Effect of molecular structures on the solubility enhancement of hydrophobic organic compounds by environmental amphiphiles
Abstract
Amphiphilic molecules, such as humic substances and surfactants, are known to increase the apparent aqueous solubility of hydrophobic organic compounds (HOCs) in the aqueous phase because of their molecular structures, which consist of hydrophilic and hydrophobic moieties. In this study, we examined the effect of the structures of humic acid and HOCs on the sorption of four polycyclic aromatic hydrocarbons (PAHs) and an organochlorine pesticide, p,p′-DDT, to humic acid. As the number of aromatic rings was increased, the extent of solubility enhancement of PAHs by humic acid was increased. Although p,p′-DDT was more hydrophobic than pyrene in this study, the extent of solubility enhancement of p,p′-DDT by humic acid was lower than that of pyrene because of the molecular structures of the solutes. Anionic surfactants with and without aromatic rings also were studied for comparison, and the dianionic surfactant with two benzene rings exhibited similar results with humic acid, unlike the surfactants without and with one benzene ring. The results from this study indicate that bulky molecules, such as p,p′-DDT sorbed with more difficulty to the aggregates of amphiphiles with larger molecules, such as humic substances and the dianionic surfactants.
INTRODUCTION
Humic substances are ubiquitous, and are found in all soils, sediments, and waters [1-3]. They represent the major fraction of dissolved natural organic matter in aquatic environments and contain various functional groups, such as phenol, carboxyl, ether, acid, and aromatic groups, and are known to have exceptionally high complexing powers. These humic substances are macromolecules because typical average molecular weights of humic substances range in mass from < 1,000 to > 100,000 Da [4-6]. Humic substances that consist of a hydrophilic part and a hydrophobic part separately in a molecule are called amphiphiles [7].
In aqueous solutions, the sorption of hydrophobic organic compounds (HOCs) to humic substances can cause an increase in apparent water solubility, thus affecting the fate and transport of HOCs in both surface-water and groundwater systems [1, 2, 8-10].
Previous studies have shown that the partition coefficients for HOCs (dissolved organic matter partition coefficients [Kdoms]) are dependent on the characteristics of the humic sorbent [1, 2]. Binding intensity of HOCs will be changed as the properties of the humic substances, such as aromaticity, molecular weight, dispersivity, and aliphatic composition, vary [11-13].
Surfactant molecules, which also consist of hydrophilic and hydrophobic moieties, form micelles over a certain aqueous concentration, called the critical micelle concentration (CMC) [14]. The central part of a micelle is hydrophobic, so the aqueous solubility will be enhanced greatly for poorly water-soluble compounds at concentrations higher than CMCs [15].
Humic substances and surfactants as environmental amphiphiles are very similar structurally and functionally. Both sorb HOCs and thus affect the fate and transport of HOCs in the aquatic environment.
Even though the sorption of HOCs to the organic phase can be explained simply with the linear relationship between the octanol-water partition coefficient of HOCs and the organic carbon content of the sorbent, more mechanisms are known to be involved than simple hydrophobic dissolution. Chiou et al. [16] calculated organic carbon partition coefficients (KOCs) for polycyclic aromatic hydrocarbons (PAHs) from the solubility in benzene and water, and indicated that the polar groups in soil organic matter should have a significant effect on the partitioning of PAHs. The aromaticity of humic acid played a significant role in binding of aromatic pollutants, as shown by Gauthier et al. [17]. Rouse et al. [18] also suggested that the existence of aromatic rings in surfactants and HOCs can affect the binding between them.
However, little has been done on the systematic evaluation of the effect of aromaticity in sorbent and sorbate on the sorption of HOCs. Therefore, the objective of this research is to determine the effect of molecular structures on the solubility enhancement of HOCs, especially PAHs, of humic substances. Anionic surfactants with no, one, and two aromatic rings in a monomer also were studied as model amphiphiles whose molecular structures are known. The results from the surfactant experiment will help to understand the results from the humic substance experiment. Overall, the results from this research will provide important information for understanding the fate and transport of HOCs in groundwater as well as in surface water.
Solubility enhancement by environmental amphiphiles
 (1)
(1) (2)
(2)| Surfactant | Molecular structure | Molecular weight | CMCa (mg/L) |
|---|---|---|---|
| Sodium dodecyl sulfate | C12H25SO Na+ Na+ |
288.4 | 2,100 |
| Sodium dodecylbenzene sulfonate | 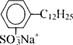 |
348.0 | 1,000 |
| Monoalkylated disulfonated diphenyl oxide | 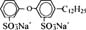 |
575.0 | 113 |
- a CMC = critical micelle concentration.
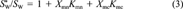 (3)
(3)MATERIALS AND METHODS
Materials
The humic substance chosen for this study was the soil humic acid (reference 1R102H) acquired from the International Humic Substance Society (St. Paul, MN, USA). Generally, humic substances are composed of humic acid, fulvic acid, and humin, among which humic acid is the major component [5]. The reported values of the percent elemental composition from the International Humic Substance Society on a dry and ash-free basis are C: 58.90%, H: 3.38%, O: 33.46%, N: 4.31%, S: 0.41%, and P: 0.42%.
Sodium dodecyl sulfate (SDS) and sodium dodecyl benzene sulfonate (SDDBS) were purchased from Sigma Chemical (St. Louis, MO, USA) and Aldrich (Milwaukee, WI, USA), respectively. Monoalkylated disulfonated diphenyl oxide (MADS-12) was obtained from Dow Chemical (Midland, MI, USA). All three surfactants were used without further purification. The chemical structures and properties of these anionic surfactants are listed in Table 1 [15, 18]. Unlike the single-anion SDS and SDDBS, MADS-12 has two hydrophilic heads per single hydrophobic tail. This structure with more aromatic rings per molecule is somewhat analogous to humic acid than to the other surfactants in this research [18].
Table 2 shows the characteristics of HOCs in this study [20]. The four PAHs were naphthalene, phenanthrene, pyrene, and perylene, of 99, 98, 98, and 99+% purity, respectively, whereas p,p′-DDT was 98% pure. All compounds were purchased from Aldrich and used without any further purification.
Methods
Humic acid was dissolved into 500 ml of distilled water with 1 ml of 0.1 N NaOH [13]. Humic acid solution was added to a glass vial with a nominal volume of 40 ml and a Teflon®-lined septum cap. Then, excess amounts of HOCs were added to each vial. The vials were mixed in a rotary shaker at 250 rpm and 25 ± 0.5°C for 72 h, and the solution subsequently was filtered through a 0.1-μm inorganic membrane filter (Anotop 10 plus, Whatman, Maidstone, UK) mounted on a 10-ml syringe, to remove the undissolved HOC. The concentration of HOC in humic acid solution was measured with a high-performance liquid chromatograph (HPLC; Waters model 515, St. Milford, MT, USA). The analysis was conducted at an ultraviolet wavelength of 254 nm, a flow rate of 1.8 ml/min, a mobile phase of 80% acetonitrile (Fisher Scientific, optima grade, Pittsburgh, PA, USA) and 20% water, and a 3.9 × 300-mm μ-bondapak C18 reverse phase column (Waters). The retention times of humic acid and naphthalene were about 0.6 and 2.6 min, respectively, so no hindrance for the analysis occurred from these.
Surfactant solution at the concentration above the CMC was prepared with distilled water in a 500-ml volumetric flask at 25°C. Then, excess amount of HOCs were added to the solution in the vial as described above. After equilibration and filtration, the concentration of HOC in surfactant solution was measured with an HPLC. The analysis of HOC in anionic surfactant solutions was conducted at a wavelength of 254 nm with a flow rate of 1.0 ml/min and a mobile phase of 100% acetonitrile. The retention times of SDDBS, MADS-12, and naphthalene were about 1.4, 1.1, and 3.4 min, respectively, whereas no specific peak could be found for SDS. All experiments were at least duplicated.
RESULTS AND DISCUSSION
First, the sorption of HOCs to humic acid was studied. Plots of (S*W/SW - 1) for HOCs in this research versus the concentrations of humic acid at 25°C are presented in Figure 1, where the slopes of the fitted linear lines are KHAs. Even though a single value of KHA was assumed for each compound by fitting a linear line, KHA seems to increase somewhat as humic acid concentration increases, especially for perylene. Similar results also were found with surfactants in the study of Park and Jaffe [21], which showed that the partition coefficients of HOCs increased initially with increasing concentrations of micellar surfactants and became constant at higher surfactant concentrations. It is likely that the aggregates of amphiphiles at lower concentrations are less efficient in sorbing HOCs than at higher concentrations.
| Molecular structure | Molecular weight | Water solubility (mg/L) | Log K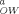 |
|
|---|---|---|---|---|
| Naphthalene | 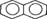 |
128.16 | 3.1–3.4 × 10 (25°C) | 3.37 [22], 3.36 [24, 25] |
| Phenanthrene | 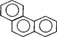 |
178.22 | 1.6 (15°C) | 4.57 [8, 22-24] |
| Pyrene | 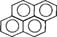 |
202.26 | 1.6 × 10−1 (25°C) | 5.18 [24-26] |
| Perylene | 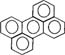 |
252.32 | 4.0 × 10−4 (25°C) | 6.50 [8, 26] |
| p,p′-DDT | 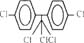 |
354.50 | 3.0 × 10−3 (25°C) | 6.36 [1, 8] |
- a KOW = octanol-water partition coefficient.
By using the results from Figure 1, the relationship between log KOC and log KOW is plotted in Figure 2. For PAHs, the log KOC values increased with increasing number of aromatic rings in a molecule, from naphthalene to perylene.
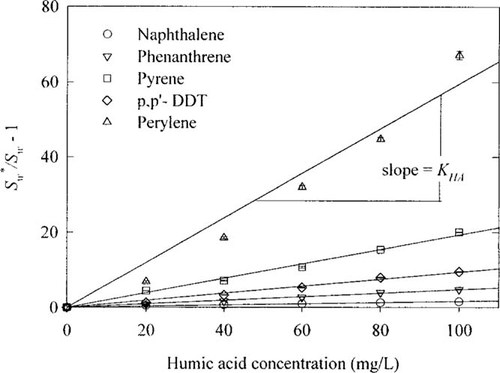
Plot of (S*W/SW - 1) for hydrocarbon organic compounds versus humic acid concentrations, where S*W is the solubility of the solute in a humic substance solution and SW is the aqueous solubility of the solute in pure water.
Interestingly, however, the KOC value of p,p′-DDT is even lower than that of pyrene, although p,p′-DDT, which consists of two aromatic rings and five chlorines, is more hydrophobic than pyrene. Table 3 contains the measured KOC values of five HOCs in humic acid solutions in this research.
Partitioning of HOCs into the organic phase was regarded as the main cause for the sorption of HOCs to humic acid by other researchers [1, 2, 8-10]. However, the relationship between log KOC and log KOW may not be linear for solutes of different molecular structures and configurations, for example, PAHs and DDT. The PAH molecule has a fused-ring structure and is planar, whereas p,p′-DDT has two separate rings and a bulky three-dimensional structure [12]. Therefore, partitioning into the hydrophobic parts of humic acid would be much easier for a PAH molecule than for a p,p′-DDT molecule, if two molecules are similarly lipophilic.
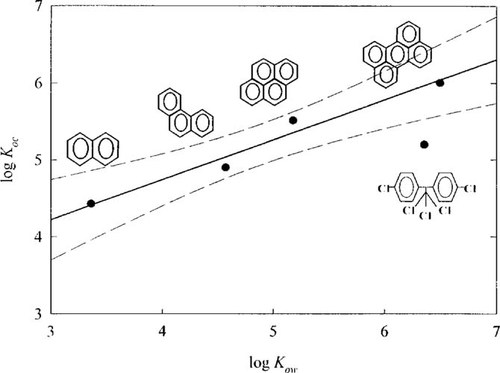
Relationship between log partition coefficient of humic acid (KOC) versus log octanol—water partition coefficient (KOW) of hydrocarbon organic compounds.
K (L/kg) (L/kg) |
Log KOC | |
|---|---|---|
| Naphthalene | 2.67 × 104 | 4.43 |
| Phenanthrene | 4.68 × 104 | 4.70 |
| Pyrene | 3.28 × 105 | 5.52 |
| Perylene | 1.01 × 106 | 6.00 |
| p,p′-DDT | 1.59 × 105 | 5.20 |
- a KOC = partition coefficient of humic acid.
The same experiments were performed with three surfactants and the result is shown in Table 4. Enhancement of the solubilities of five HOCs by three anionic surfactants, where KW is the partition coefficient on a gram weight basis and was derived from the measured Kmc, are shown in Figure 3. A linear relationship was found between log KW and log KOW for four PAH solutes solubilized in SDS and SDDBS solutions. This result is somewhat different from the result in humic acid solution (Fig. 2). However, the value of log KW for p,p′-DDT with MADS-12, which contains two benzene rings in a molecule, was below the 95% confidence interval of the regression line between log KW and log KOW for the PAH (Fig. 3c). The log KW value of p,p′-DDT is lower than that of perylene (Fig. 3), which is similar to the result with humic acid (Fig. 2). Thus, as more aromatic rings exist in the amphiphile molecules, such as humic acid and surfactant, the molecules seem to become less efficient in sorbing HOC with larger and bulky molecules.
The partition coefficients of p,p′-DDT are lower than those of perylene by 15.8% with humic acid and by 9.3% with MADS-12. This can be explained by the molecular sizes of humic acid and MADS-12. The single molecular size of macromolecules such as humic acid is much larger than that of MADS-12 molecules, so humic acid molecules with more aromatic rings are structurally less flexible than those of MADS-12 molecules, which results in inefficient absorption of the larger molecules, such as p,p′-DDT.
The attraction between the aromatic parts of the sorbent and HOCs has been regarded by other researchers as a factor that contributes positively to the sorption of aromatic HOCs [12, 17]. However, the aromatic interaction does not seem to be conspicuous in this research. If the contribution from the benzene ring to the sorption of HOCs in micelles was important, the log KW values with MADS-12 should be larger than those with SDS and SDDBS for PAHs. The log KW values for PAHs are similar for three surfactants, so the contribution from the benzene ring to the sorption of HOCs seems to be minimal. The log KW values with MADS-12 are found to decrease somewhat in respect to those with SDS and SDDBS, where the reduction seems to increase with increasing solute size. This suggests that the micelles formed by MADS-12, which has a bulky polar head, are less efficient for solute solubilization, because the micelle core in this case may have a less dense partitioning medium. This effect seems to be magnified for p,p′-DDT, relative to PAHs, because p,p′-DDT has a bulky molecular structure and contact with the micelle core is likely to be reduced because of this.
| Surfactant | K (L/kg) (L/kg) |
Log KW | |
|---|---|---|---|
| Naphthalene | SDS | 1.53 × 103 | 3.18 |
| SDDBS | 1.74 × 103 | 3.24 | |
| MADS-12 | 1.02 × 103 | 3.01 | |
| Phenanthrene | SDS | 9.33 × 103 | 3.97 |
| SDDBS | 1.05 × 104 | 4.02 | |
| MADS-12 | 1.02 × 104 | 4.01 | |
| Pyrene | SDS | 3.31 × 104 | 4.52 |
| SDDBS | 3.80 × 104 | 4.58 | |
| MADS-12 | 3.55 × 104 | 4.55 | |
| p,p′-DDT | SDS | 6.76 × 105 | 5.83 |
| SDDBS | 6.92 × 105 | 5.84 | |
| MADS-12 | 1.51 × 105 | 5.18 | |
| Perylene | SDS | 4.37 × 105 | 5.64 |
| SDDBS | 5.25 × 105 | 5.72 | |
| MADS-12 | 5.13 × 105 | 5.71 |
- a KW = partition coefficient of surfactants; SDS: sodium dodecyl sulfate; SDDBS = sodium dodecylbenzene sulfonate; MADS-12 = monoalkylated disulfonated diphenyl oxide.
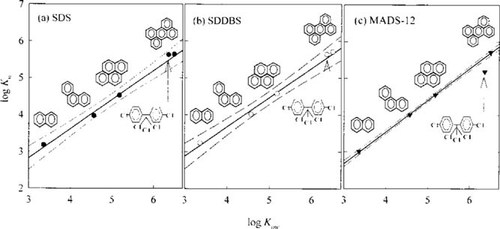
Relationship between log partition coefficient of three anionic surfactants (KW) versus log octanol-water partition coefficient (KOW) of hydrocarbon organic compounds. (a) Sodium dodecyl sulfate (SDS); (b) sodium dodecylbenzene sulfonate (SDDBS); and (c) monoalkylated disulfonated diphenyl oxide (MADS-12).
In summary, enhancement of solubilities of four PAHs and bulky molecules such as p,p′-DDT by humic acid was studied in this research. Three different anionic surfactants, which, unlike humic acid, are amphiphilic and contain known molecular structures, were used for better understanding the sorption behavior to humic acid, the exact molecular structure of which is difficult to define. Surfactant and humic acid molecules may be structurally quite different, because the hydrophobic parts of surfactant micelles and humic acid are mainly composed of long alkyl chains and of aromatic rings, respectively. However, the approach in this study seems reasonable because surfactants and humic acid commonly are amphiphilic and act as sorbents for HOCs in water. The results from this study indicate that sorption of HOCs can be determined by their molecular structures as well as their hydrophobicity.
Acknowledgements
The funding for this research was provided by the National Research Laboratory Program of the Korean Ministry of Science and Technology. We are grateful to Lisa Quencer from Dow Chemical for the donation of MADS-12, and to Cary Chiou from the U.S. Geological Survey for helpful advice.




