Investigation of an onsite wastewater treatment system in sandy soil: Sorption and biodegradation of linear alkylbenzene sulfonate
Abstract
The objective of this work was to determine the sorptive and biodegradable characteristics of linear alkylbenzene sulfonate (LAS) in a soil below a Florida, USA, septic system drainfield. Three distinct soil samples were collected from the septic system drainfield study site. These soils were used in laboratory sorption and biodegradation studies. Different concentrations of LAS were added, in radiolabeled and unlabeled forms, to a series of test vessels that contained upgradient groundwater and the soils collected from the study site. The sorption test was designed to determine the partitioning of LAS between groundwater and soil in each sample. Results indicated that the sorption distribution coefficient (Kd) decreased from 4.02 to 0.43 L/kg and that the rate of ultimate biodegradation (first-order rate constant, k1) decreased from 2.17 to 0.08/d with increasing distance (0.7–1.2 m vertically below ground surface [BGS] and 0 to 6.1 m horizontally) from the drainfield. The three soils showed 49.8 to 83.4% LAS mineralization (percentage of theoretical CO2) over 45- or 59-d test periods. These results demonstrate that subsurface soils in this system have the potential to sorb and biodegrade LAS.
INTRODUCTION
This study was part of a program that investigated the fate and transport of household cleaning product surfactants in onsite wastewater treatment systems (OWTS) [1, 2]. Sorption and biodegradability characteristics are two important parameters for determining the fate of a material in the soil environment. The primary objective of this work was to determine the potential for LAS to sorb and biodegrade in soil beneath and downgradient of a septic tank drainage field. A secondary objective was to determine the sorption distribution coefficients and biodegradation rate constants of LAS for incorporation into a predictive model for OWTS.
Sorption of organic compounds to natural soil is important to their fate, bioavailability, and remediation. The principal determinant of the degree of sorption of organic compounds to natural soils is the soil organic matter content [3-5]. The partitioning of an organic compound between a liquid and solid phase once equilibrium conditions are established is generally expressed as a distribution coefficient (Kd). The relative distribution of an organic compound between solid and liquid phases depends on the physical and chemical properties of the organic compound and the solid phase.
The sorption of LAS has been previously reported on sediment [6] and in soil and aquifer material beneath a septic tank tile field in Ontario, Canada [7]. The current study was designed to measure the sorptive capacity and distribution of LAS to soils with different characteristics and climatic conditions collected below a septic system drainfield in Jacksonville (FL, USA).
Biodegradation is an important loss mechanism for determining the fate of an organic compound in the soil environment. The biodegradation process reduces the exposure concentration in the soil environment to higher-level organisms, thus improving the overall environmental safety of a chemical. One method of assessing the biodegradability of an organic compound is to conduct a CO2 production test [8-10]. In this study, a 14C-labeled LAS compound was used to evaluate its rate and extent of mineralization as was done previously to determine the ultimate biodegradability of LAS in soil and aquifer water beneath a septic tank field in Ontario, Canada [8]. The LAS sorption and biodegradation data obtained from this study were used as inputs to a predictive model for an on-site wastewater treatment system [2].
MATERIALS AND METHODS
The methodologies used in this study for both sorption and biodegradation were based on methods provided by the U.S. Environmental Protection Agency (EPA) Toxic Substance Control Act (TSCA) [9, 11] and Organization for Economic Cooperation and Development (OECD) [10, 12]. These studies were performed in compliance with all pertinent U.S. EPA Good Laboratory Practice regulations [13].
Test materials
Both unlabeled and uniformly ring-labeled [14C] LAS (dodecylbenzene sulfonic acid, sodium salt) were obtained from Sasol North America (Austin, TX, USA). The molecular weight of the LAS used in this study was 348 g/mole. The specific activity of the 14C-radiolabeled material was 77.0 μCi/mg, the radiochemical concentration was 93.3 μCi/ml, and the radiochemical purity was >92% based on radioactive thinlayer chromatography.
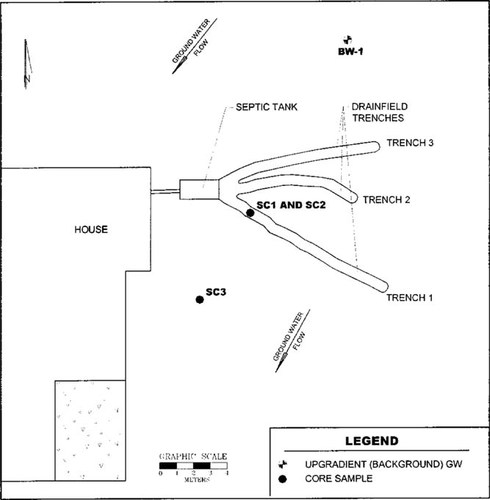
Diagram of septic system site showing the three soil and upgradient water collection points. SC = soil core; BW = background well.
Soil sample collection
The soils used in this study were collected from the Jacksonville study site on January 16, 1996. Soil core samples were collected at three locations below and downgradient of the drainfield using a stainless-steel soil recovery auger with polycarbonate sample liners (see Fig. 1 for diagram of site and sampling locations). The samples were packed in dry ice and shipped overnight to the testing laboratory. Soil core 1 (SC-1) was a composite sample of unsaturated soil collected from locations along the infiltrative surface of trench 1 approximately 71 to 91 cm BGS. Soil core 2 (SC-2) was a composite sample from the same boreholes as SC-1, but the cores were taken from the saturated zone below SC-1 from 122 to 142 cm BGS. Soil core 3 (SC-3) was a composite sample of the soil approximately 6.1 m downgradient (southwest) of trench 1. The SC-3 core was taken from the same relative elevation as SC-2 (122–142 cm BGS) and was in the saturated zone at the time of sampling. The multiple samples from each designated site were composited, passed through a 2-mm screen to remove coarse materials, and then refrigerated at approximately 4°C until use within 48 h.
 (1)
(1)Upgradient water collection
The groundwater used in this study was collected upgradient of the septic system drainfield in well BW-1 (Fig. 1). The groundwater was collected on January 16, 1996, packed in ice, and shipped by overnight delivery to the testing laboratory. The groundwater was refrigerated at approximately 4°C until use within 48 h.
Analytical methods
An aliquot of each composited soil sample was analyzed for the following parameters: pH, cation exchange capacity, organic carbon, organic matter, water content, water holding capacity, texture (sand, silt, clay), and bulk density according to Standard Methods of Soil Analysis [14, 15].
Liquid scintillation counting (LSC) was used to quantify the amount of 14C activity in the study samples. Stock solutions, solvent extracts, and aqueous and combustion samples were combined with Ultima Goldr̀ scintillation cocktail (Packard Instruments, Meriden, CT, USA) and analyzed using a Packard Instruments model A2500 Tri Carb LSC analyzer. The radiochemical purity was determined by radioactive thin-layer chromatography using a Bioscan System 200 TLC Imaging Scanner (Bioscan, Washington, DC, USA) [16].
Sorption determination
The test apparatus for the sorption study consisted of 30-ml Teflon™ centrifuge tubes. All tubes, except the LAS controls (no soil added), received 7 g of dry-weight-equivalent composited soil and 7 ml of upgradient groundwater. The LAS controls received 7 ml of upgradient groundwater. Radiolabeled or unlabeled LAS was dosed to duplicate tubes for each soil at concentrations of 40, 200, 700, and 2,000 μg/L. No LAS was added to the soil blank tubes.
The test vessels were equilibrated for 3 h on a shaker table rotating at 180 rpm. The vessels were then centrifuged for 40 min at 5,600 g. Triplicate aliquots of each centrate were assayed by LCS. A mass balance was determined on one tube for each concentration from each soil site by extracting the soil with methanol. The mass of test substance present on the soil was assayed by LCS. Linear alkylbenzene sulfonate sorbed to the tube walls was extracted with methanol and measured. Aliquots of the soil pellets from three test vessels were combusted to confirm the effectiveness of the methanol extraction.
Biodegradation determination
The test apparatus consisted of 500-ml Erlenmeyer glass flasks each containing 100 g of dry-weight-equivalent soil from each site. The 14C-labeled LAS was dosed to triplicate flasks for each site at concentrations of 50 and 2,000 μg/kg. The LAS concentrations chosen were representative of the range of LAS concentrations estimated in the saturated soil of the septic system drainfield as measured by methylene blue active substance [17]. The water content of each treatment was adjusted to approximately 30% with deionized water [18].
During the study, the flasks were aerated continuously with a CO2-free air source. The flasks were weighed to determine water loss and adjusted daily with deionized water to maintain 30% water content. The CO2 evolved from the flasks was trapped in a 1.5-N potassium hydroxide solution. Samples from the base traps were periodically collected on days 0.25, 1, 3, 5, 7, 10, 14, 18, 21, 28, 35, and 45, with additional samples on days 52 and 59 for site 3 to account for lag time. These samples were assayed for 14C activity by LSC. After 45 d, the contents of the flasks containing soil from sites 1 and 2 were acidified with 1 N hydrochloric acid (HCl) and aerated for 3 d. A final set of base trap samples was collected and analyzed by LSC on day 48. The flasks from site 3 were acidified with 1 N HCL on day 59 and aerated for 3 d, and the base traps were analyzed by LSC.
| Other typical soilsa | |||||||
|---|---|---|---|---|---|---|---|
| Study soils | |||||||
| Parameter | SC-1b | SC-2 | SC-3 | Alflsol | Spodosol | Entisol | Loamy sand |
| PH | 6.1 | 6.3 | 6.9 | 5.5–6.5 | 4.0–5.0 | 6.6–8.0 | 4.6 |
| CECc (mequiv/100 g) | 0.99 | 0.75 | 0.78 | 10–15 | <10 | >10 | 32.7 |
| Organic carbon (%) | 0.13 | 0.03 | <0.01 | 1.0–1.5 | 1.5–3.5 | 1.0–4.0 | 9.25 |
| Organic matter (%) | 0.22 | 0.05 | <0.01 | − | − | − | − |
| WHCd (%) 1/3 bar | 2.12 | 1.34 | 1.22 | − | − | − | − |
| WHCd (%) 15 bar | 1.03 | 0.82 | 0.37 | − | − | − | − |
| Sand (%) | 93.2 | 93.2 | 93.2 | − | − | − | 81.6 |
| Silt (%) | 4.0 | 4.0 | 4.0 | − | − | − | 12.6 |
| Clay (%) | 2.8 | 2.8 | 2.8 | 10–20 | ≤10 | 11–25 | 6.0 |
| Soil classification | Sand | Sand | Sand | − | − | − | − |
| Bulk density (g/cm3) | 1.45 | 1.51 | 1.47 | − | − | − | − |
- a Soils used in U.S. Environmental Protection Agency and Organization for Economic Cooperation and Development biodegradation studies.
- b SC = soil core.
- c CEC = cation exchange capacity.
- d WHC = water holding capacity.
A mass balance was determined on one flask per each triplicate set. Each flask was centrifuged for 30 min at 5,600 g. Triplicate aliquots (1 ml) of the centrate were assayed by LCS. The weight of the remaining soil pellet was documented, and five aliquots (0.1 g) were combusted using a sample oxidizer (Packard Instruments model 307 oxidizer). Combustion of soil pellets was performed to determine the amount of 14C radio-activity in the soil [17].
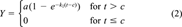 (2)
(2)RESULTS
The soils in this study were comprised primarily of sand with smaller amounts of organic carbon and clay (Table 1). Higher amounts of organic matter were found in soil collected closest to the leaching field (SC-1 and SC-2). The organic carbon, clay content, and cation exchange capacity values for the soils in this study were markedly lower than the corresponding values for the four typical soils used in U.S. EPA TSCA [11] and OECD [13] soil biodegradation studies (Table 1).
Sorption
The amount of 14C activity measured in the centrate (liquid phase after centrifugation) and in the soil methanol extracts was used to determine the sorption distribution coefficients (Kd). A summary of the sorption data for the three soils collected at the study site is shown in Table 2. The amount of 14C activity in the centrate increased or remained the same, whereas the amount of 14C activity in the soil decreased from site 1 to site 3. Consequently, the Kd values were lower in the soil from site 3 when compared to the soil from site 1. Test substance recoveries in these experiments ranged from 62 to 104%.
Biodegradation
The production of CO2 is an indication of the amount of test substance that is ultimately biodegraded or mineralized by microorganisms in a soil matrix test system. In this study, the 14C activity mass balance for all the soils tested averaged 103.5 ± 6.0% and ranged between 92.2 and 109.5%. A summary of the biodegradation data is presented in Table 3, while the 14CO2 production-versus-time curves are shown in Figures Fig. 2., Fig. 3..
Examination of these biodegradation data results in four observations. A direct correlation was observed between the level of organic carbon content in the soils and the initial LAS biodegradation activity. The organic carbon content decreased from 0.13 to <0.01% from site 1 to site 3 (Table 1), while the highest first-order biodegradation rates observed in these soils decreased from 2.17 to 0.08/d, and the lag times increased from 0.11 to 9.8 d (Table 3). An increase in LAS concentration from 50 to 2,000 μg/kg negatively affected the initial LAS biodegradation activity. In all soils, the initial first-order biodegradation rates stayed constant or decreased, and the lag times increased at the higher LAS concentration (Table 3). The LAS biodegradation patterns in soil from sites 1 and 2 were biphasic, while the pattern in the soil from site 3 was monophasic. Despite the initial differences in the LAS biodegradation rates and lag times, the final amount of LAS mineralization was extensive and very similar in all the soils with final mineralization values (% T 14CO2) ranging from 49.8 to 83.4% with most (15 of 16) >68% (Table 3).
| Site no. | Dose (μg/L) | Centrate concn. (μg/L)a | Solids concn. (μg/kg) | Kd (L/kg) measured |
|---|---|---|---|---|
| SC-1b | 40 | 12.67 | 15.62 | 1.23 |
| SC-1 | 40 | 11.58 | 13.31 | 1.15 |
| SC-1 | 200 | 46.86 (46.46) | 130.8 | 2.79 |
| SC-1 | 700 | 128.3 (136.4) | 452.5 | 3.53 |
| SC-1 | 2,000 | 345.4 (344.4) | 1,387 | 4.02 |
| SC-2 | 40 | 21.62 (21.02) | 19.98 | 0.92 |
| SC-2 | 200 | 86.36 (78.34) | 75.02 | 0.87 |
| SC-2 | 700 | 275.9 (262.3) | 301.4 | 1.09 |
| SC-2 | 2,000 | 836.0 (826.8) | 746.0 | 0.89 |
| SC-3 | 40 | 15.74 (16.70) | 12.82 | 0.81 |
| SC-3 | 200 | 75.76 (75.66) | 64.06 | 0.85 |
| SC-3 | 700 | 273.7 (286.6) | 228.3 | 0.83 |
| SC-3 | 2,000 | 1,115 (1,057) | 476.8 | 0.43 |
- a Values in parentheses are the aqueous concentration in μg/L for a replicate test vessel.
- b SC = soil core.
| Kinetic data (± standard error)a | |||||
|---|---|---|---|---|---|
| Soil site no. | Concn. LAS (μg/kg) | Final % T14CO2 | Asymptote % T14CO2 | Rateb (per day) | Lag (d) |
| SC-1c | 50 | 68.8 | 38.2 ± 0.7 | 2.01 ± 0.28 | 0.11 ± 0.03 |
| 71.7 | 37.2 ± 1.8 | 2.09 ± 1.02 | 0.00 ± 0.14 | ||
| 70.3 | 37.1 ± 1.3 | 2.17 ± 0.77 | 0.00 ± 0.10 | ||
| SC-1 | 2,000 | 73.1 | 35.4 ± 0.9 | 0.74 ± 0.10 | 0.21 ± 0.05 |
| 75.1 | 34.6 ± 1.3 | 0.88 ± 0.19 | 0.14 ± 0.09 | ||
| SC-2 | 50 | 68.3 | 38.4 ± 3.0 | 0.41 ± 0.12 | 0.05 ± 0.22 |
| 49.8 | 23.8 ± 0.9 | 0.62 ± 0.11 | 0.20 ± 0.09 | ||
| SC-2 | 2,000 | 74.6 | 42.4 ± 5.1 | 0.20 ± 0.07 | 0.22 ± 0.34 |
| 72.8 | 32.0 ± 0.6 | 0.52 ± 0.05 | 0.71 ± 0.08 | ||
| 71.5 | 30.6 ± 0.8 | 0.55 ± 0.08 | 0.73 ± 0.10 | ||
| SC-3 | 50 | 78.4 | 77.9 ± 0.6 | 0.09 ± 0.00 | 5.2 ± 0.2 |
| 69.8 | 68.5 ± 0.9 | 0.09 ± 0.01 | 5.1 ± 0.3 | ||
| 73.3 | 75.6 ± 0.8 | 0.09 ± 0.00 | 6.6 ± 0.2 | ||
| SC-3 | 2,000 | 74.5 | 74.1 ± 0.9 | 0.08 ± 0.00 | 9.1 ± 0.2 |
| 68.3 | 67.0 ± 0.8 | 0.10 ± 0.01 | 9.1 ± 0.2 | ||
| 83.4 | 84.2 ± 0.5 | 0.08 ± 0.00 | 9.8 ± 0.1 | ||
- a These parameters are calculated from the first order portions of the biodegradation curves.
- b The data (site 1 at 2,000 μg/kg and site 2 at 50 μg/kg) were not included in the respective analyses because in both cases the final 14CO2 evolved was considerably less than other flasks of the same treatment. No visual observations were made during test setup or throughout the sampling period to explain the lower results. Knowledge of the site and interpretation of other biodegradation data determined that these data points should be eliminated as outliers.
- c SC = soil core.
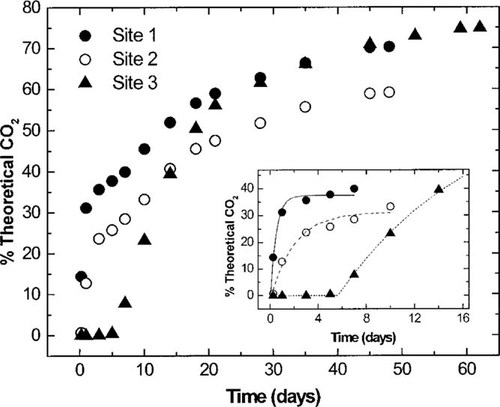
Biodegradation of linear alkylbenzene sulfonate (LAS) in a sandy soil—14CO2 evolved (LAS concentrations: 50 μg/kg) for the three sites. Each point is the average 14CO2 evolved from three flasks. Insert shows the first-order kinetic portions of the biodegradation curves along with the curve fits based on Equation 2.
DISCUSSION
The ability of a soil to sorb and biodegrade organic compounds is dependent on its characteristics. The soils in this study were primarily sandy with organic matter, clay, or silt content much less than typical soils within the United States (Table 1). A direct correlation appears to exist with the amount of organic content of a soil and its potential for sorption and biodegradation as evidenced by lower sorption and biodegradation activity at site 3 as compared to site 1, likely due to an increase in the microbial population near the effluent source.
The sorption data from this study (Table 2) are consistent with other observations reported in the literature. The sorption of LAS to aquatic sediments was shown to more closely correlate to the organic carbon content of the sediments than to the LAS homolog tested, the pH or calcium concentration of the test solution, or solids concentrations [6]. In soils beneath a septic tank drainfield in Ontario, Canada, the sorption of LAS was correlated with organic carbon and clay content in the subsurface soils [7]. The Kd for LAS in that study ranged from 1 to 20 L/kg for soils and 20 to 3,019 L/kg for sediments [19, 20].
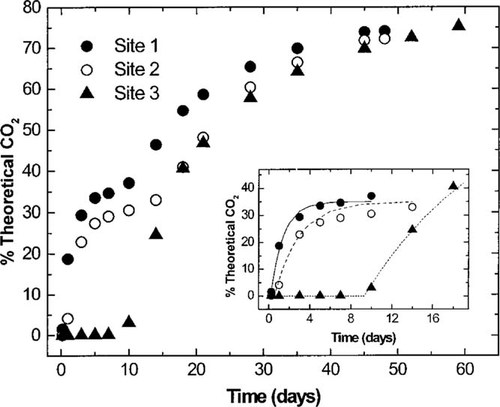
Biodegradation of linear alkylbenzene sulfonate (LAS) in a sandy soil—14CO2 evolved (LAS concentrations: 2,000 μg/kg) for the three sites. Each point is the average 14CO2 evolved from three flasks. Insert shows the first-order kinetic portions of the biodegradation curves along with the curve fits based on Equation 2.
The biodegradation data in this study are also consistent with other reports in the literature. In a biodegradation study of LAS in the previously mentioned septic tank drainfield in Ontario, Canada, it was shown that the process of adaptation of the resident microorganisms influenced the biodegradation of LAS [8]. This same phenomenon was noted in this study since, in each soil, longer lag periods were required for the microbial populations to adapt to the higher LAS concentration (Table 3).
The rapid mineralization of LAS in soils has been widely reported [19-25]. First-order rate constants for LAS mineralization in pristine, agricultural, and sludge-amended soils ranged from 0.02 to 1.25/d. Linear alkylbenzene sulfonate sorbed to various soil constituents and mixed with either a natural woodlot soil or a sludge-amended soil exhibited firstorder rates of 0.19 to 1.25/d with a maximum mineralization amount of 48.5 to 96.2% T14CO2 [22, 23], respectively. In another study by Larson et al. [24], the first-order rate constants were quite similar and averaged 0.03/d for the surface soils and 0.04/d for the subsurface soils. The results of this experiment indicate that soils immediately below the drainfield, where biological activity would be high, had rates slightly higher (2.10/d) than sludge-amended soils. Downgradient soils, which do not receive appreciable LAS concentrations and therefore would not have high biological activity, show slower rates (0.09/d) comparable with previously reported values for pristine soils.
The data shown in Figures Fig. 2., Fig. 3. indicate that two separate kinetic events occurred in the soils from sites 1 and 2. Only one kinetic event occurred with the soil from site 3. This phenomenon has been described as biphasic mineralization. The first phase is consistent with typical first-order biodegradation, and the second phase is consistent with zero-order biodegradation [26]. The zero-order phase represents the mineralization of carbon that is incorporated into biomass. This phenomenon is known as biomass turnover or endogenous respiration.
Ultimate biodegradation occurred more slowly and with a longer lag time in the soils that were further removed from the septic system drainfield (unsaturated to saturated soil) and that contained lower levels of organic carbon. It is reasonable to assume that the microbial populations were proportional to the organic content of the soils. Therefore, it was anticipated that the initial LAS mineralization rates would be higher in soil with a higher organic content. It was also expected that even in the soil from site 3, which had a very low organic matter content, some microorganisms capable of LAS biodegradation were present since the soil had been exposed to LAS for many years. These capable organisms apparently acclimated to the levels of LAS in the test, multiplied, and produced a large enough population to show detectable rates of LAS mineralization. Ultimately, the microbial population mineralized LAS to approximately the same level in the three test soils.
The sorption distribution coefficients and biodegradation rate constants were highest in the unsaturated soil immediately below the drainfield and decreased with increasing distance from the drainfield. The amount of ultimate biodegradation was quite high given the conditions of the soil, and the final percentage of ultimate biodegradation remained relatively constant for the three soil sites. The biodegradation pattern of sites 1 and 2 were consistent with biphasic mineralization, while biodegradation in site 3 could be described by simple firstorder kinetics. These results demonstrate that both sorption and biodegradation of LAS occur in the soils beneath the study site and indicate that these parameters are important factors in the removal of LAS in a subsurface soil system. Data from this paper were subsequently used to validate a mathematical model developed to predict the fate and transport of down-the-drain household chemicals in septic systems [2, 27].
Acknowledgements
The authors would like to thank John Hash and Kathleen Crapo of the Weston Environmental Fate and Effect Laboratory. We would also like to thank Ayres&Associates and A&L Great Lakes Laboratory.




