Chemical and biological availability of sediment-sorbed benzo[a]pyrene and hexachlorobiphenyl
Abstract
This study examined the chemical and biological availability of two nonpolar organic compounds, benzo[a]pyrene (BaP) and hexachlorobiphenyl (HCBP), from a spiked sediment that was aged for varying amounts of time. Chemical availability was evaluated using four different solvent combinations to extract chemicals from the sediment. The extractability of BaP and HCBP from sediment using traditional solvents was then compared to the transfer efficiency (TE) of a benthic invertebrate (Lumbriculus variegatus) to relate chemical extractability to bioavailability in the organisms. Results indicated that water was the solvent that best approximated bioavailability for BaP, whereas comparisons for HCBP were inappropriate, because TE values exceeded 100%. The inability to obtain a reasonable TE estimate for HCBP was most likely due to the fact that the oligochaetes received a major portion of their uptake from interstitial water instead of ingestion of sediment particles, which invalidated an important assumption of the TE model. Overall, the results of this study indicate that exhaustive chemical extractions may be an inaccurate representation of the bioavailable fractions for some contaminants.
INTRODUCTION
Upon entering the aquatic environment, hydrophobic contaminants such as polycyclic aromatic hydrocarbons (PAHs) and polychlorinated biphenyls (PCBs) can become sorbed to suspended material in the water column and be deposited on the sediment surface. These hydrophobic compounds are expected to remain sorbed to the sediment, bioaccumulate in aquatic organisms, and possibly biomagnify through the aquatic food web. Therefore, the availability of these sediment-sorbed compounds to benthic invertebrates is an important environmental concern.
Estimates of the bioavailable fraction of hydrophobic contaminants in sediments often depend on measurements of contaminant concentrations in bulk sediment samples using single or a series of vigorous chemical extractions [1-3]. However, these types of techniques may be limited in their usefulness for predicting bioavailable fractions of the contaminants because chemical extractions may overestimate the quantity of chemical that is bioavailable. For example, evidence exists that suggests that the amount of chemical that is available to mammals [4], terrestrial invertebrates [2, 5], marine invertebrates [6], and plants [7] is less than that obtained by vigorous chemical extractions. This relationship between chemical and biological availability is of particular importance, because environmental regulations are often based on the concentrations of contaminants determined by exhaustive chemical extractions, which may not necessarily reflect the bioavailabile fraction of those contaminants.
The purpose of this study was to evaluate the chemical and biological availability of two hydrophobic compounds, benzo[a]pyrene (BaP) and 2,2′,4,4′,5,5′-hexachlorobiphenyl (HCBP) in spiked sediment that was aged for varying amounts of time. These chemicals were chosen because of their similar lipophilicity (BaP, log Kow 6.02; HCBP, log Kow 6.7), the large body of literature available on these compounds, and because they represent two different classes of compounds (PAH and PCB). The chemical extractability of BaP and HCBP using traditional chemical solvents was compared to the transfer efficiency (TE) of an aquatic invertebrate (Lumbriculus variegatus). In addition, passive sampling devices (PSDs) were used to measure the freely dissolved fraction of the chemical in the sediment.
MATERIALS AND METHODS
Test organism
Adult L. variegatus were used in all tests and were reared onsite from cultures originally obtained from the U.S. Environmental Protection Agency, Environmental Research Laboratory, Duluth, Minnesota. Animals were cultured in accordance with standard operating procedures for static cultures [8].
Chemicals
Radiolabeled 3H-BaP (specific activity 84 Ci/mmol, 732 disintegrations per minute [dpm]/pg) and 14C-HCBP (specific activity 12.6 mCi/mmol, 7.68 × 10−2 dpm/pg) were purchased from Amersham International (Arlington Heights, IL, USA) and Sigma Chemical Company (St. Louis, MO, USA), respectively. Radiopurity of the chemicals was determined by thin-layer chromatography and liquid scintillation counting, and purity of both BaP and HCBP was > 95%. All procedures were performed under red light to minimize the photodegra-dation of BaP.
Sediment spiking
Florissant sediment was collected from Florissant, Missouri, USA, and has been characterized as a fine-grained silt loam soil with a total organic carbon content of approximately 1% [9]. It has been used as a reference material in previous sediment assays [10, 11]. Before use in the current experiments, the sediment was sieved to a particle size ≤ 1 mm, and then spiked in bulk with both BaP and HCBP in 4-L glass containers. Both chemicals were dissolved in a minimal amount (<0.5 ml) of acetone carrier, and added dropwise to a 60% sediment-water slurry. The slurry was stirred continuously for 4 h at 20°C, which allowed for volatilization of the acetone carrier. Separately spiked sediments were combined and remixed to assure an even distribution of the radiolabeled compounds. The concentrations of BaP and HCBP were 0.099 ± 0.003 and 66.4 ± 0.3 μg/kg dry weight (n = 5) sediment, respectively. Enough sediment was spiked at one time to conduct all experiments. Sediments were held in glass containers and stored in a Sherer Dual-Jet Environmental Chamber (Marshall, MI, USA) in darkness at 4°C until use.
Availability experiments
Availability experiments were performed on spiked sediment that had been allowed to age for 1, 30, 60, 90, and 120 d. Before the start of each experiment, sediments were remixed, and extractions were performed on an aliquot of bulk sediment. Bioavailability studies were then conducted with L. variegatus and PSDs using additional aliquots of the aged sediment.
Chemical extractions of the sediments were performed using sonication with four solvents including reconstituted moderately hard water, methanol, octanol, and acetonitrile. These data were then compared to a soxhlet extraction using dichloromethane:acetone (1:1, v/v), which served as a reference extraction procedure. The soxhlet procedure was used as a reference because it represents a vigorous extraction technique. At each sampling time, spiked sediment (300 mg wet weight) samples were taken for determination of dry to wet ratios and contaminant concentrations. The dry to wet ratios were obtained by weighing a wet sediment sample and drying it at 60°C to a constant weight. Contaminant concentrations in sediment were determined by placing approximately 300 mg of wet sediment (three replicates for each solvent system) into 50-ml centrifuge tubes and extracting with the various solvent systems. A 24-h soxhlet extraction was used for the dichloromethane:acetone system with the resultant extract reduced to 1 ml using a Buchi rotary evaporator (Brinkmann Instruments, Westbury, NY, USA). The 1-ml sample was then placed into 10 ml of scintillation cocktail (ScintiSafe Plus 50%, Fisher Chemical, Fair Lawn, NJ, USA) and analyzed for radioactivity on a Packard 1900TR Liquid Scintillation Analyzer (Meridian, CT, USA). Sample counts were corrected for background and quench using the external standards ratio method. Sediment extractions for the other solvent systems were performed by sonicating the samples for 60 s in pulse mode using a Tekmar sonic disruptor (model TM501, Cincinnati, OH, USA). Three 10-ml washes were conducted for each extraction. After each wash, samples were centrifuged (6,000 g), the washes were combined, and the total volume was recorded. The centrifugation step was designed to remove sediment particles from the solvents, not necessarily dissolved organic carbon. A 1-ml sample was then removed and placed into 10 ml of scintillation cocktail, and radioactivity levels of the samples were determined by liquid scintillation counting.
To determine the bioavailability of the two chemicals in the sediment, L. variegatus and PSDs were added together to a set of prepared beakers. Spiked sediment (approximately 250 g wet weight) that had been aged for predetermined amounts of time was transferred to each tall-form 600-ml glass beaker. The PSDs were then placed into the sediment, and 400 ml of moderately hard water was slowly added to the beakers. Care was taken to reduce resuspension of the sediment into the water column. Finally, 30 adult oligochaetes (approximately 160 mg wet weight) were introduced into each beaker.
The PSDs were prepared from polyethylene film having pores generally ∼10 Å in diameter, which allowed smaller freely dissolved lipophilic compounds to pass, but restricted compounds associated with particulate or dissolved organic carbon. The PSDs used in this study included only the polyethylene film; no lipids were added to the tubing. The PSD tubing (thin-walled, 80–100 μm, Brentwood Plastics, St. Louis, MO, USA) was cut into strips 2.5 cm wide × 11.0 cm long, and made into a ring using a plastic sealer. The PSD tubing was initially soaked in cyclohexane and dried with high-grade nitrogen before use in the experiments.
Sampling times for the uptake tests were 0, 6, 12, 24, 48, 96, and 168 h. At each sampling time, 1 ml of overlying water was removed from each of three replicate beakers, and added to 10 ml of scintillation cocktail for radioactivity measurement. Water samples were taken to determine overlying water concentrations and found to be consistently below detection limits. Test animals and the PSDs were collected from the sediment using a number 230 (63-μm) sieve, rinsed with deionized water, blotted dry, and weighed to the nearest 0.1 mg on a Sartorius H51 analytical balance (Westbury, NY, USA). Lumbriculus variegatus and PSD tubing were then transferred to 10 ml of scintillation cocktail and extracted using sonication for 60 s in pulse mode. After sonication, a 24-h waiting period was used to ensure ample time for radioactivity extraction into the scintillation cocktail before radioactivity measurement via liquid scintillation counting using the external standards method. Samples were stored in darkness during this waiting period. All experiments were preformed in a Precision Scientific Environmental Chamber (Chicago, IL, USA) maintained at 20°C with a 16:8 h light:dark photoperiod.
Lumbriculus variegatus were not fed additional food during the 7-d accumulation period; therefore, they relied solely on the sediment as their food source. This eliminated the possibility of selective feeding on uncontaminated substrate that could potentially affect uptake of the chemicals by the organisms. Preliminary tests demonstrated that L. variegatus did not significantly change weight after a 7-d exposure, suggesting that additional feeding was not required.
Feeding rates
The purpose of this experiment was to determine feeding rates for L. variegatus in the Florrisant sediment. Estimates of feeding activity were determined using egestion rates, and were needed for the TE calculations. Egestion rates represent a good surrogate for feeding, because assimilation efficiencies are relatively low for deposit-feeding invertebrates [12]. The design consisted of six replicate containers (50-ml centrifuge tubes) to which 20 g (wet weight) of undosed sediment, approximately 5 g of washed quartz sand, and 20 ml of moderately hard reconstituted freshwater were added. The sediment was dispensed into the containers and overlying water was carefully added to minimize the resuspension of sediment. The test sediment was allowed to settle for 24 h before the addition of the washed sand. The sand was evenly distributed over the sediment surface to a 5-mm thickness. A single oligochaete was added to each container and fecal material was collected at 0, 24, 48, 72, 96, and 168 h from the surface of the quartz sand by pipette [12, 13]. Lumbriculus variegatus burrow headfirst into the sediment, but leave the caudal end projecting above the sediment-water interface. Fecal material is then deposited on the surface of the sand and is easily collected. The overlying water that was removed during fecal collection was replaced daily. The collected fecal material was filtered through a preweighed glass-fiber filter and then dried at 60°C to a constant weight. The samples were allowed to cool to room temperature and weighed to the nearest 0.01 mg using a Cahn C-33 microbalance (Cahn Instruments, Cerritos, CA, USA). At the end of the 7-d test, animals were sieved from the sediment and wet and dry weights were determined. Replicate feeding experiments were conducted and mean values were used in all calculations.
Data analysis
Percent chemical extractability from the sediment was calculated by comparing the amount of chemical extracted using each solvent system to the total compound extracted by soxhlet in the 1-d aged sediment. Biological availability was determined by calculating the TE obtained from the ratio of the mass of contaminant accumulated in the organism divided by the mass of contaminant to which the organism is exposed.
 (1)
(1) (2)
(2)An important assumption of the TE model is that the main route of uptake into the organism must be through ingestion. If uptake through other sources, such as the aqueous phase, is significant, then the TE model may be inappropriate for those compounds, because the equation will not reflect the total mass of contaminant to which the organism is exposed.
Note that the feeding rates for L. variegatus were not directly determined for 6- and 12-h time points, thus allowing the organisms adequate time for burrowing and feeding upon introduction into the test chambers before sampling. The feeding rates for the 6- and 12-h time points were determined by extrapolation of the 24-, 48-, and 72-h data using regression analysis.
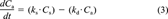 (3)
(3)Statistics
Percent chemical extractability of each individual solvent system and the TE values were compared across aging periods by a one-way analysis of variance using square root- and arcsine-transformed data. In addition, individual solvent systems were analyzed using regression analysis. Uptake data for L. variegatus and PSDs were analyzed using a z test statistic. All statistical differences were determined at the 0.05 significance level [18].
RESULTS
Chemical extractability
Both BaP and HCBP were extracted from the sediment using four separate solvents. Percent extractability for both compounds was calculated by comparing all extractions to the total compound measured in the 1-d sediment as determined by soxhlet extraction. The soxhlet extraction was used as a reference, because it represented the most exhaustive chemical extraction used. The mean percent extractability of each solvent for BaP over time is presented in Figure 1. No significant differences were found in BaP concentrations in the bulk sediment with respect to aging time as determined by soxhlet extraction (Fig. 1). The BaP exhibited a broad range of extractability to the selected solvent systems, with methanol obtaining the greatest recoveries (84.9–92.5%) and water yielding the lowest recoveries (24.7–30.4%). The extractability did not change significantly for the other solvent systems throughout the study, with the exception of octanol. Octanol recoveries declined significantly between the 1- and 60-d aged sediments (df = 4, F = 23.14, p < 0.0001), then remained relatively constant from 60 to 120 d.
Similarly to BaP, the bulk sediment concentration of HCBP, measured by soxhlet extraction, remained constant with only an 8% reduction in recovery over the 120-d aging period. The HCBP extractability also remained relatively constant over the course of the study for all other solvents (Fig. 2). The greatest recoveries for HCBP were obtained from the octanol extractions (82.7–94%), whereas water had the lowest recoveries (13.9–28.6%). Note for both BaP and HCBP, the extractability of both compounds using water was higher than expected. This is most likely due to compounds being attached to dissolved organic carbon that was not removed by centrifugation. Extractability of HCBP by the remaining solvent systems was not as solvent-specific as that of BaP (Fig. 2).
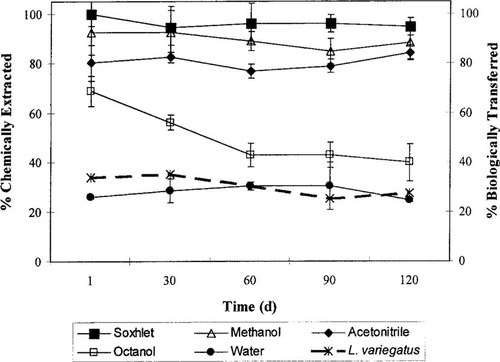
Effect of sediment aging on the chemical extractability of benzo[a]pyrene (BaP) using traditional solvents (y-axis) and the transfer efficiency of BaP from sediment using Lumbriculus variegatus (second y-axis). Error bars represent ± 1 standard deviation.
Uptake
Uptake of BaP and HCBP by L. variegatus was determined using toxicokinetic tests (Fig. 3), and a summary of ks values for L. variegatus is presented in Table 1. The uptake of BaP in L. variegatus was not significantly different between organisms that were exposed to sediment that was aged from 1 to 30 d; however, a significant decline occurred in L. variegatus uptake for both compounds in sediment that was aged at least 90 d (Table 1). Similarly, the uptake results from the PSDs indicate a gradual reduction in BaP and HCBP levels in the interstitial water with each successive aging period (Table 1 and Fig. 4).
Transfer efficiency
Bioavailability was determined by calculating the TE of the chemicals from sediments into L. variegatus using Equation 2. To calculate TE values, the feeding rates for L. variegatus needed to be determined. The feeding rate for this particular sediment was determined in two separate 7-d experiments (Fig. 5). Experiments were performed three weeks apart and the overall feeding rate was represented by the mean of the two experiments. Feeding activity was expressed as an egestion rate, with a mean L. variegatus value of 0.214 g dry feces/g dry worm/h being obtained.
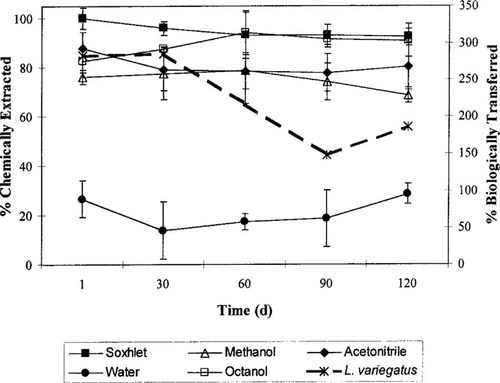
Effect of sediment aging on the chemical extractability of hexachlorobiphenyl (HCBP) using traditional solvents (y-axis) and the transfer efficiency of HCBP from sediment using Lumbriculus variegatus (second y-axis). Note second y-axis exceeds 100% because a significant amount of the HCBP uptake was from sources other than ingestion. Error bars represent ± 1 standard deviation.
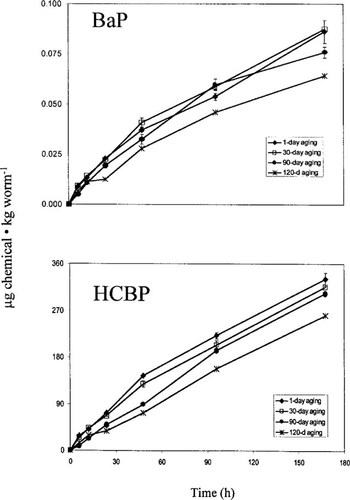
Effect of 1, 30, 90, and 120 d of sediment aging on the accumulation of benzo[a]pyrene (BaP) and hexachlorobiphenyl (HCBP) by Lumbriculus variegatus. Error bars represent ± 1 standard deviation.
Transfer efficiency values for each chemical were calculated for the time course during the uptake exposures and for the various aging periods (Table 2). The reduction in TE values over the time course of each aging period was due to a slight increase in the feeding rates. Therefore, mean TE values have been reported. The mean TE values for BaP did not change significantly over the 120-d aging period, ranging from 35 to 25%. Statistical comparisons of mean TE values for HCBP were not performed because TE values exceeded 100%; however, an obvious declining trend in TE values was noted. The TE values for HCBP dropped from 286 to 147%. The TE value for HCBP was much greater than the TE for BaP; however, it should be noted that TE values should not exceed 100% if ingestion is the only route of uptake.
| Aging (d) | ||||
|---|---|---|---|---|
| 1 | 30 | 90 | 120 | |
| Lumbriculusa | ||||
| BaPa | 0.0538A | 0.0561A | 0.0400B | 0.0434B |
| (0.0039) | (0.0036) | (0.0025) | (0.0029) | |
| HCBP | 0.4495A | 0.4533A | 0.2336C | 0.2948B |
| (0.0397) | (0.0237) | (0.0143) | (0.0098) | |
| PSDd | ||||
| BaP | 0.0063A | 0.0054A,B | 0.0045B | 0.0035C |
| (0.0006) | (0.0004) | (0.0003) | (0.0003) | |
| HCBP | 0.0350A | 0.0277A,B | 0.0218B | 0.0165C |
| (0.0041) | (0.0029) | (0.0016) | (0.0015) | |
- a Units are g dry sediment/g dry organism/h.
- b BaP = benzo[a]pyrene.
- c HCBP = hexachlorobiphenyl.
- d Units are g dry sediment/g wet PSD/h.
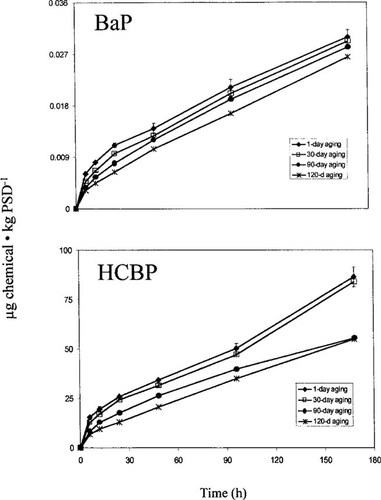
Effect of 1, 30, 90, and 120 d of sediment aging on the accumulation of benzo[a]pyrene (BaP) and hexachlorobiphenyl (HCBP) by passive sampling devices (PSDs). Error bars represent ± 1 standard deviation.
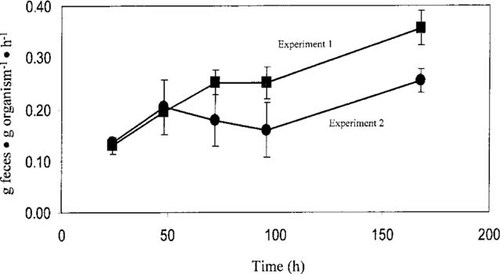
The mean feeding rates of Lumbriculus variegatus determined for Florissant sediment during two separate 7-d experiments. Experiments were performed three weeks apart and the overall feeding rate was represented by the mean of the two experiments. The error bars represent ± 1 standard deviation.
DISCUSSION
Exhaustive chemical extractions are often used in defining sediment quality criteria, because chemical concentrations are more easily quantified than are other measures of bioavailability [5, 19]. This comparison is justified, because reductions in bioavailabilty for many compounds have been suggested to be the result of a decrease in chemical extractability [4, 5]. However, reliance on exhaustive chemical extractions in establishing guidelines could overestimate bioavailability. This study demonstrates that the bioavailabilities of BaP and HCBP are not necessarily correlated with chemical availability. The bioavailability of the two compounds was higher in the shortterm aging periods compared to the longer-term aging periods. The TE values for BaP dropped slightly from 35 to 27%, whereas the TE values for HCBP dropped from 286 to 186%. Conversely, the chemical extractability of BaP and HCBP was generally unchanged with respect to sediment aging for most solvent systems. These findings are in agreement with the previous study by Landrum [20], which also demonstrated that the chemical extractability of BaP and HCBP did not change significantly over aging time, whereas bioavailability, as measured by uptake clearance, of sediment-sorbed contaminants declined. In a study comparing acute toxicity to chemical extractability, Roberston and Alexander [2] found a significant reduction in mortality of houseflies (Musca domestica), fruit flies (Drosophila melanogaster), and German cockroaches (Blattella germanica) exposed to DDT- and dieldrin-amended soils that were aged for 30 d, whereas the chemically extracted amounts declined only 15% for DDT and <10% for dieldrin. Collectively, these studies indicate that data obtained from vigorous chemical extractions do not always correlate well with the concentrations that are biologically available.
| Aging (d) | ||||
|---|---|---|---|---|
| Sampling time (h) | 1 | 30 | 90 | 120 |
| BaP | ||||
| 6 | 48.25 | 50.30 | 35.87 | 38.93 |
| 12 | 44.21 | 46.08 | 32.87 | 35.67 |
| 24 | 40.40 | 42.11 | 30.04 | 32.60 |
| 48 | 26.90 | 28.04 | 20.00 | 21.70 |
| 96 | 26.19 | 27.30 | 19.47 | 21.13 |
| 168 | 17.60 | 18.34 | 13.08 | 14.20 |
| Mean | 33.92 | 35.36 | 25.22 | 27.37 |
| SD | 12.07 | 12.58 | 8.98 | 9.74 |
| HCBP | ||||
| 6 | 403.14 | 406.54 | 209.51 | 264.39 |
| 12 | 369.35 | 372.47 | 191.94 | 242.23 |
| 24 | 337.52 | 340.38 | 175.40 | 221.36 |
| 48 | 224.74 | 226.64 | 116.79 | 147.39 |
| 96 | 218.80 | 220.65 | 113.71 | 143.50 |
| 168 | 147.02 | 148.27 | 76.40 | 92.42 |
| Mean | 283.43 | 285.83 | 147.30 | 185.88 |
| SD | 100.86 | 101.72 | 52.42 | 66.15 |
Why does bioavailability decrease over aging time of a sediment? In general, uptake of contaminants can come from the freely dissolved fraction of the chemical in the interstitial or overlying water, ingestion of sediment particles, or both. The relative role of each is dependent on partitioning properties of the chemical, the characteristics of the sediment, and the feeding habits of the organism. For example, Lamoureux and Brownawell [21] measured desorption rates of several sediment-sorbed hydrophobic organic contaminants and determined that BaP did not desorb over the time course of their experiments (20 d), whereas the majority of HCBP was desorbed despite the similarity in Kows between the two compounds. As a sediment ages, adsorption of hydrophobic chemicals onto sediment particles increases and desorption rates decrease, which in turn, decreases the fraction of chemical available to organisms in the freely dissolved phase. To measure the freely dissolved fraction of the chemical in the present study, PSD tubing was added to the sediment. The results from the PSD uptake indicated a gradual reduction in BaP and HCBP in the interstitial waters with each successive aging period (Table 1 and Fig. 4). The PSD uptake seemed to track well with uptake by L. variegatus. In other words, decreases in the interstitial water concentration of the compounds corresponded well with a decrease in uptake (ks) for L. variegatus. Therefore, the reductions in L. variegatus uptake as the aging time of the sediment increased may have been due to decreases in the freely dissolved fraction of the chemicals within the sediment matrix.
In terms of ingestion of sediment-bound contaminants, movement of chemicals into inaccessible regions of the organic or mineral matrices (e.g., micropores) is thought to limit the amount of chemical available through the diet. Basically, digestive fluids within organisms may be too weak to extract compounds that are tightly bound to sediment particles. In a study with 1,2-dibromoethane, Steinberg et al. [22] found that weathered 1,2-dibromoethane was highly resistant to microbial degradation, in contrast to freshly added compound. They concluded that the 1,2-dibromoethane was trapped in soil micro-pores because pulverizing the soils helped release the residual compound. Therefore, TEs of the chemicals during digestion also may be decreased as chemicals segregate in sediment particle interstices as a sediment ages.
Transfer efficiency values have been experimentally derived by a number of researchers using a variety of different approaches including using organic carbon as a tracer [10, 23], from the use of feeding rates [23], and dual-label techniques using a nonassimilated tracer [10, 24]. In addition, several review articles have recently been published comparing the different techniques [25, 26]. In this study, TE values were determined for L. variegatus over a period of 120 d using feeding rates. The average TE values for BaP for L. variegatus for the current study (35–25%) relate well with previous measurements. For instance, measures of assimilation efficiency of BaP for L. variegatus have ranged from 0 to 26% [23] and for other deposit-feeding invertebrates estimates range from 5.6 to 60.4% using the above methods [10, 11].
The method for determining TE values of BaP in Equation 2 was inappropriate for HCBP (e.g., TE > 100%). The average TE values presented in this work ranged from 286 to 147%. Similar results were found by Harkey et al. [11], with TE values ranging from 279 to 80% for HCBP. Literature values for the TE of HCBP to other benthic invertebrates from sediments were much lower, ranging from 36 to 52% for Diporeia spp. [23], 15 to 36% for oligochaete worms [24], and 30% for Dreissena polymorpha [27].
The source of error resulting in TE > 100% is either due to misrepresentation of feeding or uptake rates of the organisms. First, the feeding rates determined in this study are comparable with previous measurements for oligochaetes. For example, the mean feeding rate of L. variegatus (0.214 g dry feces/g dry worm/h) for the 7-d exposure in this study compares well with previous measurements for L. variegatus (0.085–0.166 g feces/g worm/h [23]; and 0.443–0.981 g feces/g worm/h [12]) and for a related oligochaete (0.054–0.249 g feces/g worm/h [24]). Therefore, the feeding rates are not considered to be the likely source of error forcing TE > 100%. Thus, the term that is most likely resulting in TE > 100% is the ks value. The concentration within the organism at the end of the exposures was calculated by using estimates of ks values. In a complex media such as sediment, the ks value describes uptake from all sources, not just sediment ingestion [10, 14]. Therefore, for a compound such as HCBP, for which interstitial water has been shown to be a major source of uptake because of the ability of HCBP to readily desorb from sediment particles [14, 18], estimates of ks may significantly overestimate the amount of material accumulated via ingestion. This conclusion is supported in the present study by the elevated HCBP levels in the interstitial water as measured by the PSDs. In addition, the ks values may be inflated, leading to high TE values because the organisms were not gut purged before radioactivity determinations. However, estimates for the predicted influence of gut contents on the measured body residues are not overly large (<20%) for compounds with log Kow > 5 [23, 28].
CONCLUSIONS
The findings of this study demonstrate that the bioavailability of BaP and HCBP are not necessarily correlated with chemical availability. The bioavailability of both compounds was significantly different from the short-term aging periods compared to the longer-term aging periods. On the other hand, the chemical extractability of BaP and HCBP was generally unchanged with respect to sediment aging for most solvent systems. These results suggest that reliance on vigorous chemical extractions when establishing sediment quality criteria may significantly overestimate the bioavailability of these chemicals as well as the environmental risk.
Finally, estimates of TEs, by way of uptake coefficients and feeding rates, work well for compounds where the contaminant contribution from sediment ingestion is much greater than from interstitial water. Such is the case for BaP, where the measured TE relates well with previous estimates. However, the TE of HCBP for L. variegatus, of which a significant portion of uptake seems to be from the water phase, is three to four times greater than in most of the other studies reported in the literature. Future studies should measure desorption rates of the compounds from the sediments and measure feeding rates at different aging times to better determine the factors controlling bioavailability.
Acknowledgements
We wish to thank Peter Landrum, Jussi Kukkonen, Don Weston, and David McDonald for their comments and suggestions on previous versions of the manuscript.




