Precautions in the use of 110mAg as a tracer of silver metabolism in ecotoxicology: Preferential bioconcentration of 109Cd by trout gills after 110mAg exposure
Abstract
An often overlooked problem in the use of radiotracers is the possibility of isotopic contamination. Commercially available silver 110mAg was used to study silver uptake and depuration in rainbow trout and European eel. Quality control by means of comparative γ and β counting brought our attention to a contamination of the 110mAg stock with 109Cd, which could be seen only because the 109Cd was markedly bioconcentrated by trout gills. The contamination could not be detected in eel gills or in other tissues of both species. The difference between trout and eel gill structure and function is the probable explanation for the marked difference in 109Cd accumulation. This contamination was identified as 109Cd by γ spectroscopy and its origin by transmutation of natural silver as a result of neutron activation is described. Failure to recognize this contamination problem would have resulted in serious misinterpretation of the data set. Guidance for avoiding this problem is given.
INTRODUCTION
The use of radioactive isotopes as tracers in ecotoxicology is a powerful tool. It enables a dynamic study of metal metabolism in vivo with a lower detection limit than most, if not all, nonradioactive assays of metal concentrations. Furthermore, by counting the radioactivity we are able to follow the fate of just the metal we have added, independent of the metal that was there beforehand. The latter can also be achieved with stable isotopes, but in a much more tedious manner. All other assays regard total metal contents, so that any changes will be measured as arithmetic differences, with associated loss of precision.
An often overlooked disadvantage is possible radioactive impurities, because the assumption commonly is made that commercially available radioisotopes are chemically pure. When biology is involved we risk a bioconcentration of even small impurities, which can disturb the final result in a significant manner. These radioactive impurities can occur even after the neutron activation of chemically pure targets, as in the present study.
The present investigation concerns the accumulation of the radioactive silver isotope 110mAg in two freshwater fish species, the rainbow trout (Oncorhynchus mykiss) and the European eel (Anguilla anguilla). The 110mAg is commercially produced at RISOE National Nuclear Research Institute by the neutron activation of natural silver, which is a mixture of 52% 107Ag and 48% 109Ag. Through the remarkable differential bioconcentrative properties of trout gills, we were able to detect that the supposedly pure 110mAg stock contained minute amounts of 109Cd. We subsequently worked out how this 109Cd resulted from the production procedure, and showed by theory that it was unavoidable, and furthermore undetectable, by the traditional quality-control counting procedures used by the manufacturer. What does this minute amount of 109Cd mean to the application of commercially available 110mAg as a radiotracer in fish ecotoxicology?
In the following, we present the use of 110mAg in a study of ambient silver uptake and depuration, covering both the rainbow trout and the European eel. In spite of only 0.16% 109Cd originally occurring in the 110mAg stock solution, results showed that trout gill tissue could contain up to seven times as much actual 109Cd activity as 110mAg activity, and that the 109Cd depuration kinetics were very different from those of 110mAg. In contrast, eel gill tissue showed no such bioconcentration. If we had not detected this 109Cd in the 110mAg stock, thanks to the differential bioconcentrative properties of trout gills, we would have seriously misinterpreted the results.
MATERIALS AND METHODS
Silver of natural composition in the form of 3.96 mg silver wool (36.7 μmol) was irradiated in a flame-sealed quartz-glass ampule in the core center facility of the Danish Reactor 3 (DR3; RISOE Laboratory, Roskilde, Denmark). The nominal fluence densities in this facility are fth = 1.5 × 1018 n/m2/s and fepi = 1.2 × 1017 n/m2/s and the irradiation time was 92.83 d. After irradiation, the target ampoule was broken and the contents were dissolved in hot 4 M HNO3, the solution was evaporated to dryness, and the residue was measured in a calibrated ionization chamber (1383A, National Physics Laboratory, Teddington, Middlesex, UK) and then redissolved in 0.2 M HNO3 to a radioactive concentration of approximately 125 MBq/ml. A sample was taken for γ-spectrometric measurement in a calibrated germanium lithium (Ge-Li) multichannel system to determine the content of 110mAg and to identify possible radioactive impurities. The γ-spectrum revealed only one impurity, 111Ag (t1/2 = 7.5 d). The activity of 110mAg differed only by 2.8% from the activity measured in the ionization chamber. The total activity at the calibration date (1.774 GBq) was taken as the mean of these two determinations.
| Sample type | Treatment | n (samples) | β Activity (cps) | γ Activity (cps; 658 keV) | β:γ (mean ± SEM)a |
|---|---|---|---|---|---|
| Water | 3 | 72 | 3.79 | 19 ± 1 | |
| Trout gills | Depuration, 2 d | 10 | 54 | 0.89 | 73 ± 16 |
| Trout gills | Depuration, 15 d | 10 | 45 | 0.40 | 135 ± 29 |
| Eel gills | Depuration, 2 d | 10 | 32 | 2.08 | 15 ± 1 |
| Eel gills | Depuration, 15 d | 10 | 15.5 | 0.85 | 19 ± 1 |
- a SEM = standard error of the mean.
Fish studies
Eels (45–75 g, mean 60 g) were caught in fyke nets in Roskilde Fjord, Denmark. All eels were at the yellow stage (adult, nonmigrating). Rainbow trout (20–30 g, mean 25 g) were obtained from Reersø Fish Farm, Kalundborg, Denmark. The fish were acclimated to a synthetic softwater medium for 16 days. Water chemistry was as follows: [Na+] = 50 μM, [Cl−] = 1 mM, [Ca2+] = 10 μM, [Mg2+] < 1 μM, pH 7.0. The fish were exposed to 110mAg in the adaptation medium for 24 h under static conditions at 170 KBq/L. During the following 67 d, the fish were depurated by exposure to running synthetic soft water, as during the adaptation process. Subcellular distribution of silver was followed by recording 110mAg radioactivity in cellular fractions separated by differential centrifugation [1].
Measurements of β and γ
The present results are part of a study [1] covering more than 5,000 tissue samples from trout and eel. Because 110mAg is both a β and γ emitter, the original protocol was based on a simple β counting of dried homogenized tissue. Tissue samples were counted on aluminium planchets (6-cm diameter, 10 ml) with a thin window (<1 mg/cm2), low-background, five-unit Geiger-Müller multicounter (RISOE) [2]. We elected to use β counting because of much higher efficiency than γ counting (e.g., β:γ ratio of 15–19 in the uncontaminated samples; Table 1).
The presence of 109Cd was not listed in the assay report of the commercially prepared 110mAg, and therefore the influence of 109Cd in these experiments was not expected. The β measurements were originally just occasionally compared to γ measurements in selected samples by 7 counting at 658 keV. The β activities were counted from the top and the corresponding γ activities of the dried samples were counted from the bottom with a liquid nitrogen-cooled Ge-Li semiconductor. These γ measurements were further calibrated by also measuring three representative trout gill samples, dissolved in fuming HNO3, in the above mentioned absolute calibrated, Ge-Li multichannel system. When 109Cd was revealed as an impurity by the bioselectivity of the trout gills, all the relevant samples were recounted by γ counting at 658 keV (110mAg) and 88 keV (109Cd).
RESULTS AND DISCUSSION
Bioconcentration of 109Cd
Table 1 presents some of the unexpected discrepancies observed between β and γ measurements. Trout gill tissue routinely showed markedly enhanced β:γ ratios, relative to ambient water because of what turned out to be an extra registration by the Geiger-Müller multicounter of significant low-energy 109Cd γ activity at 88 keV. Remarkably, the same did not apply to any other tissue of trout nor to any tissue of the eels including the gills, all of which showed β:γ ratios equivalent to that of the water. Therefore, the phenomenon was limited exclusively to trout gill tissue. Both 110mAg and 109Cd levels were below detection limits in nonexposed eel and trout, regardless of detection method.
Figure 1 illustrates the magnitude of the 109Cd bioconcentration process by trout gills. Figure 1b shows a dominant 109Cd peak in the γ spectrum of a trout gill sample (at day 8 of depuration). In clear contrast, Figure 1a presents a γ spectrum of the original 110mAg stock solution where no 109Cd activity could be detected. In this sample the 109Cd:110mAg ratio was impossible to determine because the 88 keV emission of 109Cd was too small and could not be completely resolved from emissions at other γ lines (background). This applied even with a prolonged counting time of 62 h and a decay period of more than 14 months, to enhance the 109Cd (t1/2 = 462.0 d): 110mAg (t1/2 = 249.8 d) ratio. The result was an upper limit γ 109Cd:110mAg ratio of 0.0019 in the 110mAg stock solution. This value should be compared with the calculated theoretical ratio of 0.0016 (see below).
Source and quantitative estimation of 109Cd and total cadmium in 110mAg stock
Once we had discovered that the 110mAg stock contained 109Cd, we first worked out by theory [3] how this occurred. Neutron capture by 109Ag results in 110mAg, which has a half-life of 249.7 d, and decays to stable 110Cd. An unavoidable consequence of neutron activation of the two naturally occurring silver isotopes (107Ag and 109Ag) is the concomitant neutron capture by 107Ag, resulting in 108Ag, which decays with a half-life of 2.4 min to stable 108Cd. This difference in half-life between 110mAg and 108Ag means that 108Cd accumulates together with 110mAg during neutron activation, thereby enabling a further neutron capture by 108Cd yielding radioactive 109Cd, which has a half-life of 462 d and decays to stable 109Ag. The resulting radioactive product is thus 110mAg, and inevitably contains radioactive 109Cd as the result of a neutron transmutation of the original silver target.
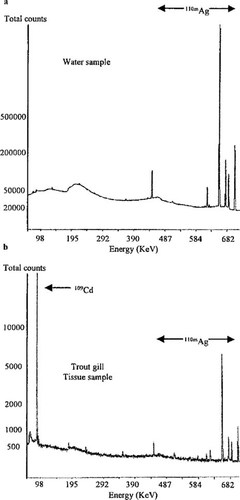
(a) Spectrum of 110mAg stock solution applied to the exposure tanks and (b) spectrum of a randomly selected rainbow trout gill sample after 8 d of depuration. Note the distinct l09Cd peak (88 keV) observed in the gill sample. No such peak was detectable in the stock solution.
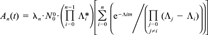
 is the initial number of target nuclides, t is irradiation time, n is number of transmutations, Λ* is formation probability for a certain nuclide, Λ is disappearance probability for a certain nuclide, and i and j are summation and multiplication indices for the formation and disappearance probabilities.
is the initial number of target nuclides, t is irradiation time, n is number of transmutations, Λ* is formation probability for a certain nuclide, Λ is disappearance probability for a certain nuclide, and i and j are summation and multiplication indices for the formation and disappearance probabilities.We calculated the amount of both cadmium and silver isotopes by using the earlier listed fluence densities and irradiation time and nuclear data together with the effective fluence density from the measured amount of 110mAg produced. These calculations were corrected for the shielding effect from the target, assuming this effect to be the same for all the transitions involved.
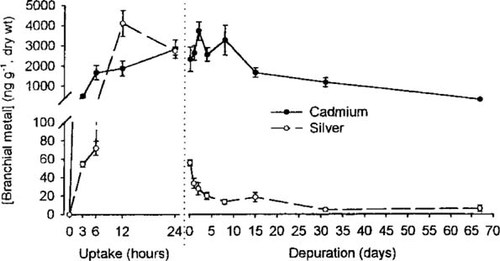
Total cadmium and silver accumulation in rainbow trout gills during 24 h of waterborne exposure, and subsequent depuration for up to 67 d after transfer to clean water. Data were calculated from the respective 109Cd and 110mAg radioactivity of the samples, together with their respective specific activities in the exposure water. Mean ± standard error of mean (n = 5–10 at each time). Note the break on the y-axis.
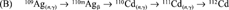
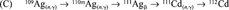
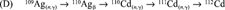
From these production routes, the amount of 111Ag activity was calculated to be 9.0% and the amount of 109Cd activity as calcualted to be 0.16% at time of calibration of the 110mAg stock solution. Adding these four lines (A-D) gives a cadmium composition in atom % of 108Cd = 19.0, 110Cd = 80.7, 111Cd = 0.27, and 112Cd = 0.0015, resulting in a total of 2.63 μmol. Similarly for silver, 107Ag = 54.35, 109Ag = 45.35, and 110mAg = 0.29, resulting in a total of 34.1 μmol (Ag:Cd ratio of 13).
Originally, 36.7 μmol of silver was present. The total amount of measured 110mAg activity was 1,774 MBq, which gives 0.16% = 2.84 MBq of 109Cd, and the following specific activities based on the above calculated values: 110mAg = 52.1 kBq/nmol Ag and 109Cd = 1.08 kBq/nmol Cd.
From the above theoretical analyses, the absolute concentration of cadmium in the exposure water was calculated to be less than 100 ng/L, which is lower than cadmium concentrations previously reported to have no or very little effect on rainbow trout even during chronic exposure in soft water [6]. In contrast, the radioactivity of 109Cd in trout gills could be as much as seven times higher than the corresponding 110mAg radioactivity, equivalent to an approximately 345 times higher absolute mass concentration measured in normalized weight units (ng/g dry wt).
Uptake and depuration of silver and cadmium in the gills
Rainbow trout gills accumulated more silver than eel gills during the initial 24-h 110mAg exposure, but, because of faster depuration of silver from trout gills compared to eel gills, the 110mAg activity levels (at 658 keV) in eel gill samples was higher than in trout gill samples throughout the 67-d depuration period, as illustrated by the selected samples in Table 1. A full comparison of the 110mAg patterns in trout versus eel gills is given by Wood et al. [1]. However, our focus here is on the 110mAg versus 109Cd patterns in trout gills. Eel gills did not take up detectable amounts of 109Cd.
Figure 2 shows a comparison of uptake and depuration patterns of ambient total silver and cadmium in trout gills, with the results calculated based on the above specific activities. Note that no 109Cd or 110mAg was present in samples obtained from nonexposed control fish. Although the uptake of silver peaks at 12 h, the concomitant uptake of cadmium continues to increase up to 24 h. However, the main difference seems to relate to the depuration process. After transfer to clean water, gill-associated silver is rapidly depleted, whereas cadmium persists in the gills in substantial amounts even after 67 d of depuration. The present results show an approximately 4,400 times greater concentration factor of branchial cadmium from the water, relative to silver from the water, in trout gill tissue after 8 d of depuration in vivo. This was due partly to an enhanced relative uptake during the 24-h incubation period (Fig. 2), resulting in comparable levels of branchial cadmium and silver despite the 13-fold difference in exposure concentration. However, more importantly, this was due to an approximately 100-fold slower depuration of cadmium compared to silver (Fig. 2).
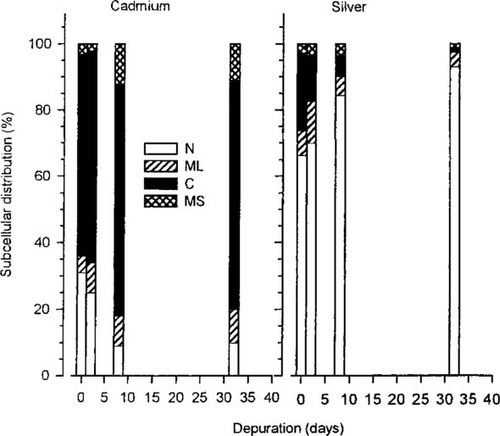
Subcellular distribution (percent of total) of cadmium and silver as calculated from the respective 109Cd and 110mAg radioactivity of the samples in rainbow trout gill samples after 0, 1, 2, 8, and 32 d of depuration. N = nuclear fraction (includes also cell debris, cell surface membranes, and mucus); ML = mitochondria and lysosomes; C = cytosolic fraction; MS = microsomes (n = 5).
The observed slow cadmium depuration is in excellent agreement with the work of Norey et al. [7], who showed very slow depuration from rainbow trout gills after transfer to cadmium-free water (time to half concentration > 60 d). This slow depuration explains why no 109Cd could be detected in any of the internal tissues of the trout; the gills served as a barrier. The very rapid depuration of branchial silver (time to half concentration < 8 h) is in accord with the rapid recovery (a few hours) of branchial Na+ transport function reported by Morgan et al. [8] and is associated with subsequent internal accumulation of silver [1].
The difference in depuration between silver and cadmium is furthermore illustrated in Figure 3, which compares the distribution patterns of the two metals among various trout gill cell components as calculated from the respective distributions of 110mAg and 109Cd. Although 110mAg was concentrated in the nuclear fraction, which includes cell nuclei, cell debris, cell surface membranes, and mucus, 109Cd was concentrated mainly in the cell cytosol. These patterns remained consistent over time, despite the great difference in depuration rates. The subcellular distribution of 109Cd is in close agreement with patterns previously reported in minnows [9], where dominating cytosolic 109Cd was largely associated with metallothioneinlike proteins. In contrast, Wood et al. [1] suggested that 110mAg was cleared rapidly from the gills because it was not retained by the cytosolic fraction; only small amounts remained bound to unidentified nuclear components.
We now know that silver is a Na+ antagonist [10], and that cadmium is a Ca2+ antagonist [11], in terms of gill transport function. Both Na+ and Ca2+ enter the gill via different routes and possibly even different cells (Na+ via pavement cells and Ca2+ via chloride cells); thus, it is not surprising that they exhibit marked differences in branchial handling. Although eel and trout take up Ca2+ via similar pathways [12], the virtual absence of exposed chloride cells in the gills of eels compared to trout [13] could well explain the absence of 109Cd accumulation in eels in response to this short-term exposure.
CONCLUSION
Obviously, one cannot be too careful in interpreting the gross uptake of metal isotopes in experimental ecotoxicology. Only thanks to the differential bioconcentrative properties of the trout gills for 109Cd and 110mAg, in combination with our comparison of γ and β detection techniques for a number of different tissues from two different species were we able to detect the 109Cd in our 110mAg study. This 109Cd in principle is unavoidable, as outlined above. Had we not detected this contamination problem, we would have completely misinterpreted the kinetics of silver loading and depuration in the trout gill. The lesson here is that β counting of 110mAg in biological samples always should be associated with control γ counting. Furthermore, it is essential that such γ counting is restricted to an energy window well above 88 keV so as to ensure no 109Cd detection. We have adopted these precautions in our recent studies [1, 10]. Nevertheless, it should be stressed that once 109Cd has been determined to be absent, direct β counting is by far the most sensitive detection method, because of a much higher counting efficiency, even when the samples are just dried tissues. Scintillation counting that applies appropriate energy windows could avoid 109Cd interference with 110mAg detection.
Acknowledgements
We are grateful for the excellent technical assistance of Alice Kjølhede, Vibeke Jørgensen, and Tove Christensen during our stay at RISOE National Laboratory, Roskilde, Denmark. Financial support was provided by a grant from the Association of University Colleges of Canada Going Global Program, KODAK Canada, Eastman Kodak (USA), and the Natural Sciences and Engineering Research Council of Canada Industrially Oriented Research Program. We thank loe Gorsuch for expediting this project and for helpful comments on the manuscript. CM. Wood is supported by the Canada Research Chair Program. M. Grosell is supported by the Danish Natural Research Council (grant 21–01–0255).




