Committee (Davis, CA, USA).
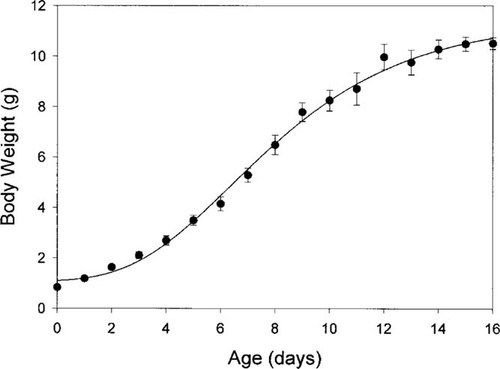
Growth of zebra finch (mean ± standard error; n = 12) fitted with a four-parameter Gompertz curve.
A separate group of 12 untreated zebra finch chicks were weighed daily from hatch until 16 d of age to establish a normal growth curve. A Gompertz four-parameter curve was fitted to these data (SigmaPlot 2000, SPSSr̀, Chicago, IL, USA) and is shown in Figure 2.
RESULTS
Oral dosing with EB produced dose-dependent increases in oviduct mass ranging from approximately 4.2 mg (mean) at the 0.1 nmol/g body weight dose to more 108 mg at the 1,000 nmol/g dose (Fig. 1), a 60-fold induction in mass over controls (1.7 mg). The 100 nmol/g dose of methoxychlor did not induce significant growth, but 1,000 nmol/g did, with average mass of 14.6 mg, a greater than eightfold induction. The 100 nmol/ g dose of genistein induced significant oviduct growth to 7.8 mg, a nearly fivefold induction. The 1,000 nmol/g dose of octylphenol induced a slight but significant oviduct growth to 3.6 mg, an approximately twofold induction. Dosing was done during the linear part of the growth curve, from day of age 5 through 11 (Fig. 2).
| Treatment group and dose (nmol/g body wt) | |||||||
|---|---|---|---|---|---|---|---|
| Canola oil | Estradiol benzoate | Genistein | Methoxychlor | ||||
| Oviduct histology group | 0 | 0.1 | 1 | 10 | 100 | 100 | 1,000 |
| A | 1.2 | 3.0 | 13.0 | ||||
| 1.2 | 3.4 | ||||||
| 1.8 | |||||||
| 2.2 | |||||||
| B | 5.3 | 9.8 | 35.8 | 4.7 | |||
| 12.8 | 42.2 | 5.3 | |||||
| 5.7 | |||||||
| 10.2 | |||||||
| C | 41.2 | 92.0 | 11.4 | ||||
| 12.1 | |||||||
| 15.8 | |||||||
| 17.5 | |||||||
| D | 77.0 | 65.8 | |||||
| 83.1 | |||||||
| 104.0 | |||||||
Two of six finches treated with EB at 100 nmol/g EB and 2 of 12 treated with EB at 1,000 nmol/g had right as well as left oviducts (24.1 ± 2.6 mg; mean ± standard error [SE]; range, 18.0–33.0 mg), with no apparent relation between oviduct mass and dose of EB. Right oviducts were not noticed in 68 females treated with canola oil, lower doses of EB, or any of the environmental estrogens. Gonads of treated chicks appeared normal by gross inspection and were not examined histologically.
Magnal portions of oviducts were categorized into four groups (A, B, C, or D) based on microscopic appearance (Table 1). Oviducts in category A had small, dense sheets of stromal cells with no mucosal tubular glands and a uniform columnar ciliated epithelium (Fig. 3a). Unblinding revealed that four of these oviducts had been treated with canola oil and three had been treated with the lower doses of EB. Oviducts in category B had rare to light numbers of tubular glands in the mucosal stroma, some of which communicated with invaginations in the surface columnar epithelium (Fig. 3b). Single necrotic cells were occasionally observed in the epithelium (Fig. 3b, arrows). These oviducts had either been treated with the three lowest doses of EB or with genistein at 100 nmol/g. Oviducts in category C had pseudostratified columnar epithelium lining the mucosal surface and multifocal to densely packed, tubular glands lined by cuboidal epithelium with centrally placed large, round nuclei and scant vesicular, basophilic cytoplasm (Fig. 3c). One of these oviducts was treated with EB at 10 nmol/g, one was treated with EB at 100 nmol/g, and four were treated with methoxychlor at 1,000 nmol/g. Oviducts in category D had luminal pseudostratified, ciliated, columnar epithelium and a markedly thickened mucosa that was densely packed with tubular glands lined by epithelial cells with basally oriented nuclei and eosinophilic, granular to vesicular cytoplasm (Fig. 3d). These oviducts had been treated with EB at either 10 or 100 nmol/g.
Mortality of chicks treated with EB or xenoestrogens was low (two deaths in 202 treated chicks [1%]). But the 1,000 nmol/g dose of methoxychlor killed 13 of 46 chicks during the treatment period (28.2%; chi-square, p < 0.01). Nine of these died during the first 2 d of treatment.
DISCUSSION
The zebra finch estrogen assay appears analogous in many ways to the immature rat uterotrophic assay. The limit of sensitivity of the zebra finch chick oviduct assay EB was at approximately 0.1 nmol/g body weight (37.65 μg/kg) or less. This is comparable to the sensitivity of oral administration of EB in rats, which is approximately 40 μg/kg (from inspection of Fig. 3 in Odum et al. [18]). Similarly, treatment with genistein at 100 nmol/g (27 mg/kg) increased oviduct weight to approximately the level of EB at 1 nmol/g. Thus, the estrogenicityof genistein in this assay appears to be approximately 0.01 that of EB. In ovariectomized rats, 3 d of oral treatment with genistein at 50 mg/kg, but not at 25 mg/kg, increased oviduct weight above controls. The finch chick oviduct may be slightly more sensitive to oral treatment with genistein than the chick oviduct of G. domesticus is to subcutaneous injections of genistein [19]. Methoxychlor at 1,000 nmol/g (346 mg/kg) induced finch oviduct growth to the approximate amount as did EB at 1 nmol/g. This is comparable to rats, in which an oral dose of methoxychlor at 500 mg/kg significantly increased uterine weight [18]. With the caveat that we only tested one dose of octylphenol, the sensitivity of zebra finch oviduct to octylphenol also appears similar or slightly less than the sensitivity of uterine growth in the immature rat. We detected an increase in oviduct weight at an oral dose of octylphenol at 1,000 nmol/g (203 mg/kg), whereras rat uterine weight is increased after 3 d of 100 mg/kg [20].
Generally, the developmental complexity of the oviduct increased with oviduct mass and dose of EB treatment, but this relationship did not hold for xenoestrogens. Methoxychlor had approximately one one-thousandth the estrogenic potency of estradiol benzoate in inducing oviduct growth but it was decidedly more potent in inducing morphological differentiation. All four methoxychlor-treated oviducts showed histologic characteristics typical of group C: development of a pseudostratified, columnar epithelium with multifocal clusters to densely packed tubular glands. In contrast, oviducts induced by EB to comparable size as methoxychlor at 1,000 nmol/g (∼14–18 mg) were more often categorized into histologic groups A and B. Even larger estradiol-induced oviducts were categorized into group B (i.e., EB at 10 nmol/g). Thus, methoxychlor appeared to induce a distinct suite of effects that differed from those induced by EB. Analogous differences have been observed in mammals. In mice, methoxychlor has approximately one one-thousandth the estrogenic activity of estradiol in stimulating uterine growth, but, compared to estradiol, this same dose nearly doubles the immunohistochemical expression of proliferating cell nuclear antigen, a marker of epithelial proliferation [21]. Several other phytoestrogens and xenoestrogens also modulate expression of estrogen-sensitive genes in rat uterus in a manner distinct from their ability to induce uterine growth [22]. Such early exposure to xenoestrogens may have long-lasting consequences. For example, chicken oviducts differentiated by diethylstilbestrol versus estradiol show a different pattern of avidin synthesis in response to progesterone challenge [23]. In the mouse uterus, early exposure to diethylstilbestrol results in inappropriate demethylation of cytosine–guanine dinucleotide sequence sites in the promoter region of the lactoferrin gene [24]. The long-term consequences of posthatch methoxychlor exposure to songbird reproductive tracts is not known, but neonatal exposure to methoxychlor alters pregnancy outcome in mice [25], an effect possibly related to altered reproductive tract physiology.
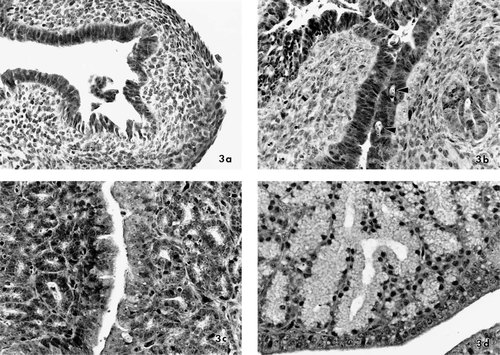
Light photomicrographs of oviducts illustrating growth and differentiation characterizing categorization into groups A, B, C, and D. All sections stained with hematoxylin and eosin; X370 magnification. (a) Group A. Oviduct from a finch treated with canola oil (estradiol benzoate at 0 nmol/g). Surface epithelium is lined by columnar ciliated epithelium and the mucosa is composed of dense sheets of small stromal cells with no tubular glands. (b) Group B. Oviduct from a finch treated with estradiol benzoate at 1 nmol/g. Rare tubular glands are present within the mucosal stroma, multifocal invagination of the surface epithelium has occurred, and occasional individual cell necrosis (arrows) is apparent. (c) Group C. Oviduct from a finch treated with methoxychlor at 1,000 nmol/g. The mucosal stroma has multifocal to dense mucosal tubular glands lined by cuboidal epithelium with central, large, round nuclei and basophilic vesicular cytoplasm. The luminal epithelium is ciliated columnar epithelium. (d) Group D. Oviduct from a finch treated with estradiol benzoate at 100 nmol/g. The mucosa is densely packed with tubular glands lined by epithelium with basally oriented small round nuclei and eosinophilic, granular to vesicular cytoplasm.
The 100 nmol/g dose of genistein appears within an order of magnitude of the dose range that could be encountered in laboratory diets that use soy protein concentrate as a protein source. Zebra finch chicks are estimated to consume 0.74 g of seed per day at 5 d of age and 2.05 g per day at 11 d of age [26]. A sample of the soy protein concentrate, Promax 70 (Central Soya, Fort Wayne, IN, USA), contains genistein at 750 mg/kg, daidzein at 375 mg/kg, and glycitein at 70 mg/kg (unpublished observation; assayed for us by the Veterinary Diagnostic Laboratory, School of Veterinary Medicine, University of Missouri, Columbia, MO, USA). If finch chicks were to consume an equal amount of 25% soy protein concentrate-based formulated diet as seed, it would equal a daily intake of genistein at 15 nmol/g. But this amount of the diet would also contain half again as much daidzein, which is estrogenic in rats [27]. Further, because mixed-seed diets often have a greater energy density than formulated feeds, the birds would be expected to consume relatively more of a soy-based than mixed-seed diet. Thus, the total amount of phytoestrogens consumed may be comparable to an equivalent dose of genistein at approximately 25 nmol/g, or one fourth of the higher dose used in the present study.
The doses of xenobiotic estrogens used in our study appear to be within the upper exposure range in field conditions. Kenaga's nomogram, as modified by Fletcher et al. [28], is a graphical algorithm for estimating residues of pesticides on various substrates based on application rate and molecular weight. At application rates that methoxychlor is used in the environment, the nomogram estimates that chicks fed 1 to 8 g of insects or seeds per day would ingest from 7 to 240 μg of methoxychlor per gram of feed ingested, for a maximum daily intake of up to 1.92 mg (calculated on a typical 1 pound per acre application rate, although it is registered for use at up to 3 pounds per acre [3.36 kg/ha] on some crops, e.g., dry beans in Colorado, USA [29]). The 100 nmol/g dose used in this study amounts to a daily dose of methoxychlor of 34.7 mg/g body weight or 121.5 to 312.3 μg/d during the linear growth phase, for a total 7-d dose of approximately 1.4 mg; the 1,000 nmol/g dose is 10-fold more. Thus, both the 100 and 1,000 nmol/g doses of methoxychlor could be encountered in the wild. The 1,000 nmol/g (346 mg/kg) dose of methoxychlor significantly increased mortality. Methoxychlor is acutely toxic in rats at 5,000 to 6,000 mg/kg, whereas rats can tolerate up to 500 mg/kg when fed chronically. However, finches seem to be more tolerant to methoxychlor than rabbits and dogs, which die at 200 and 50 mg/kg/d, respectively [30]. Examination of our data suggests that estrogenicity of oral methoxychlor as regards oviduct growth in birds is approximately 0.001 that of EB.
The 1,000 nmol/g dose of octylphenol represents a daily intake of about 1 to 2 mg for finches between 3.5 and 9 g body weight (5–11 d of age). Bennie [31] summarized several studies reporting octylphenol concentrations of 0.2 to 5 mg/g in such substrates as raw sewage, sewage treatment plant final effluent, and chemical plant effluent. Servos [32] reported bioaccumulation or bioconcentration factors for octylphenol and other related alklyphenols and alkylphenol polyethoxylates from one to several hundred in a variety of organisms, reaching several thousand in certain marine animals. Although trophic studies are needed to better assess exposure threats in terrestrial environments, nestlings fed biota grown in such highly contaminated environments could possibly encounter the load used in this study. Examination of these data indicates that the estrogenicity of oral octylphenol in this assay is approximately 0.0001 that of EB.
The estrogenic activity of octylphenol may underlie results published before this property was first described [33]. Chicken oviduct membranes contain a uridine diphosphate-N-acetylglucosamine enzyme that is stimulated by Triton X-100 (Sigma) [34]. Triton X-100 is a common laboratory detergent used to solubulize proteins that is 100% octylphenol polymerized with ethylene oxide. Estradiol activates a number of other enzymes in rat uterus, for example, uterine peroxidase [35], creatine kinase, and glucose-6-phosphate dehydrogenase [36].
High doses of EB (100 and 1,000 nmol/g) induced development of the right oviduct. It is not clear why this occurred, but a plausible account is suggested by data from G. domesticus. Embryonic ovaries of G. domesticus produce müllerianinhibiting substance, which typically is produced in abundance by testes to cause regression of the müllerian ducts. Estrogen produced by embryonic ovaries normally protects left müllerian ducts from the regressive effects of müllerian-inhibiting substance, permitting their development into mature oviducts. However, right mü llerian ducts, which have fewer estrogen receptors [37], normally are not sufficiently protected and so regress, except in rare instances [38]. Massive doses of estradiol possibly also protect right oviducts, thereby compensating for a lower number of estrogen receptors. Hypertrophy of right rudimentary oviducts also occurs in chickens treated with high doses of estradiol dipropionate (∼30–40 mg/kg by intramuscular injections) [39].
Several aspects of the zebra finch oviduct assay warrant its consideration for further validation and testing. The zebra finch is representative of the 30 genera and 155 species of Estrildinae finches distributed throughout Asia and Africa. However, the oviduct growth response to estrogen extends well beyond finches, appearing qualitatitively similar to that of mammalian species, several precocial avian species, altricial ringed turtledoves, as well as in other passerines, including the house sparrow [9] and European starling [10]. Thus, the response is likely to occur generally in passerines, which comprise almost 60% (5,712 sp.) of extant avian species. Therefore, the oviduct growth assay potentially could serve as a practical field marker for estrogen exposure for a large phylogenetic group of wild birds. A potential advantage of the avian oviduct is the wide range of sensitivity, spanning some five orders of magnitude. In contrast, the rat uterotrophic assay is sensitive to less than two orders of magnitude of dosing of EB [18]. The larger growth potential of the oviduct versus the uterus likely reflects the adult function of the magnum of synthesizing albumen proteins.
Oviduct growth assays also integrate exposure over time, which potentially is a practical advantage when time and duration of exposure to putative xenoestrogens may be transient or unknown. The finch assay is extremely simple and if oviduct weight is expressed as a percent of body weight, comparisons of exposure could potentially be made across species.
Whether early estrogen exposure in other avian species impairs reproductive performance as it does in zebra finch [17] and some mammals (e.g., rats [40]) is not yet known, but if so, oviduct growth of birds in the wild could have the added advantage of predicting adult reproductive performance. This aspect of the assay recommends testing on other passerines for its potential use in monitoring estrogen exposure in wild populations.
In summary, the growth response of zebra finch chick oviduct to estrogen provides a simple and sensitive bioassay for measuring exposure to putative xenoestrogens via an oral route, which is a natural route of exposure for wild birds. The assay appears particularly relevant for passerine species, in which sexual differentiation may remain sensitive to estrogen perturbation after, as well as before, hatching.




