Immunochemical quantification of metallothioneins in marine mollusks: Characterization of a metal exposure bioindicator
Abstract
A recombinant metallothionein CgMT1, from the Pacific oyster Crassostrea gigas, was synthesized and used as antigen in the development of antibodies and a specific enzyme-linked immunosorbent assay (ELISA). The ELISA showed that rabbit anti-CgMT1 IgG reacted with purified CgMT1 and MTs from other marine bivalves, indicating its suitability as a reagent to quantify MTs and for monitoring of metal contamination in field animals. Application of this assay to tissues excised from metal exposed C. gigas showed that MT induction reached a saturation level in gills that was not observed in digestive gland. Quantification of MTs in gills and digestive gland of field-collected C. gigas showed that the MT level depended on the metal concentrations at the collection sites and may have been influenced by salinity variations at estuarine sites. Oysters from metal-contaminated estuarine sites showed lower MT concentrations than those from nonestuarine contaminated sites.
INTRODUCTION
Metallothioneins (MTs) are ubiquitous, cysteine-rich, low-molecular-weight, metal-binding proteins [1]. These proteins play important roles in the metabolism of essential metals (zinc and copper) as well as toxic heavy metals (cadmium) [2]. The MTs are associated with protection against metal toxicity and increase the capacity for binding these metals [3, 4]. Consequently, MT levels are a major specific indicator of the biological effects of heavy metals on marine organisms. It has been shown that aquatic mollusks have a high capacity to concentrate trace metals from their environment [5, 6]. Because of their sedentary lifestyle and filter-feeding mechanisms, bivalves, such as mussels [7] or oysters [3], are commonly used as indicator organisms for monitoring heavy metal pollution of coastal waters. Evaluation of MT levels in molluscan species also offers a model to study metal accumulation because of the existence of a relationship between the MT level in the organism and metal concentrations in the environment [8, 9].
Many techniques, both direct and indirect, are used to quantify MT or metals in mollusks or in seawater. Metals can be directly measured—by methods with great precision, chemical or electrochemical—in seawater or in biological samples [10-12]. Metals can also be quantified indirectly by measurement of MT levels. Different methods have been used to determine MT concentration in organisms: flow cytometry [9], MT-mRNA levels [8], chromatographic techniques [4, 13], and immunological tests [14, 15].
Immunochemical techniques for the specific and sensitive assaying of MTs are based on antibodies against proteins isolated from mammals, which show little cross-reactivity with similar proteins in fish [16] or invertebrates [17]. Specific antibodies against proteins isolated from a marine invertebrate (the blue mussel Mytilus edulis) were developed by Roesijadi et al. [14]. In the present study, an immunochemical method was developed for quantifying MT using a specific enzyme-linked immunosorbent assay (ELISA). We report here the development of antibodies against a recombinant MT cloned in the Pacific oyster Crassostrea gigas [18] in order to specifically quantify metallothioneins in mollusks.
MATERIALS AND METHODS
Experimental design
Pacific oysters, C. gigas, were collected from a non-metal-contaminated site, La Pointe du Château, Brittany, France (Fig. 1), and maintained for one week in aerated filtered seawater before experimentation. Groups of 30 oysters were exposed to two metals, one essential (Cu2+) and the other nonphysiological (Cd2+). Each metal was applied individually at two concentrations (0.4 and 4 μM) and also in a mixture (0.2 μM each) for 36 d. Each metal was applied from a concentrated solution (100 mM). A group of 30 oysters was maintained in seawater, without metal, as a control. Seawater was changed every day, and oysters were fed with microalgae every 2 d. The metal were reapplied, at the appropriate concentrations, after every water change.
Field study sites
Pacific oysters were collected from four sites chosen for their degree of metal contamination according to the Réseau National d'Observation (National Observation Network, Institut Français de Recherche et d'Exploitation de la Mer, France): La Pointe du Bendy, Le Faou River, Royan, and Oléron (France). Tissue samples were immediately collected from 10 oysters of each sites (Fig. 1).
Protein extraction
On days 0, 6, 12, 20, 30, and 36 of the experiment, the gills and digestive glands from exposed and control oysters (n = 3 for all samples) were collected and homogenized in protein extraction buffer (150 mM NaCl, 10 mM NaH2PO4, 1 mM phenylmethanesulfonyl fluoride, pH = 7.2). Gills and digestive glands from field-collected oysters (n = 10 for each population) were treated as described previously. Samples were then centrifuged at 11,000 g for 10 min at 4°C, and supernatant fractions containing proteins were collected in fresh tubes. Total proteins were quantified by the Dc protein assay kit (Bio-Rad Laboratories, Hercules, CA, USA) using dilutions of bovine serum albumin (Sigma Chemical, St. Louis, MO, USA) as the standard. Optical density was measured at 620 nm using a microplate reader.
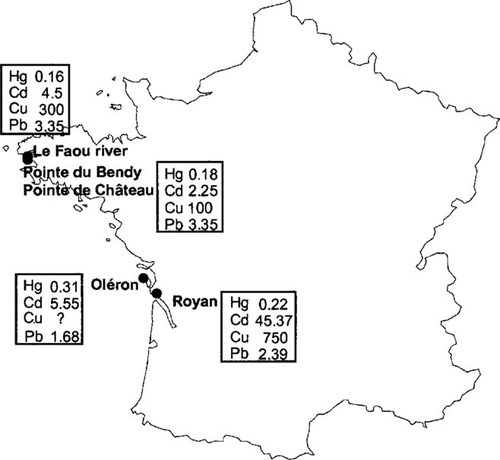
Oyster collection sites. Metal concentrations (mg/kg dry wt tissue) are provided by Réseau National d'Observation (National Observation Network, Institut Français de Recherche et d'Exploitation de la Mer, France).
Metal analysis
Pools of proteins from each samples (100 μg) were mineralized with suprapure nitric acid. Concentrations of cadmium and copper were measured in each protein sample using the potentiometric stripping method [11].
Recombinant DNA manipulations
The metallothionein CgMT1 cDNA [18], cloned in the pGEM-T vector (Promega, Madison, WI, USA), was amplified from the ATG start codon to the last AAA codon to incorporate the recognition sequence for EcoRI. Primer 1 (upstream primer with an EcoRI site) was 5′-CCCCGAATTCATGTCTGATC-3′, and primer 2 (return primer with an EcoRI site) was 5′-GGGGGAATTCTTTCTTACAG-3′. Reaction mixtures included 20 pmol of each primer, 20 ng of CgMT1 cDNA template, 100 μM dNTPs, 2 mM MgCl2, 1X Taq polymerase buffer, and 1 unit of Taq polymerase (Promega) in 50 μl total volume. The mixture was preincubated for 5 min at 94°C followed by amplification for 35 cycles: 94°C for 30 s, 55°C for 30 s, and 72°C for 30 s. The PCR product was gel-purified using the GeneClean III kit (Bio 101, Vista, CA, USA) and digested with EcoRI. The pET20B plasmid was then restricted with EcoRI and gel-purified, and the ends were dephosphorylated. The cDNA was ligated with the plasmid, and the resultant product was used to transform Escherichia coli DH5α competent cells using a protocol described by Hanahan [19]. The transformation mixture was plated on Luria-Bertani (LB) medium (AES Laboratoire, Combourg, France) with agar containing ampicillin at 100 μg/ml, and six colonies were screened by EcoRI restriction digest of alkaline lysis plasmid minipreparations. One positive clone of CgMT1 was then used to transform the protease-deficient E. coli strain BL21 (DE3)pLys competent cells (Promega) according to the manufacturer's instructions. The BL21 (DE3) cells containing CgMT1 cDNA were cultured in Luria-Bertani (LB) medium (AES Laboratoire) containing 100 μg/ml ampicillin at 37°C. Protein expression was induced at OD600 = 1 with the addition of isopropyl β-D-thiogalactoside to 2 mM.
Recombinant CgMT1 purification
The BL21 (DE3) culture was centrifuged at 3,000 g for 10 min at room temperature. The bacterial pellet was suspended in 4 ml of sonication buffer per 20 ml of cell culture (5 mM NaH2PO4, 10 mM Tris-HCl, 100 mM NaCl, pH = 8, and containing 10 mg of lysozyme per ml of 10 mM Tris-HCl, pH = 8). The mixture was incubated 30 min at RT and centrifuged at 9,000 g for 15 min at 4°C. The supernatant was collected and run through a column containing TALON® metal affinity resin (Clontech Laboratories, Palo Alto, CA, USA), following equilibration of the column with sonication buffer. After two washes with sonication buffer, recombinant proteins CgMT1 were eluted with an elution buffer (5 mM NaH2PO4, 2 mM piperazine-N,N′-bis [2-ethanesulfonic acid], 100 mM NaCl, pH = 6). Purified proteins were then precipitated in absolute ethanol and suspended in elution buffer before rabbit injection (see the following discussion) or in phosphate-buffered saline (PBS) (2.5 mM NaH2PO4, 7.5 mM Na2HPO4, 0.145 M NaCl, pH = 7.2) for ELISA (see the following discussion).
Electrophoresis
Recombinant CgMT1 was analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) (15 and 4% total acrylamide for the running and the stacking gel, respectively, with 0.4% bisacrylamide in each) using the buffer system of Laemmli [20].
Production of polyclonal antibodies
Rabbits were immunized two times at one-week intervals with 250 μg of CgMT1 suspended in Freund's complete adjuvant at 1:1.5 ml protein:adjuvant ratio. Booster injections in incomplete Freund's adjuvant (∼250 μg of protein at the same protein-to-adjuvant ratio as mentioned previously; Sigma) were given after two months. On week 9, the rabbits were killed, and blood was collected by heart puncture. Serum was separated by centrifugation (2,000 g for 10 min) and stored at −80°C until used.
Purification and biotin labeling of rabbit IgG
The immunoglobulin-G (IgG) fraction from rabbit serum was purified by affinity chromatography on protein-A column (HiTrap® 1 ml protein-A, Pharmacia, Uppsala, Sweden) using a fast-liquid chromatography system pump (Pharmacia, Uppsala, Sweden) as described by Page and Thorpe [21]. The N-hydroxysuccinimidobiotin (Sigma), dissolved in dimethyl sulfoxide (Sigma) at 1 mg/0.1 ml, was then added to purified IgG at 0.25 mg biotin/mg of IgG. The mixture was incubated at RT for 2 h prior to dialysis against three changes of phosphate-buffered saline (PBS) (pH = 7.2) at 4°C. The conjugates were diluted in 50% glycerol and stored at −20°C until used.
ELISA
Microtiter plates were coated with 0.01, 0.05, 0.1, 0.25, and 0.5 μg of recombinant CgMT1 and 0.5 μg of MT from rabbit liver (Sigma) by overnight incubation at 4°C. Active sites remaining on the plates were blocked with 200 μl per well of PBS (pH = 7.2) containing 0.1% Tween 20 (PBS-T) and 1% bovine serum albumin. After four washes with PBST, 40, 80, 120, and 180 ng of biotin labeled IgG in 100 μl of PBS-T were added to the wells and incubated for 1.5 h at 37°C. All combinations of CgMT1 and labeled IgG doses were tested to determine the appropriate reaction concentrations. A volume of 100 μl per well of ExtrAvidine peroxydase conjugate (Sigma) diluted 1:1000 in PBS-T was added after four washes with PBS-T and incubated for 1.5 h at 37°C. After two washes with PBS-T and three with PBS, 100 μl of o-phenylenediamine (Sigma), at a concentration of 0.06% in phosphatecitrate buffer (0.045 M citric acid, 0.11 M Na2HPO4, pH = 5.45) containing 0.001% H2O2, were added to the well. The reaction was stopped after 20 min with 2 N H2SO4, and OD492 was measured with a microplate reader.
Samples of 20 μg per well of total protein extracted from the digestive gland and gills from experimental and field oysters were then quantified in the same conditions with the appropriate IgG quantity.
Statistical analysis
The rates of metal and MT accumulation during the experiment and interpopulation variability were analyzed by analysis of covariance (α = 0.05) and a chi-square test (α = 0.05), respectively, using CSS Statistica (Statsoft, Tulsa, OK, USA).
RESULTS
Measurements of metal contents
Copper and cadmium concentrations in gills of C. gigas experimentally exposed to Cu2+ and Cd2+ showed significant increases (compared with controls) during the 36 d of the experiment. Increases were time and dose dependent. Copper concentrations in the gills of oysters exposed to 4 or 0.4 μM of Cu2+ increased from 14.6 to 269.4 ng of Cu/g wet-weight tissue (g/wet wt) and from 13.5 to 40.7 ng of Cu g/wet weight, respectively (Fig. 2A). Dosing with 4 or 0.4 μM of Cd2+ (Fig. 2B) resulted in concentration increases of Cd in the gills from 0.4 to 184.3 ng of Cd/g wet weight and from 0.9 to 24.4 ng of Cd/g wet weight, respectively. Measurements of residual Cd and Cu in gills of oysters exposed to Cu2+ or Cd2+ showed constant concentrations of 1.3 ± 0.7 ng of Cd/g wet weight and 25.4 ± 19.8 ng of Cu/g wet weight, respectively, during the experiment for all concentrations tested. Quantification of Cd and Cu concentration in the gills of oysters exposed to a mixture of the two metals showed increases from 0.5 to 31.6 ng of Cd/g wet weight and from 3.1 to 79.5 ng of Cu/g wet weight (Fig. 2C and D).
Electrophoresis
The SDS-PAGE of the recombinant CgMT1 reduced with 2% β-mercaptoethanol resulted in a single band with a molecular weight of about 14 kD (Fig. 3). Immunoblotting analysis of the recombinant protein CgMT1 using an antimetallothionein mouse monoclonal antibody (Stressgen, Victoria, CA, USA) confirmed that CgMT1 was a metallothionein.
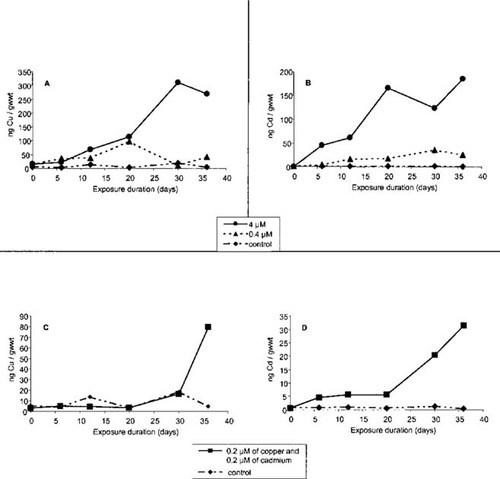
Metal concentrations, determined by potentiometric stripping analysis, in gills of Crassostrea gigas exposed to copper (A), cadmium (B), and a mixture of copper and cadmium (C, D). Each point is the results of the analysis of a pool of proteins (100 μg) from three individuals; gwwt = grams wet weight.
Quantification of metallothioneins by ELISA
Cross-reactivity of the antibodies with recombinant CgMT1 and MTs from four other marine bivalves (Ostrea edulis, M. edulis, Ruditapes decussatus, and Ruditapes philippinarum) was observed in the ELISA. No cross-reactivity was observed with rabbit MT. The ELISA developed in this study was sufficiently sensitive to detect 10 ng of purified CgMT 1 and 92 μg/g wet weight with 80 ng of purified biotin labeled IgG (MTμg =2.9959-OD492 - 0.127, R2 = 0.9973).
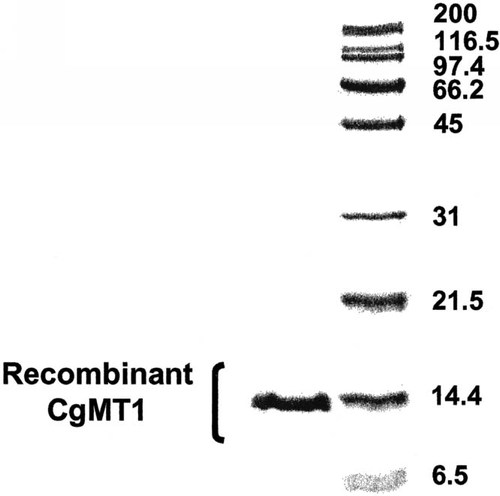
Sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) of recombinant CgMT1. Standards (BioRad): 200 kDa, myosin; 116.5 kDa, β-galactosidase; 97.4 kDa, phosphorylase b; 66.2 kDa, serum albumin; 45 kDa, ovalbumin; 31 kDa, carbonic anhydrase; 21.5 kDa, trypsin inhibitor; 14.4 kDa, lysozyme; 6.5 kDa, aprotinin.
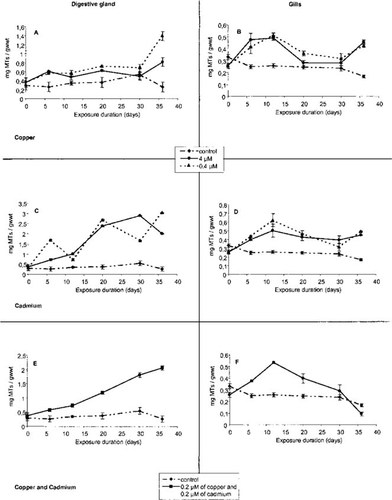
Quantification of metallothioneins by enzyme-linked immunosorbent assay (ELISA) in digestive gland (A, C, E) and gills (B, D, F) of Crassostrea gigas exposed to copper (A, B), cadmium (C, D), and a mixture of copper and cadmium (E, F). Each point is the mean of three individuals; gwwt = grams wet weight.
Application of this assay to protein samples extracted from gills and digestive glands of exposed and control oysters showed that the two organs did not display the same behavior during the experiment. The MT levels increased significantly in the digestive gland for all treatments (Fig. 4). The MT concentrations were significantly lower in the digestive gland of oysters exposed to Cu2+ (Fig. 4A) (4 μM of Cu2+: 0.365–0.814 mg/g wet wt; 0.4 μM of Cu2+: 0.365–1.392 mg/g wet wt) than in animals exposed to Cd2+ (Fig. 4C) (4 μM of Cd: 0.365–2.899 mg/g wet wt; 0.4 μM of Cd: 0.365–3.042 mg/g wet wt) or to a mixture of Cd2+ and Cu2+ (Fig. 4E) (0.365–2.057 mg/g wet wt). Concentrations of MTs in gill tissue reached a maximum value of 0.530 mg/g wet weight at day 12 for all treatments and then decreased slowly during the last 24 d of the experiment (Fig. 4B, D, and F).
Field collections of four populations of C. gigas showed significant differences in MT concentrations between sites. The highest MT concentrations were observed in gills and digestive gland of oysters collected from Oléron (gills: 0.438 ± 0.102 mg/g wet wt; digestive gland: 1.236 ± 0.255 mg/g wet wt) and Royan (gills: 0.407 ± 0.129 mg/g wet wt; digestive gland: 0.637 ± 0.223 mg/g wet wt). The lowest MT concentrations were measured in oysters from Le Faou River (gills: 0.258 ± 0.026 mg/g wet wt; digestive gland: 0.372 ± 0.047 mg/g wet wt) and La Pointe du Bendy (gills: 0.290 ± 0.044 mg/g wet wt; digestive gland: 0.387 ± 0.066 mg/g wet wt), in spite of higher metal at Le Faou than at La Pointe du Bendy.
DISCUSSION
A recombinant metallothionein CgMT1 from a marine bivalve (C. gigas) was synthesized for use as antigen in the development of antibodies and an immunochemical detection procedure. This protein, obtained by induction of CgMT1 cDNA [18] in a protein expression system, was similar in molecular mass and amino acid contents to metallothioneins isolated and described earlier in aquatic organisms [14, 22].
The cross-reactivity of the rabbit anti-CgMT1 IgG with MTs of four other marine bivalves, as well as with C. gigas, supported the suitability of the antibodies as reagents to quantify MTs in samples collected in field sites for monitoring of metal contamination. The ELISA developed in this study allowed the specific and sensitive quantification of MTs in protein extracts from mollusks exposed to metals in the laboratory or naturally contaminated in the field.
Our experiments showed a tissue-specific response: Greater MT concentrations were measured in the digestive gland than in the gills for all experiments and in field-collected oysters, as described earlier [23-25]. These observations were consistent with the involvement of these two tissues in storage and uptake of metals, respectively. The MT synthesis was similar in oysters experimentally dosed with 0.4 and 4 μM Cu2+ and Cd2+ even though the higher concentration produced greater metal accumulations. A similar observation was made in field populations of American oysters, Crassostrea virginica [4].
The MT synthesis increased during the first 12 d in gills of oysters exposed to the Cd2+ and Cu2+ concentrations used. Thereafter, MT concentrations either decreased or remained the same. These results seem to indicate that in gills, the induction of MTs is a rapid process that reaches saturation quickly and at a relatively low concentration compared with the digestive gland. These observations are in agreement with laboratory results obtained by Unger and Roesijadi [8], who showed an increase of MT-mRNA synthesis during the first 15 d of exposure to 0.44 μM of Cd, followed by a decrease. In the field populations examined, higher MT concentrations were found in the gills of oysters where contamination was higher, and these values were close to those measured in the gills of oysters experimentally exposed to different metal concentrations in the laboratory.
Our results indicated that in the digestive gland, the MT concentration increased with time of exposure by a four- to sixfold factor for Cd2+ and the Cu2+-Cd2+ mixture but by only a twofold factor for Cu2+. Similar results have been found in the European flat oyster O. edulis [26] and in C. virginica [27]. In our experiments with Cd2+ and the Cu2+-Cd2+ mixture, no MT saturation was observed, reflecting a high capacity for MT accumulation in the digestive gland. As shown earlier by Raspor and Pavicic [10, 28], MT concentrations in the digestive gland appear to be a good indicator of long-term metal exposure.
The differential accumulation rates and final concentrations of MT in the digestive gland and gills could be explained by differences in the internal turnover rate of metals in the two tissues [3, 29]. Release of Cd, as MTs were degraded, induced synthesis of new proteins, to which the metal became re-sequestered and may explain the slow rate of Cd elimination from mollusks [3, 29]. Moreover, measurements of residual Cd, which has a biological half-life in C. gigas gills of 60 d [30], in oysters exposed to Cu2+ showed constant concentrations of cadmium (1.3 ± 0.7 ng/g wet wt) during experiments. The increased rate of accumulation of Cu did not affect the retention of Cd by the oysters [6]. It has also been demonstrated that the relationship between MT induction and metal bioaccumulation is not linear [3, 31], suggesting the existence of other mechanisms for metal sequestration [32]. Roesijadi [4] demonstrated that in more highly contaminated sites, metals, and particularly Cd, was partitioned into noncytosolic compartments, including phosphate granules [33, 34], to a greater degree than in other sites.
In field populations, we observed that MT levels in the digestive gland of oysters increased in polluted sites; however, as in the gills, the highest MT concentration was not found in the most contaminated population. It is also known that Hg and Cd readily displace Zn and Cu from MTs, which serve as the intracellular sink for these essential elements and which are cofactors for various enzymes involved in immune functions as well as the metabolism of proteins and nucleic acids. A deficiency of these essential elements could compound the metal-induced disruption of metabolic processes and further diminish the body's capacities for detoxification [35]. The very high concentrations of Cd found at the Royan site could alter the metabolism of oysters, which could be reflected by abnormally low MT concentrations in gills and digestive glands. This phenomenon could also be explained by the location of Royan and Le Faou populations in estuaries. Indeed, it was demonstrated that in the digestive gland of bivalves, MTs are induced in response to bioavailable metal and particularly Cd [28]. The Cd bound to sediments or particles could also become bioavailable periodically in conjunction with changes of salinity because of the direct relationship between salinity and the chemical speciation of this metal [36, 37].
The ELISA developed in this study allowed us to measure specifically and rapidly MT concentrations in tissues from marine mollusks. Immunochemical methods such as ELISA have the advantage of detecting only MTs, unlike polarographic methods, which are not sufficiently selective because they also register the presence of proteins containing SH-groups other than MTs [28]. Other MT quantification methods, such as chromatography, which is based on the estimation of metal contents of the MT fraction [6, 13, 26], are time consuming. Moreover, this method analyzed, in general, the MT contents of a pool of oyster tissues or whole oysters rather than individual oysters or tissues, which precludes the detection of known variability within and between individuals. Finally, the ELISA developed here showed that MT induction was tissue dependent and that higher MT concentrations were measured in digestive gland, as described earlier [25].
Acknowledgements
This research program was supported by the Région Bretagne and the CE FAIR CT98–4129 program Environmental Factors and Shellfish Diseases. The authors are grateful to Claire Quintel and François Lamour for immunization and blood punctures of rabbits. Thanks are also due to Susan Ford for her useful English corrections and to Louis Quiniou for his help with use of the CSS Statistica Statsoft Software.




