Atrazine and metribuzin sorption in soils of the argentinean humid pampas
Abstract
Laboratory studies were conducted to determine the influence of surface and subsurface properties of three representative soils of the humid pampas of Argentina on atrazine and metribuzin sorption. Atrazine and metribuzin sorption isotherms were constructed for each soil at four depths. Sorption affinity of herbicides was approximated by the Freundlich constant (Kf), distribution coefficient (Kd), and the normalized Kd based on organic carbon content (Koc). Multiple regression of the sorption constants against selected soil properties indicated that organic carbon content (OC) and silt were related positively and negatively, respectively, to atrazine Kf coefficient (r2 = 0.93), while Kd coefficient of atrazine was related positively to organic carbon content and negatively to both silt and cation exchange capacity (CEC) (r2 = 0.96). For metribuzin, only organic matter content was related positively to Kf coefficient (r2 = 0.51). Lower Kf values for atrazine were obtained for all soils with increasing depth, indicating lesser sorption at greater depths. Metribuzin sorption was quite similar across all depths. Sorption constant Kf of atrazine ranged from 2.06 to 7.82, while metribuzin Kf values ranged from 1.8 to 3.52 and were lower than atrazine for all soils and depths, indicating a greater leaching potential across the soil profile.
INTRODUCTION
During 2000, herbicides represented 72% of the total value of the pesticide sales in Argentina. Nineteen percent of the main marketable herbicides were sold for application to soils. Two of the most used herbicides in Argentina, atrazine and metribuzin, are triazine herbicides applied mainly at preemergence of crops, especially atrazine in corn and metribuzin in potato and soybean. However, worldwide both atrazine and metribuzin are used as pre- and postemergence herbicides.
The fate of pesticides in the environment is governed by the processes of sorption, degradation, and transport and the interaction between those processes [1]. Sorption of a pesticide by the soil has been cited as the process that has the greatest influence in its behavior and fate in the environment [2, 3]. Sorption is the consequence of interaction between the pesticide, the soil colloids, the noncolloidal soil organic matter, and the soil solution surrounding both and may be reversible or irreversible. Properties of soil and pesticide are very important in determining the mechanisms of sorption and thereafter the bioavailability and mobility of the chemical. However, factors controlling sorption of herbicides by soils are difficult to determine because of the heterogeneous nature of soil. The extent of sorption of a herbicide in soil is generally expressed by the sorption distribution coefficient (Kd) between the aqueous and soil phases. The sorption coefficient varies widely with soil type and soil organic matter content, for this reason the sorption coefficient normalized to soil organic carbon content (Koc), has been one of the most frequently applied sorption constants. Gustafson [4] pointed out that the Koc of a pesticide may be used to predict Kd for a new soil on which Kd of the compound has never been measured and also could serve as an independent measure of the relative mobility of the pesticide in the soil. The same author in a previous work [5] classified compounds into leachers and nonleachers on the basis of an elaboration of their mobility (expressed as Koc) and persistence (soil half-life).
Soil organic matter content has been found to be the more relevant property in regulating sorption for many nonionic herbicides [6-8]. However, nonionic herbicides as the weakly basic triazines atrazine and metribuzin can be sorbed to both soil organic matter and clay minerals, and the sorption is pH dependent [8-10].
Because soil organic matter typically strongly sorbs nonionic herbicides, the pattern of organic matter distribution often indicates the relative pattern of nonionic herbicide sorption in soil [11]. For this reason, herbicides are usually highly sorbed to surficial soil layers. Subsurface soil horizons located at greater depth contain less organic matter, reducing sorption of herbicides [12], as was determined for metribuzin [13, 14] and atrazine [15, 16].
Variations in soil properties with depth influence sorption, degradation, and movement of herbicides [17]. Understanding how soil properties within a profile affect herbicide retention and degradation will result in more accurate prediction of herbicide fate and potential groundwater contamination. Soils from greater depths of the profile sorbed more metribuzin than the lower-clay-content, higher-organic-matter surface soils [18]. Greater sorption of atrazine was reported for the 0- to 30-cm zone than for soil at greater depths (30–300 cm), and this was attributed to decreasing soil organic matter content and higher soil pH at greater depths [17]. However, no correlation of sorption of atrazine was found with soil depth, clay content, or organic carbon content of five soils [19]. The same author pointed out that atrazine sorption was greater in all horizons of the fine-textured soils compared to coarse-textured soils.
| Particle size | |||||||
|---|---|---|---|---|---|---|---|
| Soil | Depth (cm) | pH | CECa (cmol/kg) | Organic carbon (%) | Clay | Silt (%) | Sand |
| Balcarce | 0–10 | 5.57 | 27.63 | 3.88 | 28.96 | 38.34 | 32.80 |
| 10–20 | 5.57 | 25.73 | 3.77 | 29.53 | 37.60 | 32.87 | |
| 20–30 | 5.80 | 25.90 | 2.54 | 30.20 | 33.83 | 35.97 | |
| 30–40 | 6.13 | 23.57 | 1.76 | 34.84 | 29.76 | 35.40 | |
| Tres Arroyos | 0–10 | 5.97 | 22.20 | 2.45 | 27.99 | 31.47 | 40.55 |
| 10–20 | 5.80 | 23.60 | 2.01 | 29.56 | 34.51 | 35.94 | |
| 20–30 | 6.03 | 23.67 | 1.37 | 35.32 | 26.65 | 38.03 | |
| 30–40 | 6.47 | 25.70 | 0.92 | 39.26 | 24.62 | 36.13 | |
| Dorrego | 0–10 | 7.02 | 24.30 | 1.92 | 21.26 | 38.29 | 40.45 |
| 10–20 | 7.45 | 22.90 | 1.68 | 20.07 | 36.36 | 43.56 | |
| 20–30 | 7.21 | 26.40 | 1.36 | 24.66 | 34.68 | 40.66 | |
| 30–40 | 6.95 | 23.90 | 1.00 | 28.10 | 32.05 | 39.85 | |
- a CEC = cation exchange capacity.
The objective of this research was to determine the influence of surface and subsurface properties of three representative soils of the humid pampas of Argentina on atrazine and metribuzin sorption.
MATERIALS AND METHODS
Soils used for sorption studies
Soil samples were collected in three different locations (Balcarce, Tres Arroyos, and Coronel Dorrego) within the humid pampa region in the southeast of Buenos Aires province (Argentina). The Balcarce soil is classified as a silty clay loam (fine, thermic, illitic, Typic Argiudoll), the Tres Arroyos soil is a clay loam (fine, thermic, illitic, Typic Argiudoll), and the Coronel Dorrego soil is a loam (fine, thermic, mixed illitic-mortmorillonitic, Typic Argiudoll). Soil samples were collected from four layers: 0 to 10 cm, 10 to 20 cm, 20 to 30 cm, and 30 to 40 cm. Soils of Balcarce and Tres Arroyos have a similar distribution in depth for each horizon, corresponding 0 to 10 cm to Ap, 10 to 20 cm to Ap-A2, 20 to 30 cm to A2, and 30 to 40 cm to AB horizons. In the case of Coronel Dorrego, the distribution of horizons according to each layer is 0 to 10 cm to Ap, 10 to 20 cm to Ap, 20 to 30 cm to B2t, and 30 to 40 cm to B2t horizons.
Soil samples were collected between September and October 2000 from research plots with no history of atrazine or metribuzin application. Three subsamples of each depth were mixed to obtain a representative sample of that specific soil. Soil samples were air dried for 48 h at room temperature and sieved through a 2-mm screen and thoroughly mixed. Physical and chemical properties of each layer are described in Table 1. The pH was measured by electrode in a soil:water ratio of 1:2.5. Cation exchange capacity was determined by displacement with 1 M ammonium acetate at pH = 7 [20]. Organic carbon was analyzed by oxidation with chromic acid [21]. Textural analysis was done by the method of successive pipetting to determine particle size fractions by gravimetric settling [22].
Sorption experiments
Atrazine and metribuzin were purchased as analytical standards with 99% purity from Chem Service (West Chester, PA, USA). Sorption was determined by the batch equilibrium method [23] separately for both herbicides and in each of the soil samples. Ten grams of air-dried soil was first equilibrated using a reciprocal shaker with 25 ml of 0.01 M CaCl2 solution for 12 h at 20°C. After this equilibration step, seven increasing amounts of atrazine or metribuzin were added dissolved in a minimal quantity of methanol in order to facilitate dissolution as suggested by the Organization for Economic Cooperation and Development (Paris, France) [23]. The concentrations for this experiment were chosen taking into account both the analytical working range in the lab, which was basically influenced by the detection limit of the analytical method, and the expected concentration of the herbicides in the top soil layer when they are applied in the field. From those considerations, the following concentrations were used for the sorption experiments: 0.04, 0.1, 0.2, 0.5, 1.0, 2.0, and 5.0 mg/L of herbicide in solution. Triplicate replications were prepared for each soil and concentration, and the batches were equilibrated in 50-ml-capacity glass Erlenmeyer flasks with ground-glass stoppers for 24 h on a reciprocal shaker at 20°C to ensure equilibrium was reached. Preliminary studies showed that both atrazine and metribuzin reached equilibrium in all the soils studied after approximately 12 to 16 h. After equilibration, the samples were vacuum filtered through a membrane (0.2-μm pore size, 47-mm diameter), and 12 ml of the filtrate were preconcentrated onto a Varian BondEluter̀ Herbicide cartridge (Varian, Walnut Creek, CA, USA). Finally, the eluate was analyzed by means of a Hewlett-Packard 1100 system high-performance liquid chromatography (HPLC) (Agilent Technologies, Hewlett-Packard, Waldbronn, Germany), consisting of a quaternary pump system, a diode array detector programmed at 224 nm (atrazine) and 214 nm (metribuzin), and an autosampler, using a Varian Microsorb-MVr̀ 5-μm C8 column with dimensions of 4.6 by 150 mm with a solvent system of 50/50 (v/v) acetonitrile/HPLC-grade water at a flow rate of 1 ml/min at 40°C.
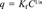
| Linear isotherm | Freundlich isotherm | ||||||
|---|---|---|---|---|---|---|---|
| Soil | Depth (cm) | K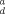 |
r2 | K |
k |
l/ |
r2 |
| Balcarce | 0–10 | 9.39 ± 0.99A | 0.98 | 242BCD | 7.82 ± 0.58A | 0.82 ± 0.03BCD | 0.99 |
| Balcarce | 10–20 | 8.77 ± 1.70A | 0.93 | 233CD | 7.64 ± 0.71A | 0.80 ± 0.04BCDE | 0.99 |
| Balcarce | 20–30 | 5.78 ± 1.52BC | 0.92 | 227BCD | 4.48 ± 0.35D | 0.70 ± 0.04F | 0.99 |
| Balcarce | 30–40 | 5.31 ± 0.76CD | 0.96 | 302B | 5.29 ± 0.47C | 0.85 ± 0.04AB | 0.99 |
| Tres Arroyos | 0–10 | 7.27 ± 0.60B | 0.99 | 297BC | 6.09 ± 0.29B | 0.71 ± 0.02F | 0.99 |
| Tres Arroyos | 10–20 | 5.04 ± 0.63D | 0.98 | 251BCD | 4.46 ± 0.36D | 0.75 ± 0.04CDEF | 0.99 |
| Tres Arroyos | 20–30 | 5.53 ± 0.78CD | 0.96 | 404A | 4.96 ± 0.35CD | 0.86 ± 0.04AB | 0.99 |
| Tres Arroyos | 30–40 | 3.37 ± 0.38E | 0.98 | 366A | 2.97 ± 0.84E | 0.91 ± 0.15A | 0.99 |
| Dorrego | 0–10 | 4.82 ± 0.46D | 0.99 | 251BCD | 3.03 ± 0.24E | 0.71 ± 0.04EF | 0.99 |
| Dorrego | 10–20 | 4.92 ± 0.69D | 0.98 | 293BC | 3.16 ± 0.23E | 0.74 ± 0.04CDEF | 0.99 |
| Dorrego | 20–30 | 2.56 ± 0.35E | 0.97 | 188D | 2.64 ± 0.20EF | 0.73 ± 0.04DEF | 0.99 |
| Dorrego | 30–40 | 2.88 ± 0.40E | 0.98 | 288BC | 2.06 ± 0.18F | 0.83 ± 0.05BC | 0.99 |
- a Linear adsorption coefficient with 95% confidence interval.
- b Kd normalized for organic carbon.
- c Freundlich adsorption coefficient with 95% confidence interval.
- d Freundlich slope with 95% confidence interval.
In order to estimate the distribution coefficient Kd, the data of the first four points of the isotherm, where the Freundlich exponent was close to 1, were used in a linear relationship, resulting Kd from the ratio between adsorbed herbicide to the concentration in equilibrium solution.
RESULTS AND DISCUSSION
The atrazine and metribuzin sorption data were evaluated quantitatively by the linearized Freundlich sorption isotherm and linear sorption isotherms. The Freundlich parameter 1/n is a measure of the nonlinearity of the sorption isotherm. If 1/n is less than 1, the Freundlich equation describes adequately the sorption isotherm. Sorption of atrazine was well described by the Freundlich equation for almost all soils and depths. Atrazine sorption showed a better fit (r2 = 0.99) than metribuzin (r2 = 0.94–0.99) (Tables 2 and 3).
Considering each soil profile, atrazine sorption (both Kd and Kf) was significantly (p = 0.05) of greater magnitude in the upper 0- to 20-cm layer of Balcarce and Dorrego soils, whereas in Tres Arroyos soil sorption was significantly (p = 0.05) greatest in the 0- to 10-cm depth (Table 2). In agreement with these results, previous reports [11, 17] cited that sorption of atrazine was higher in the surface soil compared to the greater soil depths. Sorption of atrazine in soil from each depth of Dorrego soil was generally lower than in the other soils, indicating greater potential of leaching through this soil. For atrazine, Kf values ranged from 7.82 to 2.06, and the order was Balcarce > Tres Arroyos > Dorrego, with similar patterns of decreasing atrazine sorption with increasing soil depth. Values found here were in general higher than previous reports for other Mollisolls [17, 19], probably because the previously reported soils had lower OC content than the soils in our experiment. Lower Kf values for atrazine with increasing depth would indicate a lower sorption at greater depths and therefore a greater leaching potential for this herbicide once it has passed throughout the upper layers of the soil. Besides this, the microbial population levels tend to decrease with depth in the profile, and thus biodegradation of pesticides is much slower than in surface soil [12, 15, 16]. Thus, shallow subsoils would appear to provide increased opportunity for further leaching of compounds escaping from the surface layer [14, 24]. With the exception of Dorrego soil, metribuzin sorption was quite similar across all depths (Table 3). Metribuzin sorption showed no clear tendency between depths into each soil. Metribuzin Kf values ranged from 1.8 to 3.52 and were lower than atrazine for all soils and depths, indicating a greater leaching potential across the soil profile. This could be partially explained by the lower Kow of metribuzin with respect to atrazine (1.6 and 2.7 respectively), indicating a low affinity of metribuzin for sorption to organic matter. However, considering the 95% confidence intervals for 1/n, metribuzin sorption is truly linear, except for 0- to 10-cm and 20- to 30-cm depths of Tres Arroyos soil and 0- to 20-cm and 30- to 40-cm depths of Dorrego soil, although we did not find significant differences (p = 0.05) for 1/n between the different soils (Table 3). To further investigate the linearity of 1/n, we proceeded with a statistical analysis of the hypothesis that 1/n ≠ 1. The results showed that although in the case of atrazine 1/n differed significantly (p = 0.01) from 1 for all the soils and the three upper layers, in the case of metribuzin we observed no significant difference (p = 0.05) of 1/n from 1 in all the soils and depths (Table 4). When 1/n values are not different from 1, the estimation of metribuzin sorption should be made using the Kd [19]. For pesticides, the exponent 1/n tends to be less than 1 with a mean value of 0.87 [25]. The average value of 1/n for the three soils used here were 0.93 and 0.78 for metribuzin and atrazine, respectively. Distribution coefficients for metribuzin ranged from 2.27 to 4.52, and, as well as Kf, they were quite higher than other reported values [18, 26, 27]. The higher values of Kd of atrazine and metribuzin for our soils suggest that movement of these chemicals should be significantly less than in other reported soils and must be considered if results from this study are to be extrapolated to other soils.
| Linear isotherm | Freundlich isotherm | ||||||
|---|---|---|---|---|---|---|---|
| Soil | Depth (cm) | K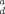 |
r2 | K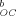 |
k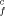 |
l/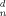 |
r2 |
| Balcarce | 0–10 | 3.17 ± 0.90ABC | 0.86 | 82F | 2.94 ± 0.44B | 0.99 ± 0.08A | 0.97 |
| Balcarce | 10–20 | 4.52 ± 0.56A | 0.97 | 120F | 3.51 ± 0.41A | 0.97 ± 0.06A | 0.98 |
| Balcarce | 20–30 | 2.92 ± 0.69BC | 0.93 | 115F | 3.35 ± 0.40A | 0.99 ± 0.07A | 0.98 |
| Balcarce | 30–40 | 4.01 ± 0.64AB | 0.95 | 228BC | 2.96 ± 0.43B | 0.94 ± 0.08A | 0.97 |
| Tres Arroyos | 0–10 | 3.26 ± 0.87ABC | 0.92 | 133EF | 2.41 ± 0.25D | 0.86 ± 0.06A | 0.98 |
| Tres Arroyos | 10–20 | 4.04 ± 0.39AB | 0.99 | 201 CD | 2.85 ± 0.45BC | 0.95 ± 0.09A | 0.96 |
| Tres Arroyos | 20–30 | 2.27 ± 0.77C | 0.81 | 165CDE | 1.80 ± 0.33E | 0.87 ± 0.10A | 0.94 |
| Tres Arroyos | 30–40 | 2.99 ± 0.59BC | 0.93 | 325A | 2.49 ± 0.42BCD | 0.96 ± 0.09A | 0.96 |
| Dorrego | 0–10 | 3.22 ± 0.71BC | 0.94 | 168DE | 2.38 ± 0.26D | 0.91 ± 0.06A | 0.98 |
| Dorrego | 10–20 | 3.31 ± 0.39BC | 0.98 | 197CD | 2.31 ± 0.31D | 0.85 ± 0.07A | 0.97 |
| Dorrego | 20–30 | 2.91 ± 0.34BC | 0.98 | 214BCD | 2.50 ± 0.19D | 0.96 ± 0.04A | 0.99 |
| Dorrego | 30–40 | 2.57 ± 0.32C | 0.98 | 257B | 1.84 ± 0.16E | 0.89 ± 0.05A | 0.99 |
- a Linear adsorption coefficient with 95% confidence interval.
- b Kd normalized for organic carbon.
- c Freundlich adsorption coefficient with 95% confidence interval.
- d Freundlich slope with 95% confidence interval.
| Atrazine | Metribuzin | |||||
|---|---|---|---|---|---|---|
| Depth (cm) | Balcarce | Dorrego | Tres Arroyos | Balcarce | Dorrego | Tres Arroyos |
| 0–10 | ** | ** | ** | NSa | NS | NS |
| 10–20 | ** | NS | NS | NS | ||
| 20–30 | NS | NS | NS | |||
| 30–40 | NS | NS | NS | NS | NS | |
- a NS = not significant at p = 0.05.
- ** Significant at p = 0.01.
- * Significant at p = 0.05.
The calculated Koc values from linear isotherm ranged from 188 to 404 for atrazine and from 82 to 325 for metribuzin. Our atrazine Koc agreed with cited values of 100 to 200 depending on pH [10], 100 [28, 29], and 50 to 2,950 [19]. Metribuzin Koc values obtained were higher than the Koc of 60 cited by several authors [10, 26-28, 30].
Considering the 95% confidence intervals for 1/n, sorption isotherms of atrazine for all soils and depths, with the exception of the 30- to 40-cm layer of Tres Arroyos soil, had values for 1/n less than 1 (Table 2), indicating that the percentage of atrazine sorbed decreased with increasing solution concentration [17, 31]. Reported metribuzin isotherm exponents were in general close to 1 (Table 3) and statistically not different from 1 (Table 4), indicating that the percentage adsorbed to soils from all depths was nearly independent of initial concentration [17, 31].
Correlations between atrazine or metribuzin sorption coefficients (Kf and Kd) and slopes (1/n) and selected soil properties were calculated (Table 5). The highly significant correlation between clay and silt (r2 = −0.79) exists because the sand content in all the soils in this experiment was approximately constant. Atrazine's Kd values were highly correlated (p = 0.01) negatively with pH and positively with organic matter content, while metribuzin's Kd values were not significantly correlated (p = 0.05) with any soil properties. However, metribuzin's Kf had a highly significant correlation with organic carbon content (Table 5). The high negative correlation of atrazine sorption with pH and the (although not significant) negative correlation of metribuzin sorption with pH could be explained by the fact that protonated species are more readily sorbed onto exchange sites in the soil. Our experimental results are in good agreement with those found by Capriel et al. [32], who states that atrazine formed more links with the organic fraction (90%) than the mineral fraction (10%) in soils with neutral pH, and with Jenks et al. [18], where soil organic matter content was cited as a good predictor of atrazine sorption (r2 = 0.98) followed by soil pH (r2 = 0.82), while Sonon et al. [11] found that clay and organic carbon content appeared to have the main influence on the sorption of atrazine.
| Atrazine | Metribuzin | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| CECa | Clay | Silt | OCb | Kd | Kf | l/n | Kd | Kf | l/n | |
| PH | −0.234 | −0.55* | 0.04 | −0.67* | −0.74** | −0.83** | −0.13 | −0.36 | −0.59 | −0.46 |
| CEC | 0.14 | 0.27 | 0.41 | 0.20 | 0.24 | 0.15 | −0.04 | 0.47 | 0.84** | |
| Clay | −0.79** | −0.14 | 0.02 | 0.22 | 0.75** | −0.05 | 0.09 | 0.26 | ||
| Silt | 0.63* | 0.41 | 0.26 | −0.63* | 0.39 | 0.41 | 0.24 | |||
| OC | 0.93** | 0.88** | −0.26 | 0.52 | 0.72** | 0.45 | ||||
- a CEC = cation exchange capacity.
- b OC = organic carbon.
- ** Significant correlation at p = 0.01 level.
- * Significant correlation at p = 0.05 level.
Stepwise multiple linear regression was used to further evaluate the relationship between atrazine or metribuzin sorption and soil properties. This procedure indicated that soil organic carbon content was positively related to atrazine Kf coefficient, while silt content was negatively related to atrazine (r2 = 0.93, Kf = 6.87 + 2.37OC - 0.217silt). The Kd coefficient for atrazine was positively related to organic carbon content and negatively related to both silt content and CEC (r2 = 0.96, Kd = 11.78 + 2.68OC - 0.14silt - 0.28CEC). Jenks et al. [17] obtained the highest r2 value, combining organic matter content and CEC through multiple regression of the sorption constants against selected soil properties. In the case of metribuzin, only organic matter content was related to metribuzin sorption Kf coefficient (r2 = 0.51, Kf = 1.8 + 0.39 OC), although this correlation coefficient was much lower than that for atrazine. These results agree with several authors [33, 34] who found that differences in mobility of metribuzin in several soils were the consequence of different capacity of sorption of soils, and this was significantly correlated to organic matter content of soil. However, Harper [18] determined that though sorption of metribuzin was best related to clay content, pH, and organic matter, sorption was not highly related to organic matter content alone. On the other hand, Peek and Appleby [34] did not find correlations between soil properties and metribuzin sorption.
In summary, the higher affinity of atrazine and metribuzin for organic carbon observed in our study is in agreement with the general sorptive behavior of many pesticides in soils [7]. However, the presence of organic carbon could be a greater impediment for atrazine than metribuzin movement, indicating greater leaching potential of metribuzin across the soil, which was supported by several authors [30, 35-37].
Acknowledgements
Funding for this work was provided by Fondo Nacional de Ciencia y Tecnología Argentina (PICT 4581), Universidad Nacional de Mar del Plata (AGR 107/00), and Instituto Nacional de Tecnología Agropecuaria (PI 663). The authors want to thank Marcelo Huarte and PROPAPA Project for permitting access to analytical facilities.




