Simultaneous measurement of uptake and elimination of cadmium by caddisfly (Trichoptera: Hydropsychidae) larvae using stable isotope tracers
Abstract
The use of stable isotopes coupled with inductively coupled plasma—mass spectrometry provides a unique opportunity to investigate pathways of metals in aquatic invertebrates. In this study, the simultaneous uptake and elimination of cadmium (Cd) in stream-dwelling caddisfly (Trichoptera: Hydropsychidae) larvae was measured in laboratory experiments with stable isotopes of Cd. In one experiment, animals were exposed to low levels (0.5 μg/L) of 114Cd in filtered river water and uptake was measured for 9 d, followed by 9 d during which the larvae were exposed to either 114Cd, 113Cd, or filtered river water. In a second experiment, the initial exposure concentration of 114Cd varied from 0.1 to 1.6 μg/L for 5 d, followed by 5 d during which the larvae were exposed to the same respective levels of 113Cd. The purpose of the two experiments was to test whether or not elimination of 114Cd from Hydropsyche larvae was the same in the presence or absence of Cd in the water and also whether or not elimination rates were dependent on exposure concentration. The results indicated that elimination of Cd by Hydropsyche larvae (elimination rate constant ∼ 0.21–0.24/d) in the presence of Cd in the water was the same as the depuration when no metal was present. Furthermore, the elimination rate was independent of exposure concentrations, ranging between 0.1 and 1.6 μg/L. Thus, the stable isotope tracer technique enabled the simultaneous measurement of uptake and elimination of Cd in hydropsychid larvae and may have the potential to facilitate the labeling of various compartments (e.g., water, sediment, and food) in the aquatic environment.
INTRODUCTION
Experimental studies of uptake and elimination of metals by aquatic invertebrates are abundant in the literature. These studies often have involved exposing the organisms to metal concentrations many times greater than background [1-5]. In part, this was dictated by the relatively high detection limits of the available analytical methods compared with background or environmental concentrations. However, this approach has the drawback that by increasing ambient metal levels in the water, the solubility of the metal may be exceeded and/or the uptake and depuration kinetics of the organism may be altered [6].
A second problem with many previous uptake and elimination experiments involving aquatic invertebrates is that usually discrimination between background and added doses of the metal in terms of accumulation and loss has not been possible, nor has any differentiation been made among the various external (e.g., food, sediment, and water) or internal (e.g., blood, tissue, and gut) uptake pools. Although the use of radioisotopes allows individual animals and compartments to be labeled, and thus has gained popularity for use in experiments involving aquatic invertebrates [7-12], generally only one radioisotope is appropriate for a given metal [13]. Thus, in a given experiment, only one compartment can be labeled and distinguishing among various uptake pathways is not possible.
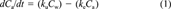 (1)
(1)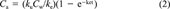 (2)
(2)The term ke, the elimination rate constant, represents the fractional elimination of the metal from the organism, while the animal is still exposed to the metal. Although ke can be estimated during the uptake part of the experiment, traditionally it is measured during an independent experiment where the test animal is placed into clean water.
This approach has two problems. First, as Landrum and coworkers [14] pointed out, elimination from an organism in the absence of any metal in the water actually represents the depuration rate constant, kd. In the past, ke and kd have been used interchangeably, under the assumption that elimination is dependent on the metal concentration in the organism, Ca, rather than on its concentration in the water (i.e., Cw). However, the suggestion has been made [15, 16] that depuration rates of certain aquatic crustaceans may be dependent upon exposure concentrations. Despite this, no tests of the assumption of the independence of elimination on water concentrations have been made, most likely because of the logistics of previous uptake and depuration experiments.
The second problem with studies in which measurements of uptake and loss are separated in time [17, 18] is that it is necessary to assume that the condition of the organisms has remained the same during both phases of the experiment. The assumption that the uptake of metal has not caused any physiological changes that might affect the organism's cycling of the metal is not always justified because the process of elimination of a metal from an organism may be different after exposure to metal concentrations that are above ambient levels.
The use of stable isotopes is one of the most recent developments in metal biogeochemistry [19] and has been used successfully to investigate pathways of metals in aquatic invertebrates [20, 21]. The approach relies upon the fact that for most metals, a number of chemically identical but isotopically distinct forms exist, and that very low concentrations of the different isotopes can be detected and discriminated by inductively coupled plasma-mass spectrometry (ICP-MS).
Similar to the radiotracer approach, the high sensitivity of modern ICP-MS instrumentation allows for experiments to be conducted on individual organisms, rather than groups of two or more individuals, at environmentally realistic concentrations. Thus, fewer test organisms are required for each experiment and an estimate of animal-to-animal variability is obtained. However, unlike the radiotracer technique, several stable isotopes are available commercially for most metals of interest, even though they are of low abundance in the natural environment. For example, cadmium (Cd) has eight stable isotopes (107Cd, 108Cd, 110Cd, 111Cd, 112Cd, 113Cd, 114Cd, and 116Cd) but only one useful radioactive isotope (109Cd). The availability of several stable isotopes makes it possible to use one isotope as an internal standard, as opposed to the traditional external calibration or standard curve approach, and then to apply the principle of isotopic dilution [22] to determine concentrations within the organism. This increases both accuracy and also precision in the experimental data. Also, distinguishing between the metal taken up during the experiment and preexisting tissue concentrations is not a problem. Furthermore, the use of two or more stable isotopes, properly applied, allows for the simultaneous measurement of the concurrent processes of uptake and elimination. This approach is thus unique.
The objectives of this study were to use environmentally realistic concentrations of stable Cd isotopes (i.e., 110Cd, 111Cd, 112Cd, 113Cd, and 114Cd) to measure simultaneously and, to our knowledge, for the first time, uptake and loss of the metal from stream-dwelling caddisfly larvae {Hydropsyche spp.; Trichoptera: Hydropsychidae); and to determine the effect of various exposure concentrations in the water on the elimination of Cd by these invertebrates.
Caddisfly larvae were selected for the study because they are important decomposers of organic matter, they are a food source for fish and birds, and they are widely distributed in nature [23]. In addition, they are relatively tolerant of many trace metals and their collection for study is simple and inexpensive [24-26]. In recent years, hydropsychid larvae in particular have been used to study the uptake and effects of various contaminants under laboratory conditions [4, 23, 26, 27] and also as in situ biomonitors [24, 28]. The members of the family Hydropsychidae that were selected for the present study live in cool or warm lotic waters and are included in the groups that spin nets. The nets function as shelter and also serve to collect food for these suspension feeders.
Cadmium was selected for several reasons. Measured concentrations in most surface waters generally range from 2 to 50 ng/L [29; and references therein] although values greater than 500 ng/L were reported for two lakes near the Copper Cliff smelter in Ontario, Canada [29]. Thus, Cd has become an important contaminant of some lakes but still occurs in the low nanogram per liter level in many surface waters so that tests can be conducted at low concentrations. Furthermore, Cd is readily accumulated by both marine and freshwater organisms [3, 8-10, 30] and is known to be toxic to certain zooplankton at low concentrations (eg. <1 μg/L) [31]. Finally, as mentioned previously, several stable isotopes of Cd are available, providing the necessary means to make separate but concurrent measurements of uptake and loss.
In general, our technique involved a two-phase exposure of the organisms to stable isotopes of Cd. During the first phase, the organisms were exposed to one isotope of the metal. After a given period of time, the isotope used for exposure was changed. During this second phase, the uptake of the second isotope was measured concurrently with the loss of the first isotope. Throughout the experiment, the exposure concentration of the metal was held constant, so that the only variable was the specific isotope that had been added to the experimental systems.
MATERIALS AND METHODS
Study area and collection of test organisms
Caddisfly larvae (5th instars) of the genus Hydropsyche (mostly H. betteni) were collected from a riffle zone in Thompson Creek, Peterborough County, Ontario, Canada (44°17′N, 78°19′W), which originates as an overflow from the Otonabee River. Individual larvae were collected with forceps from overturned rocks and placed directly into Trent tubes (∼50 per tube). The Trent tube was described and illustrated by Balch and Evans [25]. Briefly, it consists of an S-shaped transparent polyvinyl chloride tube of 5 cm inner diameter, the central straight section of which contains a chamber with 50 individual compartments delimited with drinking straws, into which the larvae are placed. The central chamber is covered with netting to prevent the organisms from escaping or moving to adjacent compartments, while still permitting the flow of water and food. The Trent tubes containing the Hydropsyche were placed directly into covered exposure tanks for transportation back to the laboratory. No immediate mortality of larvae occurred due to sampling and transport.
Acclimation and pretreatment holding of animals
At the laboratory, the animals were held in their exposure tanks without food for 1 to 3 d, at approximately 10°C, in Otonabee River water that had been filtered through sand to remove particulates and organic matter. Acclimation provided for the removal of any organisms that were injured during collection and transport. Approximately 12 to 24 hours before the commencement of the experiment, the larvae were fed a mixture of TetraMin™ (TetraWerke, Melle, Germany) flake fish food, freeze-dried Tubifex, and live Artemia.
Experimental design
Mixing tanks. Large, rectangular, 25-L-capacity, polyethylene mixing tanks were used to prepare and supply river water or Cd solutions to the various exposure tanks (four exposure tanks per mixing tank). Two Teflon® hoses entered each mixing tank. One hose carried filtered river water supplied directly from the Otonabee River and the other delivered concentrated Cd stock solution, which varied from 1 to 16 μg/L, depending on the experiment, from a carboy. Note that during the acclimation period, although all pumps were running, only filtered river water was circulating through the mixing and exposure tanks. The flow rates, approximately 25 ml/min for the river water and approximately 3 ml/min for the Cd solution, were regulated by two peristaltic pumps. Although flow rates for each individual peristaltic pump did not vary over time, variability occurred among the various pumps. Therefore, the actual Cd concentrations in each mixing tank were regulated manually to offset any flow rate differences among the three or four pump pairs. Within each mixing tank, a small (0.15-amp) submersible pump circulated and mixed the water, which was maintained at a constant volume of water (∼12 L).
Exposure tanks. The exposure tanks used to hold the Trent tubes and the organisms were 7-L-capacity, rectangular, polyethylene containers. Four exposure tanks were used for each mixing tank. By using peristaltic pumps to regulate flow rates, water was delivered to the exposure tanks via Teflon hoses connected to the mixing tanks. The exposure system would be best described as a flow-through system with a replacement rate of two to three volumes of water in a 24-h period. The volume of water within each exposure tank was maintained at approximately 4 L during the experiments.
As described above, each exposure tank held one Trent tube, each containing approximately 50 Hydropsyche larvae. Attached to the inside wall of each exposure tank was a small submersible aquarium pump, which continuously provided a flow of water necessary for aeration of the organisms inside the Trent tube.
Experimental procedure
Experiment 1: Simultaneous measurement of uptake and elimination of Cd. This experiment was designed to measure, simultaneously, the uptake and elimination of Cd by Hydropsyche larvae, at a single Cd concentration (0.5 μg/L). This concentration was selected, although it is at the high end of Cd levels found in lakes and rivers in Ontario [29], because it is within the no-effect level in terms of acute toxicity to most aquatic invertebrates. The experiment lasted for 18 d, during which time the animals were exposed to 114Cd at 0.5 μg/L for the first 9 d, followed by a 9-d exposure to either 113Cd at 0.5 μg/L (treatment A), 114Cd at 0.5 μg/L (treatment B), or filtered river water (treatment C). Thus, the experiment included three treatments and three mixing tanks, with each representing a different depuration scenario. Because four exposure tanks were used per treatment (i.e., mixing tank), a total of 12 exposure tanks was included.
Before the beginning of the experiment, three animals were removed from each exposure tank (for a total of 36 animals) and analyzed to provide information on background concentrations of 110Cd, 111Cd, 112Cd, 113Cd, and 114Cd. Similarly, background concentrations of the same Cd isotopes in the Otonabee River were taken from samples of the river water before it entered the mixing tanks. At t = 0, appropriate volumes of stock 114Cd solution were added to each mixing tank to provide final concentrations of 0.5 μg/L. In addition, each mixing tank and each exposure tank was spiked initially with <2 ml of the concentrated stock solution necessary to immediately achieve the desired exposure concentration.
On days 3, 6, and 9, three live larvae were removed from each of the 12 exposure tanks. The animals were blotted with paper towels to remove surface water and inspected to ensure that no netting adhered to the organism. Each animal was placed into an individually labeled 15-ml graduated polystyrene centrifuge tube and allowed to air dry at room temperature for approximately one week. The tubes were then sealed. Also on these days, 10 ml of water was collected from each exposure tank. Each of these water samples was also put into a 15-ml graduated polystyrene centrifuge tube and acidified to pH < 2 with concentrated nitric acid (trace metal grade, Seastar Chemicals, Sydney, BC, Canada). The tubes were then sealed.
In the morning of day 9, after the animals and water had been sampled, food was supplied to the remaining organisms by adding a 250-μl volume of a slurry containing TetraMin fish flake, freeze-dried Tubifex, and live Artemia. Approximately 1 h after feeding all the tubing, the tanks (mixing and exposure), the Trent tubes (containing the remaining test organisms) and the carboy containing the stock 114Cd solution were rinsed thoroughly with river water. This procedure was designed to remove any 114Cd and food particles from the experimental system. Approximately 4 h after the initial sampling of the animals and the water on day 9, the experiment was restarted. Treatment (mixing tank) A now contained 113Cd at 0.5 μg/L, treatment B contained 114Cd at 0.5 μg/L, and mixing tank C contained Otonabee River water. Similar to the start of the experiment, the mixing tanks and their exposure tanks were spiked initially with concentrated stock solution to achieve the desired exposure concentration and thus avoid any delay in the equilibration period. Within an hour of restarting the experiment, 10-ml water samples were obtained from each exposure tank. Samples of both organisms and water were taken on days 12,15 and 18 as described above for the samples taken during the first half of the experiment.
The Cd isotopes used in the experiments were supplied in the form of CdO for 112Cd and 114Cd and as CdCl2 for 113Cd. The temperature of the water in each exposure tank was monitored daily, and dissolved O2 was determined on every third day. Dissolved oxygen during the experiments was generally above 80% saturation (at ∼15°C).
Experiment 2: Effect of Cd concentration on uptake and elimination. In this experiment, the uptake and elimination of 114Cd by Hydropsyche larvae in the presence of varying concentrations of Cd was measured. The experiment lasted for 10 d, during which time the organisms were exposed to various concentrations of 114Cd for the first 5 d, followed by exposure to the same respective concentrations of 113Cd for the last 5 d. Four mixing tanks were included, with each representing a treatment and each containing a different concentration of Cd so that treatment D = 0.1 μg/L, treatment E = 0.4 μg/L, treatment F = 0.8 μg/L, and treatment G = 1.6 μg/L. As in experiment 1, four exposure tanks were included per mixing tank, resulting in a total of 16 exposure tanks. The procedures for experiment 2 were generally similar to those followed in experiment 1, except that in experiment 2, the water and the Hydropsyche larvae were sampled on a daily basis, rather than every 3 d. A summary of the design of the two experiments is given in Table 1.
Analyses
Air-dried individual animals were oven-dried in the uncapped centrifuge tubes overnight at 60°C. The tubes were then capped. Before analysis, each animal (dry wt ∼ 4.753 mg) was weighed individually to the nearest 100 ng. Animals were then placed into individually labeled 5-ml Teflon digestion vials with screw caps and a 50-μL spike of 112Cd was added to each sample. This procedure is necessary for isotopic dilution determination of Cd isotope concentrations [22]. This was followed by the addition of 250 μl of concentrated nitric acid. Vials containing the samples were heated at 90 to 100°C for approximately 3 h or until all tissue was solubilized. The cooled digest was made up to the 10-ml mark in the original graduated centrifuge tube with distilled deionized water, and then 50 μl of rhodium (103Rh) was added as an internal standard.
| Treatment | |||
|---|---|---|---|
| Day | A (0.5 μg Cd/L) | B (0.5 μg Cd/L) | C (0.5 μg Cd/L) |
| Experiment 1 | |||
| 0–9 (AM) | 114Cd | 114Cd | 114Cd |
| 9 (PM)-18 | 113Cd | 114Cd | River water |
| Treatment | ||||
|---|---|---|---|---|
| D (0.1 μg Cd/L) | E (0.4 μg Cd/L) | F (0.8 μg Cd/L) | G (1.6 μg Cd/L) | |
| Experiment 2 | ||||
| 0–5 (AM) | 114Cd | 114Cd | 114Cd | 114Cd |
| 5 (PM)-10 | 113Cd | 113Cd | 113Cd | 113Cd |
Both the (acidified) water and the animal samples were aspirated directly from the centrifuge tubes into an Elan 6000 ICP-MS (Perkin-Elmer Sciex, Concord, ON, Canada) and analyzed for 110Cd, 111Cd, 112Cd, 113Cd, 114Cd, and 103Rh. Typical instrument settings were radio frequency (RF) power = 1,000 W, torch gas = 15 L/min, auxiliary gas = 0.8 L/min, and nebulizer gas ∼ 0.9 L/min. Four replicates were measured for each sample (i.e., each individual animal or water sample). A replicate consisted of 32 individual measurements that were averaged.
Quality assurance and quality control
Trace metals in drinking water Standard Reference Material (SRM 1643a, National Institute of Standards and Technology, Gaithersburg, MD, USA) were run with all water samples at the rate of approximately one per 15 to 20 water samples. Ground oyster tissue (SRM 1566a) samples (10 mg) were analyzed, typically, at the rate of one for every 20 to 30 animals. These samples were treated with the same digestion technique as for the animal tissue samples, except that 750 μl of HNO3 was used for the digestion instead of 250 μl. The 103Rh was used as an internal standard to check for instrument drift. Calibration standards were reanalyzed after the measurement of approximately every 30 samples to check for instrument drift and change in sensitivity.
RESULTS
Background concentrations of Cd averaged 0.08 ± 0.04 μg/g dry weight (n = 35) in the animals and approximately 55 ng/L in the water. The chemical form of Cd in the river water was not determined; however, the chemistry of the water (pH = 7.6–7.9, dissolved organic carbon ∼ 5 mg/L, inorganic carbon = 60 mg/L) and speciation calculations conducted with Mineql+ [32] suggest that >90% of the Cd is present as free aquo ions or carbonate complexes.
| Day | 113Cd nominal concn. | Measured mean ± 1 SD | 114Cd nominal concn. | Measured mean ± 1 SD |
|---|---|---|---|---|
| Treatment A | ||||
| 0 | 0 | 0.02 ± 0.00 | 0.5 | 0.17 ± 0.02 |
| 3 | 0 | 0.01 ± 0.00 | 0.5 | 0.52 ± 0.01 |
| 6 | 0 | 0.01 ± 0.00 | 0.5 | 0.61 ± 0.01 |
| 9 (AM) | 0 | 0.01 ± 0.00 | 0.5 | 0.44 ± 0.01 |
| 9 (PM) | 0.5 | 0.38 ± 0.02 | 0 | 0.23 ± 0.01 |
| 12 | 0.5 | 0.61 ± 0.01 | 0 | 0.08 ± 0.01 |
| 15 | 0.5 | 0.60 ± 0.01 | 0 | 0.04 ± 0.00 |
| 18 | 0.5 | 0.61 ± 0.01 | 0 | 0.03 ± 0.00 |
| Treatment B | ||||
| 0 | 0 | 0.02 ± 0.00 | 0.5 | 0.21 ± 0.01 |
| 3 | 0 | 0.01 ± 0.00 | 0.5 | 0.52 ± 0.01 |
| 6 | 0 | 0.01 ± 0.00 | 0.5 | 0.62 ± 0.01 |
| 9 (AM) | 0 | 0.01 ± 0.00 | 0.5 | 0.48 ± 0.01 |
| 9 (PM) | 0 | 0.01 ± 0.00 | 0.5 | 0.46 ± 0.01 |
| 12 | 0 | 0.00 ± 0.00 | 0.5 | 0.55 ± 0.01 |
| 15 | 0 | 0.00 ± 0.00 | 0.5 | 0.52 ± 0.01 |
| 18 | 0 | 0.00 ± 0.00 | 0.5 | 0.54 ± 0.01 |
| Treatment C | ||||
| 0 | 0 | 0.01 ± 0.00 | 0.5 | 0.98 ± 0.22 |
| 3 | 0 | 0.01 ± 0.00 | 0.5 | 0.57 ± 0.02 |
| 6 | 0 | 0.01 ± 0.00 | 0.5 | 0.64 ± 0.01 |
| 9 (AM) | 0 | 0.01 ± 0.00 | 0.5 | 0.67 ± 0.03 |
| 9 (PM) | 0 | 0.00 ± 0.00 | 0 | 0.31 ± 0.01 |
| 12 | 0 | 0.00 ± 0.00 | 0 | 0.18 ± 0.01 |
| 15 | 0 | 0.00 ± 0.00 | 0 | 0.13 ± 0.01 |
| 18 | 0 | 0.00 ± 0.00 | 0 | 0.11 ± 0.00 |
Quality assurance and quality control
The average measured concentration of Cd in the oyster tissue standard (SRM 1566a) was 3.76 ± 0.30 μg/g (n = 36) and was within the certified value of 4.15 ± 0.38 μg/g. Cadmium concentrations in the trace metals in drinking water standard (SRM 1643a) were within 5% of the certified value.
Experiment 1: Simultaneous measurement of uptake and elimination of Cd
The measured average 113Cd and 114Cd concentrations in the water for each treatment on each sampling day, together with the nominal (i.e., target) Cd concentrations, are given in Table 2. Despite our attempts to instantaneously achieve the target concentration, 114Cd concentrations measured on day 0 were less than half (i.e., ∼0.2 μg/L) the target concentration of 0.5 μg/L for treatments A and B, and almost double the target concentration for treatment C. However, from days 3 to 9 (AM), 114Cd concentrations averaged (±1 standard deviation; SD) 0.52 ± 0.09 μg/L in treatment A, 0.54 ± 0.07 μg/L in treatment B, and 0.63 ± 0.05 μg/L in treatment C, and were not significantly different among the three treatments. After the change in isotope treatments on day 9, as expected, 114Cd levels in treatments A and C decreased dramatically but remained relatively constant (i.e., average = 0.52 ± 0.04 μg/L for days 9 [PM], 12, 15, and 18) in treatment B, where 114Cd continued to be added to the test water. The 113Cd concentrations remained in this range for the duration of the experiment in all treatments except, as expected, from days 9 (PM) to 18 for treatment A, where they averaged 0.55 ± 0.11 μg/L; these are not significantly different from the 114Cd levels used in the first half of the experiment.
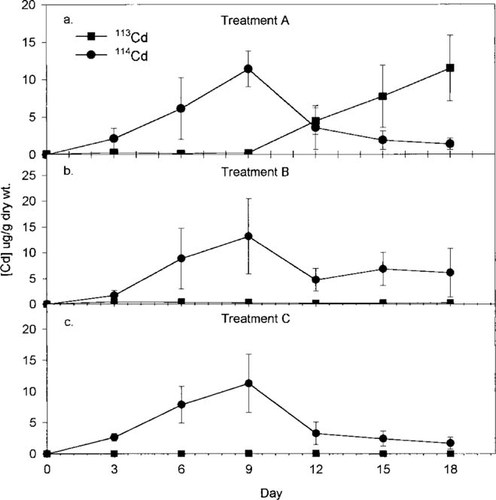
Uptake and elimination of 114Cd (circles) by Hydropsyche larvae and uptake of 113Cd (squares) by the larvae in (a) treatment A. (b) treatment B, and (c) treatment C. Error bars represent ± 1 standard deviation (SD). Values > 3 SDs from the mean of the other points have been excluded. See Table 1 for details regarding each treatment.
The uptake of 114Cd and 113Cd and depuration of 114Cd by the Hydropsyche larvae for treatments A, B, and C are shown in Figure 1a, b, and c, respectively. For the most part, each point represents the average ± 1 SD of 12 individual larvae (i.e., four replicate exposure tanks × three larvae/tank). However, for these data, and all subsequent results that will be presented, any values >3 SDs from the mean of the other points were considered to be outliers and thus excluded from further statistical analyses. By using this approach, for experiment 1, <5% of the data were eliminated.
As is evident from Figure 1, the uptake of 114Cd from days 0 to 9 was similar among the three treatments. The same can be said for the uptake of 113Cd from days 9 to 18 in treatment A. The slopes of the lines of Ca (i.e., 114Cd concentration in the larvae) versus time from day 0 to day 9 are 1.25 μg/g dry weight/d (n = 44), 1.55 μg/g dry weight/d (n = 48), and 1.31 μg/g dry weight/d (n = 47) for treatments A, B, and C, respectively. No significant differences were found in these slopes among the three treatments (analysis of covariance, p > 0.05). These slopes also do not differ (p = 0.35) from the slope of Ca versus time (1.20 μg/g dry wt/d, n = 48) obtained from days 9 to 18 with 113Cd in treatment A.
Also from Figure 1a and c, loss of 114Cd from the Hydropsyche larvae from days 9 to 18 appears to be similar in treatments A and C, even though Cd, in the form of 113Cd, was present in the system in treatment A. To test whether kd (i.e., depuration of Cd from the Hydropsyche in the absence of any metal in the water) is actually the same as ke (i.e, elimination in the presence of Cd in the water), ke and kd were calculated from the slope of the line of ln Ca versus time (d), with the 114Cd data obtained from treatments A and C, respectively. The results of the regression analyses indicate that the slope of the line, ke, for treatment A (i.e., -0.21/d, n = 45; Fig. 1a) is identical to the slope of the line, kd, for treatment C (i.e., -0.21/d, n = 47; Fig. 1c). These results indicate that elimination of this metal by this organism (Hydropsyche) is the same whether or not Cd is present in the water.
In treatment B, where 114Cd remained in the test water throughout the experiment, 114Cd concentrations in the Hydropsyche larvae averaged approximately 5 to 13 μg/g dry weight from day 6 to day 18, inclusive. Immediately after the changeover on day 9, some indication was found of a decrease in 114Cd levels in the larvae (see Fig. 1b). Mean concentrations of 114Cd in the larvae on days 12, 15, and 18 were significantly different from the mean on day 9 (one-way analysis of variance; p < 0.05). The decrease in 114Cd in the animals may be due to dilution of the isotope after the feeding that occurred on day 9. This dilution would occur also in treatments A and C; however, the effect is small relative to the losses occurring from depuration. Although in all three treatments, a trend was found toward decreasing animal dry weight as the experiment progressed (data are not shown), the dry weights of the larvae generally did not vary significantly among the three treatments for each sampling day (one-way analysis of variance; p > 0.05 for six of the seven sampling days), nor did they vary from day to day within each treatment (one-way analysis of variance; p > 0.05 for each of the three treatments).
Experiment 2: Uptake and elimination of Cd at various Cd concentrations in the water
The purpose of this experiment was to test the assumption of the independence of ku (the uptake clearance) and ke (the elimination rate constant) on Cd concentration in the water. The water concentrations of 114Cd and 113Cd in the four mixing tanks (i.e., treatments) on each day of the experiment are given in Table 3. Once again, despite our attempts to immediately achieve the target concentrations of 114Cd at the beginning of the experiment, and also to maintain these concentrations at a constant level, 114Cd concentrations increased significantly (p < 0.05, n = 5 for each treatment) from day 1 to day 5 (AM) in treatments D, E, and G. Similarly, a significant and linear decrease was found in 114Cd concentrations from day 5 (PM) to day 10 (p < 0.01, n = 6 for each treatment). The 114Cd probably was sorbed onto the walls of the carboy, the mixing tanks, the exposure tanks, and the Trent tubes during the first phase of the experiment and then released to the water during the second phase, despite the thorough rinsing of the experimental apparatus between the two phases of the experiment.
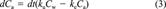 (3)
(3)| Treatment D (0.1 μg Cd/L) | Treatment E (0.4 μg Cd/L) | Treatment F (0.8 μg Cd/L) | Treatment G (1.6 μg Cd/L) | |||||
|---|---|---|---|---|---|---|---|---|
| Day | [114Cd] | [113Cd] | [114Cd] | [113Cd] | [114Cd] | [113Cd] | [114Cd] | [113Cd] |
| 1 | 0.07 | 0.01 | 0.22 | 0.01 | 0.12 | 0.01 | 1.12 | 0.01 |
| 2 | 0.08 | 0.01 | 0.26 | 0.01 | 0.59 | 0.01 | 1.33 | 0.01 |
| 3 | 0.09 | 0.01 | 0.33 | 0.01 | 0.58 | 0.01 | 1.53 | 0.01 |
| 4 | 0.09 | 0.01 | 0.38 | 0.01 | 0.69 | 0.01 | 1.65 | 0.01 |
| 5 (AM) | 0.09 | 0.01 | 0.39 | 0.01 | 0.75 | 0.01 | 1.60 | 0.01 |
| 5 (PM) | 0.04 | 0.06 | 0.14 | 0.26 | 0.26 | 0.61 | 0.29 | 1.41 |
| 6 | 0.04 | 0.07 | 0.13 | 0.25 | 0.26 | 0.50 | 0.31 | 1.26 |
| 7 | 0.02 | 0.08 | 0.08 | 0.31 | 0.17 | 0.63 | 0.18 | 1.42 |
| 8 | 0.02 | 0.09 | 0.06 | 0.34 | 0.13 | 0.71 | 0.15 | 1.51 |
| 9 | 0.01 | 0.09 | 0.04 | 0.36 | 0.09 | 0.75 | 0.11 | 1.54 |
| 10 | 0.01 | 0.10 | 0.04 | 0.37 | 0.07 | 0.76 | 0.09 | 1.56 |
The final (best) modeled Ca values versus the observed results are plotted in Figure 2 for treatments D, E, F, and G (Fig. 2a, b, c, and d, respectively). As is evident from Figure 2, the model does an excellent job of simulating the actual behavior of 114Cd in this experiment. The best-fit values for ke are 0.21, 0.24, 0.23, and 0.23/d for treatments D, E, F, and G, respectively, whereas ku ranges between 3.4 and 4.6 L/g dry weight/d. Elimination of 114Cd from these hydropsychid larvae thus is independent of the exposure concentration. Also, no trend in ku with increasing 114Cd levels is apparent.
The same approach was used to determine ku and ke values for experiment 1. The results are shown in Figure 3a, b, and c for treatments A, B, and C, respectively. Although the modeled versus observed fit is not as good as that obtained in experiment 2, possibly because dt is 3 d rather than only 1 d, similar to the results found in experiment 2, the best-fit ku values average about 4 L/g dry weight/d and elimination is approximately 0.24/d.
DISCUSSION
The ICP-MS analytical technique is particularly useful because of its ability to measure very low concentrations and discriminate isotopes on the basis of their atomic weight. At Cd concentrations of only 0.1 μg/L, individual animals could be analyzed, and thus an estimate of true interanimal variability was obtained. From Figures Fig. 1.-Fig. 3., the variability in Cd concentrations among animals, within each treatment, evidently is large. For example, in experiment 1 (Fig. 1), even with the elimination of outliers (i.e., those values greater than 3 SDs from the mean), the coefficients of variation (CV = SD/mean × 100) range from 21 to 81% for treatment A, 47 to 78% for treatment B, and 24 to 56% for treatment C. Part of this reflects true biological variability among the animals and part may be due to cannibalism, which has been observed by us and reported by others [2].
Although caddisfly larvae have become important as biomonitors of environmental pollution in situ [24, 28, 34] and also as test organisms in laboratory [2, 4, 23] and field experiments [28, 35], little information is available concerning Cd kinetics in Hydropsyche larvae in particular. Spehar and coworkers [36] exposed Hydropsyche and three other insects to Cd in an intermittent-flow system with a multichannel toxicant injection system for a period of 28 d. Timmermans and coworkers [4] exposed water mites (Limnesia maculata) and caddisfly larvae {Mystacides spp.) to Cd at 0.1 mg/L in a semistatic experimental setup for a period of up to 32 d. From their data, we estimated uptake of Cd by the caddisflies to be approximately 7.5 μg/g dry weight/d (from their Fig. 2), with a ku value of 56/d (see their Table 2).
| Day | Observed Cw (μg/L) | Modeled dCa (μg/g dry wt) | Modeled total Ca (μg/g dry wt) | Observed total Ca (μg/g dry wt) | Observed SD (μg/g dry wt) | (Observed - modeled)2 |
|---|---|---|---|---|---|---|
| 0 | 0 | 0.03 | 0.01 | |||
| 1 | 0.07 | 0.13 | 0.13 | 0.15 | 0.09 | 0.00 |
| 2 | 0.08 | 0.25 | 0.37 | 0.58 | 0.28 | 0.04 |
| 3 | 0.09 | 0.22 | 0.59 | 0.61 | 0.21 | 0.00 |
| 4 | 0.09 | 0.20 | 0.79 | 0.72 | 0.35 | 0.01 |
| 5 | 0.09 | 0.16 | 0.95 | 0.88 | 0.38 | 0.01 |
| 5.25 | 0.04 | 0.01 | 0.96 | |||
| 6 | 0.04 | −0.04 | 0.92 | 0.61 | 0.32 | 0.09 |
| 7 | 0.02 | −0.08 | 0.84 | 0.90 | 0.21 | 0.00 |
| 8 | 0.02 | −0.11 | 0.73 | 0.86 | 0.37 | 0.02 |
| 9 | 0.01 | −0.10 | 0.64 | 0.65 | 0.28 | 0.00 |
| 10 | 0.01 | −0.09 | 0.55 | 0.74 | 0.34 | 0.00 |
| t 0.21 |
- a Cw = 114Cd concentration in the water (0.1 μg/L), ku = 3.7 L/g dry wt/d; kc = 0.21/d; SD = standard deviation.
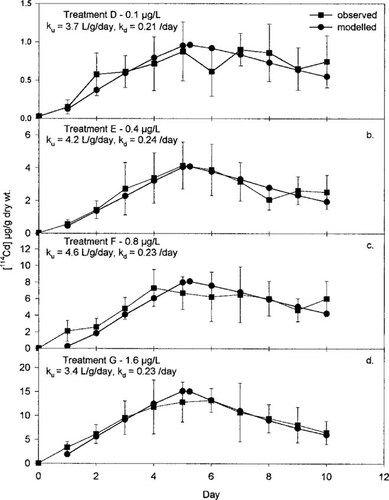
Modeled (circles) from Equation 3 versus observed (squares) 114Cd concentrations in the Hydropsyche larvae (Ca in μg/g dry wt) for the four treatments in experiment 2. Error bars represent ± 1 standard deviation (SD).
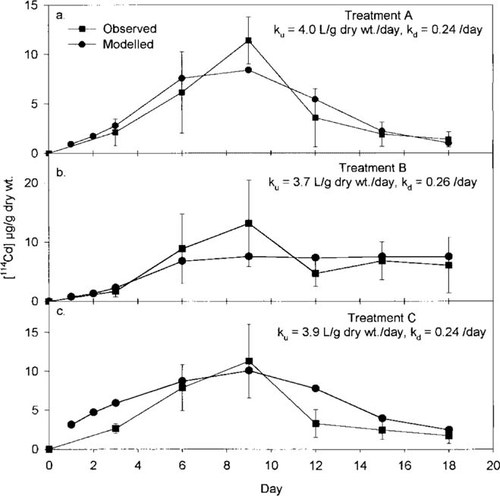
Modeled (circles) from Equation 3 versus observed (squares) 114Cd concentrations in the Hydropsyche larvae (Ca in μg/g dry wt) for the three treatments in experiment 1. Error bars represent ± 1 standard deviation (SD).
More data have been published regarding Cd dynamics in other aquatic insects such as the midge Chironomus riparius and also the mayfly Hexagenia rigida. For example, in a two-week static experiment (with daily renewal of the water) in which C. riparius was exposed to 750 nM (i.e., Cd at 86 μg/L), Postma and coworkers [6] estimated that uptake of Cd by the Chironomus larvae (minus gut contents) was 0.25 to 0.63/d, depending on the population. This corresponds to approximately 25 to 63 L/g dry weight/day if one assumes that 1 ml of water = 1 g wet weight of tissue and that the wet weight to dry weight ratio of the tissue is 10. Elimination rates of the Cd from the larvae ranged between 0.2 and 0.25/d. Bervoets and coworkers [9] reported that uptake of Cd (at 15 or 20°C) by C. riparius larvae, acclimatized at 15 or 20°C, was about 1 nmol/g dry weight/h (i.e., ∼ 3μg/g dry wt/d), which is about double the value of 1.5 μg/g dry weight/d that we obtained. Timmermans and coworkers [37] measured an uptake rate (kλ) of 2,260 ± 420/d and an elimination rate (kd) of 0.33 ± 0.07/d during a four-week experiment in which C. riparius larvae were exposed to Cd at 0.10 mg/L. In each of these studies, uptake rates were generally considerably higher than those reported in the present study. This could simply reflect that fact that different organisms were used or possibly may indicate a problem of surface adsorption at high metal concentrations.
However, concerning Cd loss, in our two experiments the elimination rate constant (∼0.23/d) is very similar to that determined by Postma and coworkers [6] (i.e., 0.2–0.25/d) and also by Timmermans and coworkers [37] (i.e., 0.33 ± 0.07/d) for Chironomus larvae and more than double the value of 0.088 ± 0.028/d reported by Hare and coworkers [11] for Hexagenia nymphs. The fact that these published ke values are reasonably similar to those obtained by us, despite the range in Cd concentrations used in the experiments, supports our observations that ke seems to be independent of water concentration. However, this is in contrast to results reported by others [15, 16], who suggested that uptake and depuration of Cd by aquatic crustaceans may be dependent upon exposure concentration.
In conclusion, the present study demonstrates the ability of the stable isotope tracer technique to measure uptake and loss kinetics at environmentally realistic metal concentrations. The technique also enables the simultaneous measurement of uptake and elimination and may facilitate the labeling of individual compartments (e.g., water, sediment, and food) in the environment. The results support the hypotheses that elimination of Cd by Hydropsyche larvae in the presence of Cd in the water is the same as the depuration of the Cd when no metal is present, and that elimination (ke) is independent of exposure concentration. In the future, application of the technique to a wider range of metals and aquatic organisms would be useful.
Acknowledgements
This work was funded by grants to R.D. Evans and P.M. Welbourn from the Natural Sciences and Engineering Research Council of Canada, Noranda Metallury, and Inco Limited. Richard Hughes conducted many of the ICP-MS analyses and Don Mackay provided assistance with the modeling.




