Environmental risk limits for polychlorinated biphenyls in the Netherlands: Derivation with probabilistic food chain modeling
Abstract
Environmental risk limits (ERLs) for individual congeners of polychlorinated biphenyls (PCB 77, 105, 118, 126, 153, 156, 157, and 169) are derived. After lipid normalization, toxicity data for birds, mammals, and aquatic organisms were converted to equivalent concentrations in soil or sediment organic carbon (OC). Accumulation in the food chain was taken into account. Field-derived data on the environmental fate of PCBs, e.g., biomagnification factors and biota-to-sediment accumulation factors, were used in the calculations. The variability in these data was incorporated by using probabilistic techniques. Parameters that are difficult to measure for these hydrophobic compounds, such as the bioconcentration factor or the sediment/water partition coefficient, were avoided where possible. Probability distributions for various species were combined per congener when statistically appropriate; ERLs were based on the fifth percentile of these combined distributions. Congener patterns occurring in various sediments and invertebrates in The Netherlands were used for determining a mixture ERL for non- and mono-ortho PCBs. The PCB 118 was selected as a guiding congener. If the concentration of PCB 118 is less than 5 μg/kg OC, Dutch ecosystems are assumed to be protected for effects of the whole mixture of non- and mono-ortho-substituted PCBs. Concentrations associated with adverse effects in field studies were comparable to concentrations that would result if all congeners would be present at the ERL level.
INTRODUCTION
Polychlorinated biphenyls (PCBs) have been banned in industrialized countries since the 1980s but still enter the environment by leakage, recycling, transboundary influx via the major rivers, and long-range atmospheric transport [1]. Relatively high concentrations can still be encountered in sediments and soils as an inheritance of the past. The PCBs are biomagnified in the food chain and are found in top predators such as otters, seals, and fish-eating birds [2, 3]. Many toxic responses have been described for the planar dioxin-like PCBs as well as for the nonplanar PCBs. The toxic responses include hepatotoxicity, body weight loss, thymus atrophy, impairment of immune responses, reproductive toxicity, disruption of the endocrine system, alterations in vitamin A and thyroid hormone metabolism, (developmental) neurotoxicity, teratogenicity, and promotor activity in carcinogenesis [4-8]. The non-ortho PCBs and, to a lesser extent, the mono-ortho PCBs are thought to exert their toxicity via the cytosolic aryl hydrocarbon receptor (AhR) [9, 10]. The structure-dependent effects of these congeners are believed to be concentration additive. Multiple ortho-substituted PCBs and hydroxylated PCBs show effects like reproductive toxicity, promotor activity, neurotoxicity, effects on vitamin A metabolism, and alterations in thyroid hormone levels [5, 11-13]. These latter effects probably are not directly exerted via the AhR-mediated pathway. For the non-ortho and mono-ortho-substituted PCB congeners (such as, respectively, 77, 126, 169 and 105, 118, 156, 157), much more toxicity data are available than for the multiple-ortho-substituted congeners. Concerning information on en vironmental fate (e.g., the n-octanol/water partition coefficient, the aqueous solubility, the bioconcentration factor, and the biomagnification factor) and on environmental concentrations (e.g., in sediment), information is available on the congeners that are included in routine monitoring programs. In The Netherlands, these are congeners 18, 28, 44, 49, 52, 101, 118, 138, 153, 170, 180, and 187, which occur in relatively high concentrations and can be analyzed with high accuracy. Of these, only PCB 118 is a planar AhR-binding congener.
For proper management of contaminated sediments and soils, environmental risk limits (ERLs) are needed to evaluate the possible risk of PCBs to the ecosystem. In The Netherlands, ERLs are based on information concerning the ecotoxicology and the environmental chemistry of substances. In The Netherlands, ERLs have been derived for several compound classes, including heavy and trace metals, several volatile compounds, substances with a potential for secondary poisoning, chlorophenols, pesticides, polycyclic aromatic hydrocarbons, and aniline derivatives [14]. The purpose of the present work is to derive ERLs for PCBs. These ERLs for PCB should protect the ecosystem and are derived for sediments and soils. Sediments and soils are considered as the major sinks, and exposure of any organism occurs via (pore) water and food from those sinks. In addition, most monitoring activities for PCBs focus on sediments and soils.
The ERLs are derived for those congeners that exert their effects by binding to the AhR, and in addition for congener 153. It is recognized that not all the possible toxic responses of PCBs are being considered. However, the lack of data on these responses and on the additivity of these effects does not justify an integral risk assessment. The individual congeners 77, 105, 118, 126, 156, 157, and 169 were selected. To account for the concentration addition of those congeners working via the AhR, a mixture ERL was derived and expressed as the concentration of PCB 118 that is routinely monitored. In addition, an ERL for PCB 153 was derived. This congener is thought to represent other toxic mechanisms exerted by PCBs that cannot adopt a planar configuration. Also for these mechanisms, concentration addition with other congeners is plausible. Because of a lack of toxicological data for other congeners, however, no mixture toxicity could be taken into account for these effects.
Toxicity data were converted into an equivalent toxic concentration in the organic carbon of sediment/soil [15]. For the compartments water and air, no ERLs were derived. Water and air, in contrast to organic carbon, are not important primary routes of exposure for highly hydrophobic compounds [16-19] and levels in water and air are extremely low. Accumulation via the food chain was taken into account. Data on environmental fate of PCBs, such as biomagnification factors and biota-to-sediment accumulation factors, were used in the calculations. Probabilistic techniques were used to incorporate the variation in the above-mentioned environmental fate data in the ERLs.
METHODS
Data collection and selection
The ERLs were derived for congeners 77 (3,3′,4,4′-CB), 105 (2,3,3′,4,4′-CB), 118 (2,3′,4,4′,5-CB), 126 (3,3′,4,4′,5-CB), 153 (2,2′,4,4′,5,5′-CB), 156 (2,3,3′,4,4′,5-CB), 157 (2,3,3′,4,4′,5′-CB), and 169 (3,3′,4,4′,5,5′-CB). Data were collected on the acute and chronic toxicity of these individual PCB congeners to aquatic and terrestrial organisms, mammals, and birds. Effects on growth, reproduction, and survival were used in the derivation of ERLs. Data on adverse biochemical or histopathological effects were not included in the derivation of ERLs. No observed effect concentration values (NOEC), but also other percentages of effect such as median effective concentrations (EC50s), were included. We included different dosing methods (diet studies, dosing per gavage or per injection). A toxicity study was considered reliable if it was in agreement with internationally accepted guidelines such as the Organization for Economic Cooperation and Development (Paris, France) guidelines or with criteria developed at the National Institute of Public Health and the Environment (Bilthoven, The Netherlands). Per compound, the most sensitive toxicity test was selected for each species to form the basis to derive the ERL.
Information about the lipid content of organisms was collected to lipid normalize the toxicity data. Data were collected on the environmental fate of the PCBs, i.e., data on the bio-concentration factor (BCFL, the lipid normalized concentration ratio between an organism and the surrounding water), the biomagnification factor (BMFL, the lipid-normalized concentration ratio between predator and prey), the biota-to-sediment/ soil accumulation factor (BSAFL, the lipid- and organic carbon-normalized concentration ratio between an organism and sediment or soil), the organic carbon-normalized partition coefficient between sediment/water or soil/water (Koc), and the partition coefficient between n-octanol and water (Kow).
Data sources
Sources used for the collection of data were the library of the National Institute of Public Health and the Environment's (Bilthoven, The Netherlands), on-line databases containing evaluated data ([20] available free through http://www.epa.gov/ecotox [21-23]), and on-line bibliographic databases Biosis® (Biosis, Philadelphia, PA, USA) and Toxline and Chemical Abstracts (http://toxnet.nlm.nih.gov) as well as literature searches of the open literature and reviews.
Calculation of toxic concentrations in the soil/sediment
The toxicity data of the different species were converted to equivalent toxic concentrations in the organic carbon of soils or sediments using the procedures described below.
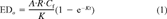 (1)
(1)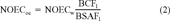 (2)
(2)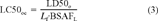 (3)
(3)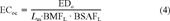 (4)
(4)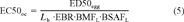 (5)
(5)Probabilistic modeling to account for variability
Considerable variability in the literature data on the parameters as used in equations 2 through 5 (Lfish egg, Lbird egg, Lm, EBR, BCFL, BMFL, and BSAFL) was encountered because of intra- and interspecies differences, sediment differences, use of different test methods, etc. The variability in these data can be included in the calculations using probabilistic modeling.
Distributions were fitted to the literature data of the mentioned parameters using the software package Crystal Ball 4.0 (Decisioneering, Denver, CO, USA). These distributions, instead of discrete values, were used as input to the calculations. For the parameters BCFL, BMFL, and BSAFL, whether there were significant differences among congeners was tested by single-factor analysis of variance (α < 0.05) or by a two-sample t test assuming equal variances. If there were statistically significant differences among congeners, these fits were performed per congener.
The calculations using equations 2, 3, 4, or 5 were then performed; the distributions for the different parameters used in the equations were sampled with a Latin hypercube sampling. Each calculation was performed 1,000 times. This resulted in probability distributions of the equivalent toxic concentration in the organic carbon of soil/sediment for each original toxicity value. In this way, all types of toxicity studies could be readily compared as all data were expressed in the same concentration axis (in organic carbon) and integrated into one ERL.
Deriving ERLs
ERLs for individual congeners. From each individual probability distribution of the equivalent toxic concentration in the organic carbon, 1,000 data points were extracted. All data were combined per congener, and new distributions were fitted to the combined data. These were normal distributions based on the log-transformed data. Combined distributions were fitted for all toxicity data together or only for the data on birds and mammals. The goodness of fit of the distributions to all data or to all mammal and bird data was tested by the Kolgomorov–Smirnov test. If the s-value was less than 0.1, the distribution was judged to be an acceptable basis for the ERL. Otherwise, the most sensitive probability distribution was chosen as the basis for the ERL.
The ERL values reported are the fifth percentiles of the selected distributions, back transformed into μg/kg OC.
Mixture ERL. The mixture ERL is supposed to be protective for the mixture of AhR-binding PCBs that were considered in this study (i.e., congeners 77, 105, 118, 126, 156, 157, and 169). The mixture ERL aims to protect ecosystems from these planar congeners. It was expressed on the basis of PCB 118 since this planar congener is monitored routinely. The fraction of the toxicity of the mixture of planar PCBs that is explained by PCB 118 was calculated, and the ERL for the single congener PCB 118 was multiplied by this fraction to yield the mixture ERL.
For calculating the fraction of the toxicity explained by PCB 118, both information on the relative concentrations of the congeners and information on the relative potency is needed. For deriving the mixture ERL, the congener pattern of planar PCBs in The Netherlands was used. The concentration of each individual AhR-binding PCB was expressed as a percentage of the summed concentration of planar PCBs. The distributions forming the basis for the ERL of the individual congeners were used to scale the toxicological importance of the congeners. Means were used instead of ERLs (fifth percentiles) since means are not influenced by the dispersion (as, e.g., measured by standard deviation) in the distributions.
RESULTS
Toxicity data
The toxicity data used to calculate the ERL values are listed in Table 1. For an extensive overview of all the toxicity data collected, we refer to the underlying report [24].
Lipid content of the different species
The BCFL, BSAFL, and BMFL data are expressed on a lipid basis, while toxicity data (except for L[E]C50s) are expressed on a wet weight basis. So toxicity data must be lipid normalized before converting them into equivalent toxic concentrations in the organic carbon (equations 3–5). Lipid content varies between species but also within a species depending on the season, reproductive phase, food availability, etc. [25]. In addition, the lipid extraction method used influences the amount of lipids found [26]. Table 2 shows extractable lipid contents in various species and their eggs and the variability therein.
For the lipid content of fish eggs, a normal distribution with a mean of 10.1 and a standard deviation of 0.2% was used in the calculations [27]. For lipid content of bird eggs, a normal distribution with a mean of 7.7 and a standard deviation of 0.8% was used [28]. These values are consistent with the values reported in Table 2. Concerning the lipid content of mammals, two alternative distributions were considered. First, a normal distribution with a mean of 9.4 and a standard deviation of 7.7%, based on a compilation of literature data on laboratory-raised test organisms [29], was used. This value is high compared to the range of lipid contents observed in mammals taken from the field (Table 2). The laboratory-raised organisms as used in toxicity studies are generally well fed and do not have much movement, so they can be assumed to have a higher lipid content than free-ranging organisms. Second, a uniform distribution of the lipid content between 2 and 30% was used. These two alternatives were included to check the importance of the mammal lipid content; the variability in the literature data is high, so uncertainty in the distribution assumed is a consequence.
The PCB concentrations in bird eggs are translated to concentrations in the parent birds by assuming an egg-to-parent ratio of a mean of 0.60 with a standard deviation of 0.11 on a lipid basis. This value is derived from a field study on herring gulls [28]; no significant differences between homologue groups of PCBs were reported in that study. Few data on egg-to-parent bird ratios are available for PCBs (see also [30, 31]). However, the absence of an influence of the substitution pattern on the partitioning between parent and egg lipids implies that the PCBs partition nonselectively between the various lipid pools in the avian body. The cited value of 0.60 is also assumed to be applicable to other bird species [31].
Data on environmental fate
Bioconcentration. Data on bioconcentration for fish, mollusks, and crustaceans derived from laboratory studies and field studies were reviewed (Table 3). In Figure 1, lipid-normalized BCFL values from laboratory or field studies were related to log Kow values (data from [32]). For the congeners considered, there was a significant relationship between BCFL and log Kow (F > Fcritical, p = 0.01, r2 = 0.63). Data points were within the range where, according to the literature, linearity is no longer observed [33-37]. In addition, standard deviations for the individual congeners were rather high (Fig. 1). For PCB 153, no significant differences were observed in BCFL values derived from laboratory or field studies (t test). Therefore, laboratory and field data were combined for fitting distributions. The available lipid-based BCFL data did not differ significantly between congeners (analysis of variance, α < 0.05) both for laboratory data only and for the combination of laboratory and field data. Therefore, the log-transformed laboratory and field BCFL data for fish and mollusks were combined for all congeners.
| Aquatic organisms | Mammals | Birds | |||||||
|---|---|---|---|---|---|---|---|---|---|
| PCB | Species | Toxicity endpoint | Ref. | Species | Toxicity endpoint | Ref. | Species | Toxicity endpoint | Ref. |
| 77 | Salvelinus namaycush, eggs | Survival, LD50 | [56] | Mus musculus | Reproduction, NOEC | [57] | Falco sparverius | Survival, LD50 | [58] |
| Oncorhynchus mykiss, eggs | Survival, LD50 | [59] | Meleagris gallopavo | Survival, LD60 | [47] | ||||
| Daphnia magna | Growth, NOEC | [60] | Gallus gallus | Reproduction, NOEC | [61] | ||||
| 105 | Brachydanio rerio | Survival, LC50 | [62] | Rattus rattus | Growth, ED50 | [63] | Gallus gallus | Growth, LOEC | [45] |
| 118 | Rattus rattus | Growth, NOEC | [64] | Gallus gallus | Survival, LD45 | [47] | |||
| 126 | Oryzias latipes | Survival, NOEC | [65] | Rattus rattus | Growth, NOEC | [66] | Meleagris gallopavo | Survival, LD36 | [46] |
| Oncorhynchus mykiss, eggs | Survival, LD50 | [59] | Gallus gallus | Reproduction, NOEC | [61] | ||||
| Falco sparverius | Reproduction, NOEC | [61] | |||||||
| 153 | Rattus rattus | Growth, NOEC | [67] | ||||||
| Mustela vison | Growth, NOEC | [68] | |||||||
| 156 | Mus musculus | Reproduction, LOEC | [69] | Gallus gallus | Survival, LD50 | [70] | |||
| Rattus rattus | Growth, NOEC | [71] | |||||||
| 157 | Rattus rattus | Growth, ED50 | [63] | Gallus gallus | Survival, LD50 | [70] | |||
| 169 | Cavia porcellus | Survival, LD50 | [72] | Gallus gallus | Survival, LD80 | [47] | |||
| Mus musculus | Reproduction, NOEC | [48] | |||||||
| Rattus rattus | Reproduction, NOEC | [73] | |||||||
| Mustela vison | Growth, LOEC | [74] | |||||||
- a NOEC = no observable effect concentration; LOEC = lowest observable effect concentration.
| Species | Lipid % whole body, mean ± SD | Lipid % egg, mean ± SD | Ref. |
|---|---|---|---|
| Birds | |||
| Larus argentatus | 10.3 ± 2.2 | 7.7 ± 0.8 | [28] |
| Larus argentatus | 8.2–9.9 | [75] | |
| Aythya fuligula | 3.6 ± 0.7 | [76] | |
| Podiceps cristatus | 4.2 ± 0.5 | [76] | |
| Ardea cinerea | 2.7 ± 1.5 | [76] | |
| Phalacrocorax carbo sinensis | 3.5 ± 0.5 | [76] | |
| Phalacrocorax carbo sinensis | 3.5–4.5 | [77] | |
| Birds | 8.9 ± 9.0 | [29] | |
| Altricials | 5.9 ± 1.5 | [78] | |
| Semialtricials | 6.3 ± 0.7 | [78] | |
| Semiprecocials | 9.5 ± 2.3 | [78] | |
| Precocials | 10.3 ± 1.4 | [78] | |
| Mammals | |||
| Martes martes (muscular tissue) | 3.4 ± 1.8 | [79] | |
| Stoat (liver) | 4.1 ± 0.7 | [80] | |
| Weasel (liver) | 3.2 ± 0.6 | [80] | |
| Polecat (liver) | 5.4 ± 2.0 | [80] | |
| Red vole | 2.9 ± 0.5 | [80] | |
| Wood mouse | 4.6 | [80] | |
| Common vole | 2.7 | [80] | |
| Shrew | 2.7 | [80] | |
| Common hare | 0.95 | [80] | |
| Mammals | 9.4 ± 7.7 | [29] | |
| Fish | |||
| Silver bass | 5.5 | [75] | |
| Smallmouth bass | 11.4 | [75] | |
| Walleye | 10.1 ± 0.2 | [27] | |
Biomagnification. Data on biomagnification can be found in Table 4. All available laboratory studies concerned fish species. Field studies concentrated on biomagnification in mammals and birds. In Figure 2, the BMFL values for the different congeners are depicted for both the laboratory and field studies. Significant differences between the congeners were observed for the field-based BMFLs. For birds, less data were found than for mammals. The largest number of BMF data available was for PCB 153. No significant differences between the BMFL for birds or mammals were observed for this congener. For each congener, the probability distributions for BMFL were fitted to the data, combining data for birds and mammals. The distributions were log normal and based on the field data.
| Laboratory studies | Laboratory studies, crustaceans | Field studies | |||||||
|---|---|---|---|---|---|---|---|---|---|
| PCB | Organism | log BCFL, l.w. | Ref. | Organism | log BCF, wet wt | Ref. | Organism | log BCFL, l.w. | Ref. |
| 77 | Dreissena polymorpha | 6.63 | [81] | Daphnia magna | 4.12 | [60] | |||
| Brachydanio rerio | 6.89 | [82] | 4.04 | [60] | |||||
| Poecilia reticulata | 5.92 | [83] | Hyalella azteca | 3.54 | [84] | ||||
| 6.11 | [85] | 3.58 | [84] | ||||||
| 3.38 | [84] | ||||||||
| 3.41 | [84] | ||||||||
| 105 | Brachydanio rerio | 6.53 | [86] | ||||||
| Gadus morhua | 6.72 | [86] | |||||||
| 118 | Daphnia magna | 3.84 | [60] | Mytilus edulis | 7.20 | [21] | |||
| 4.42 | [60] | 7.11 | [21] | ||||||
| 6.70 | [21] | ||||||||
| 6.60 | [21] | ||||||||
| 126 | Brachydanio rerio | 7.34 | [82] | ||||||
| 153 | Crassostrea virginica | 6.64 | [61] | Selenastrum capricornutum | 4.48 | [87] | 6.82 | [88] | |
| Dreissena polymorpha | 7.71 | [81] | Daphnia magna | 4.11 | [60] | 7.62 | [88] | ||
| 6.58 | [81] | 4.16 | [60] | Platichtus flesus | 7.85 | [88] | |||
| Mytilus edulis | 7.66 | [89] | Mysis relicta | 5.64 | [90] | ||||
| Strophitus rugosus | 5.72 | [91] | Potoporeia hoyi | 5.00 | [90] | ||||
| Brachydanio rerio | 7.18 | [82] | |||||||
| Cottus bairdi | 5.99 | [91] | |||||||
| Cyprinodon variegatus | 6.65 | [92] | |||||||
| Oncorhynchus mykiss | 6.20 | [93] | |||||||
| Pimephales promelas | 7.23 | [94] | |||||||
| 7.23 | [94] | ||||||||
| 6.18 | [94] | ||||||||
| 7.11 | [94] | ||||||||
| Poecilia reticulata | 6.78 | [95] | |||||||
| 7.00 | [83] | ||||||||
| 6.15 | [96] | ||||||||
| 6.78 | [95] | ||||||||
| 6.36 | [85] | ||||||||
| 169 | Brachydanio rerio | 7.51 | [82] | ||||||
- a BCF = bioconcentration factor; BCFL = lipid-normalized bioconcentration factor; l.w. = lipid weight.
Biota-to-sediment accumulation. Data on biota-to-sediment accumulation (BSAFL) are listed in Table 5. Most laboratory studies used the bivalve Macoma nasuta. For PCB 153, BSAFL data were found for other species as well; these did not differ significantly from the Macoma data. For BSAFL values derived from field studies, data for various species were found. No clear relationship was observed between type of species and BSAFL values. For biota-to-soil accumulation, few data were found, and these did not seem to deviate much from the sediment data.
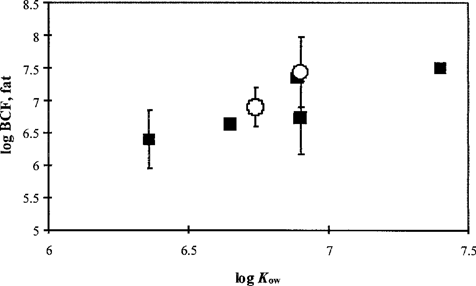
The bioconcentration factor (BCFL) (± SD) of selected polychlorinated biphenyls (PCBs) for fish and mollusks related to the log Kow. ▄, data from laboratory studies; ◯, data from field studies.
The BSAFL values derived from field studies were higher than those from studies conducted in the laboratory (Fig. 3). There were significant differences between the congeners for the field data. Normal distributions were fitted to the field-derived BSAFL data for each congener. For congeners 126 and 169, no standard deviation could be determined because of a lack of data. For these congeners, the standard deviation was estimated on the basis of the variation in the data for other congeners, as described by Luttik and Aldenberg [38]. For several congeners (especially 153 and 169), a significant part of the left tail fell below zero in the normal distribution. This resulted in an unrealistically high probability density at and near zero. Therefore, as an alternative, a log-normal distribution was fitted to the data and was included in the calculations (equations 2–5).
Environmental risk limits for individual PCB congeners in organic carbon
Probability distributions of effect concentrations in organic carbon. For each individual congener, probability distributions were calculated for concentrations in the organic carbon associated with adverse effects on survival, growth, or reproduction. Examples are given in Figure 4 for PCB 126 and in Figure 5 for PCB 169. It can be seen that probability distributions for different species sometimes overlapped (PCB 169, Fig. 5), indicating a lack of differences in sensitivity among species. For other congeners (see Fig. 4 for PCB 126), sensitivity differences between the species were more distinct.
| Laboratory studies | Field studies | |||||
|---|---|---|---|---|---|---|
| PCB | Organism | BMFL,a lipid/lipid | Ref. | Organism | BMFL, lipid/lipid | Ref. |
| 77 | Oncorhynchus kisutch | 4.2 | [97] | Fish to otter | 2.5 | [98] |
| Fish diet to otter | 1.4 | [2] | ||||
| Mammal/amphibians to weasel | 17 | [80] | ||||
| Mammal/amphibians to stoat | 6 | [80] | ||||
| Mammal/amphibians to polecat | 4 | [80] | ||||
| 105 | Fish to otter | 7.9 | [98] | |||
| Fish diet to otter | 12 | [2] | ||||
| Mammal/amphibians to weasel | 10 | [80] | ||||
| Mammal/amphibians to stoat | 38 | [80] | ||||
| Mammal/amphibians to polecat | 31 | [80] | ||||
| 118 | Oncorhynchus mykiss | 6.00 | [82] | Fish to otter | 35 | [98] |
| Fish diet to otter | 15 | [2] | ||||
| Mammal/amphibians to weasel | 7 | [80] | ||||
| Mammal/amphibians to stoat | 25 | [80] | ||||
| Mammal/amphibians to polecat | 20 | [80] | ||||
| 126 | Gasterosteus aculeatus | 4.94 | [99] | Fish to otter | 130 | [98] |
| Fish diet to otter | 70 | [2] | ||||
| Mammal/amphibians to weasel | 20 | [80] | ||||
| Mammal/amphibians to stoat | 112 | [80] | ||||
| Mammal/amphibians to polecat | 31 | [80] | ||||
| 153 | Gasterosteus aculeatus | 12.0 | [99] | Fish to otter | 28 | [98] |
| Oncorhynchus kisutch | 9.2 | [97] | Fish diet to otter | 15 | [2] | |
| Poecilia reticulata | 46.5 | [100] | Mammal/amphibians to weasel | 5 | [80] | |
| 22.5 | [100] | Mammal/amphibians to stoat | 26 | [80] | ||
| Oncorhynchus mykiss | 16.0 | [82] | Mammal/amphibians to polecat | 180 | [80] | |
| 156 | Fish to otter | 37 | [98] | |||
| Fish diet to otter | 30 | [2] | ||||
| Mammal/amphibians to weasel | 7 | [80] | ||||
| Mammal/amphibians to stoat | 37 | [80] | ||||
| Mammal/amphibians to polecat | 64 | [80] | ||||
| 157 | Fish to otter | 84 | [98] | |||
| Fish diet to otter | 19 | [2] | ||||
| Mammal/amphibians to weasel | 3 | [80] | ||||
| Mammal/amphibians to stoat | 22 | [80] | ||||
| Mammal/amphibians to polecat | 58 | [80] | ||||
| 169 | Gasterosteus aculeatus | 4.65 | [99] | Fish to otter | 108 | [98] |
| Fish diet to otter | 348 | [2] | ||||
- a BMFL = lipid-normalized biomagnification factor.
It was found for congener 77 that using either a normal or a uniform distribution for the parameter Lm in the calculations yielded comparable results (data not shown). In addition, it was found for PCB 77, 153, and 169 that using a normal or a log-normal distribution for the parameter BSAFL did not lead to different results (data not shown). Apparently, the calculated probability densities are not sensitive to parameters that are uncertain. In further calculations for all congeners, normal distributions for BSAFL and lipid content were used. For all congeners for which toxicity data on aquatic organisms were available (77, 105, 126), mammals and especially birds appeared more sensitive than aquatic species (see Fig. 4 for PCB 126).
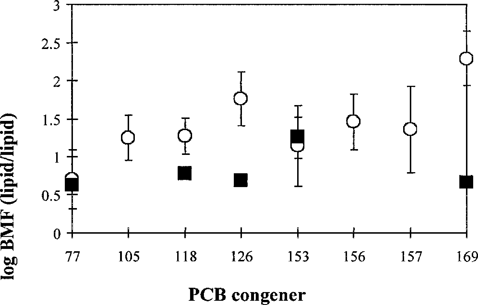
Biomagnification factors (BMFs) (± SD) of selected polychlorinated biphenyls (PCBs). ▄, data from laboratory studies on fish species; ◯, data from field studies on mammals and birds.
Contribution of individual parameters to the overall variance. An analysis was conducted to determine the relative contribution to the variance by the various underlying parameters, as used in equations 2 through 5. For the conversion of aquatic toxicity data into equivalent effect concentrations in OC (Equation 2), the BCFL was most important. For the fish egg injection studies (Equation 3), the BSAFL contributed most to the variance. For the transformation of mammal toxicity studies into effect concentrations in OC (Equation 4), all the parameters used (Lm, BMFL, and BSAFL) contributed to the variance. The importance of the various parameters varied by congener. For Equation 5 used for the bird toxicity studies, BMFL and BSAFL were most important for the resulting variance.
Derivation of ERLs. As a result of the Kolgomorov–Smirnov test (see Methods), the ERLs for all individual congeners were based on a distribution of all mammal and bird data combined. One exception was congener 77, where a combined distribution based on all toxicity data showed a good fit. Another exception was congener 105, for which neither of the combined distributions showed a good fit. The ERL for congener 105 was calculated from the most sensitive probability distribution based on a single toxicity value. The ERLs and the statistics of the underlying distributions are presented in Table 6.
| Laboratory studies | Field studies | |||||
|---|---|---|---|---|---|---|
| PCB | Organism | BSAFL,a lipid/organic carbon | Ref. | Organism | BSAFL, lipid/organic carbon | Ref. |
| In sediment | ||||||
| 77 | Dreissena polymorpha | 3.32 | [80] | |||
| Pseudanodonta complanata | 3.21 | [80] | ||||
| Pooled fish | 1.10 | [101] | ||||
| 105 | Macoma nasuta | 1.63 | [101] | Dreissena polymorpha | 5.09 | [80] |
| 2.87 | [101] | Pseudanodonta complanata | 4.56 | [80] | ||
| 3.85 | [101] | Pooled fish | 5.50 | [101] | ||
| 0.22 | [102] | |||||
| 0.39 | [102] | |||||
| 118 | Macoma nasuta | 2.02 | [101] | Anodonta cygnea | 3.80 | [103] |
| 3.28 | [101] | Pseudanodonta complanata | 0.51 | [103] | ||
| 4.74 | [101] | Dreissena polymorpha | 7.02 | [80] | ||
| 0.73 | [102] | Pseudanodonta complanata | 3.55 | [80] | ||
| 0.54 | [102] | Mercenaria mercenaria | 1.32 | [104] | ||
| Yoldia limatula | 4.26 | [104] | ||||
| 5.73 | [104] | |||||
| Anguila anguila | 4.57 | [103] | ||||
| 4.88 | [103] | |||||
| 5.88 | [103] | |||||
| Abramis brama | 5.47 | [103] | ||||
| 4.00 | [103] | |||||
| Esox lucius | 5.06 | [103] | ||||
| 4.75 | [103] | |||||
| Rutilus rutilus | 4.71 | [103] | ||||
| 6.63 | [103] | |||||
| 1.75 | [103] | |||||
| 7.00 | [103] | |||||
| Pooled fish | 7.10 | [101] | ||||
| 126 | Dreissena polymorpha | 4.17 | [80] | |||
| Pooled fish | 5.70 | [101] | ||||
| 153 | Macoma nasuta | 1.57 | [101] | Anodonta cygnea | 6.75 | [103] |
| 2.66 | [101] | Pseudanodonta complanata | 0.78 | [103] | ||
| 4.05 | [101] | Dreissena polymorpha | 4.95 | [105] | ||
| 0.71 | [102] | 0.003 | [80] | |||
| 0.40 | [102] | Pseudanodonta complanata | 6.36 | [80] | ||
| 1.75 | [89] | Mercenaria mercenaria | 1.49 | [104] | ||
| Ictalurus nebulosus | 0.23 | [106] | Yoldia limatula | 5.10 | [104] | |
| 0.93 | [106] | 8.25 | [104] | |||
| 0.76 | [106] | Anguila anguila | 14.34 | [105] | ||
| 5.94 | [106] | 5.97 | [103] | |||
| 5.87 | [103] | |||||
| 7.69 | [103] | |||||
| Abramis brama | 9.23 | [103] | ||||
| 5.38 | [103] | |||||
| Esox lucius | 4.20 | [103] | ||||
| 3.92 | [103] | |||||
| Rutilus rutilus | 6.43 | [103] | ||||
| 10.08 | [103] | |||||
| 2.69 | [103] | |||||
| 11.33 | [103] | |||||
| Pooled fish | 10.00 | [101] | ||||
| 156 | Macoma nasuta | 0.61 | [102] | Dreissena polymorpha | 5.00 | [80] |
| 0.16 | [102] | Pseudanodonta complanata | 3.22 | [80] | ||
| Pooled fish | 6.10 | [101] | ||||
| 157 | Dreissena polymorpha | 20.00 | [80] | |||
| Pseudanodonta complanata | 10.35 | [80] | ||||
| Pooled fish | 3.00 | [101] | ||||
| 169 | Pooled fish | 2.50 | [101] | |||
| In soil | ||||||
| 118 | Eisenia andrei | 4.3 | [17] | |||
| 153 | Eisenia andrei | 4.1 | [17] | |||
| 156 | Eisenia andrei | 3.9 | [17] | |||
- a BSAFL = lipid-normalized biota-to-sediment/soil accumulation factor.
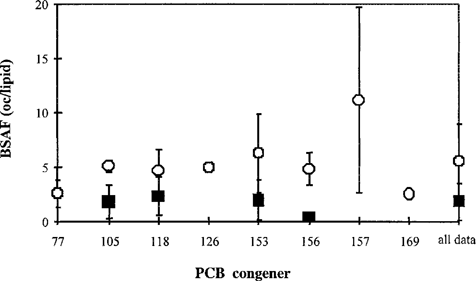
Biota-to-sediment accumulation factors BSAFs (± SD) of selected polychlorinated biphenyls (PCBs). ▄, data from laboratory studies; ◯, data from field studies.
Environmental risk limit for the mixture of planar PCBs
Congener pattern of planar PCBs in The Netherlands. The congener pattern provides the contribution of a specific congener to the total concentration of planar PCBs. Both the congener pattern and the toxicological potency of each congener determine the toxicity of the mixture of AhR-binding PCBs.
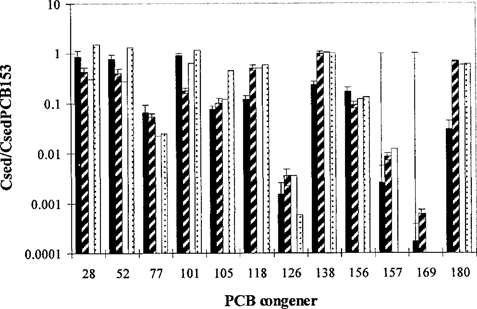
The PCB patterns normalized to PCB 153 in sediments from different areas in The Netherlands are given in Figure 6. The most toxic congeners, 126 and 169, occur at the lowest concentrations (103-104 times less than PCB 153). There is less than a sevenfold difference in 153-normalized concentrations between different locations (except for congener 180, for which 153-normalized concentrations differ up to 23-fold).
Non- or mono-ortho PCBs occur in low concentrations and are rarely measured. Only four datasets on planar PCB in sediment were available for The Netherlands. More information, and from different locations, was available on concentrations in organisms low in the food chain. Congener patterns in arthropods and mollusks should reflect those in the sediment because of their low metabolic capacity [39]. When congener patterns in sediments, arthropods, and mollusks from various Dutch locations were compared (Fig. 7), patterns were highly similar and consistent.
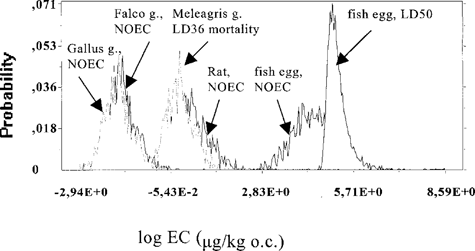
Probability distributions of organic carbon concentrations (OC) in sediment or soil associated with critical levels of polychlorinated biphenyl (PCB) 126 in different species. On the x-axis, the logarithm of the equivalent adverse effect concentration (in μg/kg organic carbon) is given; on the y-axis, the probability is given that this concentration has a certain value.
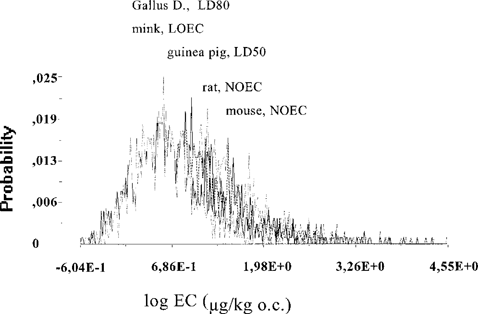
Probability distributions of organic carbon concentrations (OC) in sediment or soil associated with critical levels of polychlorinated biphenyl (PCB) 169 in different species. On the x-axis, the logarithm of the equivalent adverse effect concentration (in μg/kg OC) is given; on the y-axis, the probability is given that this concentration has a certain value.
Deriving the mixture ERL. The mixture ERL addresses the toxicity of the mixture of congeners 77, 105, 118, 126, 156, 167, and 169. To derive this mixture ERL, the data depicted in Figure 6 were averaged and expressed as percentage of the summed concentration of AhR-binding PCBs (second column in Table 7). To scale the toxicological importance of each congener, the mean of the distribution that was the basis for the ERL (see Table 6) was back-transformed (third column in Table 7). The fractions was divided by the scaling factor of that specific congener (fourth column in Table 7). The ERL for the single congener 118 (25 μg/kg OC, Table 6) was then multiplied by the fraction of the total toxicity explained by 118 (0.21), yielding the mixture ERL. The mixture ERL for 118 of 5 μg/kg OC aims to protect the ecosystem for the total mixture of planar PCB congeners.
It should be noted that, at a specific location, the congener pattern of planar PCBs may deviate from the congener pattern used here. A mixture ERL for that specific situation can then be calculated in a similar way as described here.
| PCB | ERL (μg/kg OC) | Mean ± SD of log-transformed data | Based upon |
|---|---|---|---|
| 77 | 7.2 | 4.04 ± 1.93 | All toxicity data |
| 105 | 26 | 1.87 ± 0.28 | Most sensitive toxicity value |
| 118 | 25 | 2.57 ± 0.72 | All mammal and bird data |
| 126 | 0.042 | 0.07 ± 0.88 | All mammal and bird data |
| 153 | 151 | 3.86 ± 1.03 | All mammal and bird data |
| 156 | 55 | 2.87 ± 0.69 | All mammal and bird data |
| 157 | 32 | 3.00 ± 0.92 | All mammal and bird data |
| 169 | 0.83 | 0.98 ± 0.65 | All mammal and bird data |
DISCUSSION
Methods for including the risk for secondary poisoning
In the present study, toxicity data were transformed into equivalent toxic concentrations in the organic carbon of sediment or soil using bioconcentration factors, biota-to-sediment concentration factors, and biomagnification factors. This allowed data from different types of studies to be readily compared and integrated into one ERL. Parameters such as BCFL and Koc that are difficult to determine for hydrophobic compounds were avoided where possible. We used data from field studies to obtain biota-to-sediment accumulation and biomagnification factors because a steady state is hard to establish in the laboratory for very hydrophobic substances such as PCBs. In addition, it is difficult to simulate all potential pathways of contaminant uptake in a laboratory. The use of BCFL/BSAFL instead of the organic carbon normalized sediment/ water or soil/water partition coefficient (Koc) also has the advantage that possible biotransformation of the compound is taken into account.
It should be mentioned that the food chains taken into account for this report are relatively simple ones. However, these are sufficient for estimating accumulation in the dominant aquatic food chain in The Netherlands, i.e., organic matter, herbi-detritivores (invertebrates), primary carnivores (fish), and secondary carnivores (birds, mammals) [40]. Elsewhere, additional trophic levels may be distinguished [41].
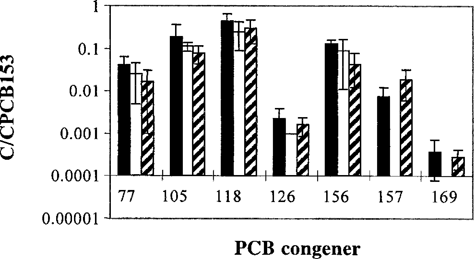
Polychlorinated biphenyl (PCB) patterns in Dutch sediments, arthropods/plankton, and mollusks, normalized to PCB 153. ▄, sediment; □, Arthropoda; hatched bar, Mollusca. Data for sediment are from Figure 6; data for arthropods are from [2] and Reinhold et al. (unpublished data) and are measured in lake ‘Zandmeer’ and the Biesbosch. Data for the mollusks are from [55] and [2] and are measured in the rivers Rhine and Meuse and in the lakes Ijsselmeer and Zandmeer.
| PCB | (A) Fraction in pattern (% of sum) | (B) Scaling factors | (A/B) (% of sum) | Mixture ERL (μg/kg organic carbon) |
|---|---|---|---|---|
| 77 | 4.73 | 11,000 | 0.06 | |
| 105 | 21.4 | 74 | 41 | |
| 118 | 56.2 | 370 | 21 | 5 |
| 126 | 0.28 | 1.2 | 34 | |
| 156 | 15.0 | 740 | 2.8 | |
| 157 | 2.29 | 1,000 | 0.32 | |
| 169 | 0.057 | 9.5 | 0.85 |
The probabilistic approach as sketched requires a sufficiently large dataset on toxicity and on the environmental behavior of the compounds studied.
Probabilistic modeling
The variability in the environmental fate parameters was taken into account. Distributions were fitted based on literature information, and these distributions (rather than absolute values) were incorporated in the calculations. Considerable spread in literature data on these parameters was encountered because of intra- and interspecies differences, sediment differences, differences in test methods, etc.
Inclusion of different dosing methods in the derivation of ERL
For toxicity studies on mammals and birds, diet studies are considered most reliable. Other and more common dosing methods include gavage or injection. The validity of using these data is evaluated by comparing the results among different types of studies.
It is unclear for the bird toxicity studies if different dosing methods influence the results, as all studies available for the extrapolation into ERLs were based on egg injection studies. Injection took place into the yolk sac or in the air chamber of the eggs. Contaminants first have to pass membranes, and the subsequent transport of the PCBs will probably mainly occur via the blood circulation of the developing embryo. We assumed in our calculations that the injected PCBs partition over the egg (mainly the lipids) and that equilibrium was reached relatively soon after the exposure. These egg injection studies generally done on 4- to 7-d-old eggs through hatching. In a study by Näf et al. [42], it was shown for PAHs, which are hydrophobic compounds with a Kow range comparable to PCBs, injected into the yolk on day 4 that 94% of the PAHs were metabolized by day 18. This indicates that the PAHs were available for uptake within this period as metabolization took place in the developing embryo.
For mammals, for those congeners where multiple studies were available (153, 156, 169), the sensitivities differed among dosing types by a maximum of 1.5 log units (means of probability distributions were compared). Therefore, the dosing method for mammals does not seem to greatly influence the results.
Concerning fish, results from different dosing methods (i.e., exposure via injection and exposure via water) for the same congener were available for PCBs 77 and 126. The results obtained by fish egg injection were more sensitive in the case of congener 77, while the opposite held for congener 126. Therefore, no conclusion can be drawn on the influence of the dosing method.
For assimilation efficiency in dietary studies with warm-blooded animals, a value of roughly 60 to 100% is often mentioned in the literature [43, 44]. Less information is available for studies where dosing takes place via gavage or injection. However, assuming a 100% availability of the administered dose will probably not be a gross overestimation.
Inclusion of multiple endpoints and effect levels in the derivation of ERL
Effects on growth, reproduction, and survival were integrated in the derivation of ERLs. The most sensitive toxicity test was selected for each species and for each compound. We included NOEC values as well as other levels of effect such as EC50s. The influence of this was evaluated by studying the steepness of the dose–response curves and the differences in sensitivity among endpoints. We examined the steepness of the dose–response curve for several studies [45-48]. The steepness of the curve has to be examined within a study and not between studies, as in the latter case interlaboratory differences in sensitivities of the test animals and in the experimental design obscure the results.
For PCBs 77, 105, and 126, Powell et al. [45] showed that the ratio between the LD50 for survival and the lowest adverse effect level for growth in chickens ranged from 0.7 to 18. In a study in Meleagris gallopavo for congener 126, the concentration exerting 100% mortality was three times the concentration causing 36% mortality [46]. The concentrations needed to exert 17% and 100% lethality in chicken embryos exposed in ovo to PCB 77 differed by a factor of five [47]. Marks et al. [48] showed that an eightfold increase in dose resulted in an increase in response from 10 to 60% for reproduction in mice exposed to congener 169.
These results show that dose–response curves for individual congeners are steep [46-48] and that the differences in concentration needed to obtain different types of effects (survival, growth) are not overly large [45]. The differences in sensitivity between species are large (see Fig. 4) relative to the aforementioned differences within a species among endpoints or effect levels. Therefore, it is believed that the inclusion of diverse endpoints and diverse levels of effects in the extrapolation of ERLs is acceptable.
Vulnerability of different organism groups
The vulnerabilities of different groups of organisms to PCB contamination of sediments or soils can be compared directly since the equivalent effect concentrations are expressed in a comparable unit (i.e., μg/kg OC). Although different end-points, different levels of effect, and studies with different dosing methods were combined in the derivation of the ERLs, patterns in vulnerability of different species groups could still be discerned. For those congeners where information on aquatic species was available (77, 105, 126), the aquatic organisms invariably were the least vulnerable. Avian NOECs for growth or reproduction, expressed as equivalent (no) effect concentrations in the organic carbon of soil or sediment, turned out to be the most vulnerable parameters (see 77, 105, 126). For congeners 118, 153, 156, 157, and 169, no information was available on avian NOECs for growth or reproduction, which may result in an underestimation of the ERL. New studies on these endpoints in birds may lead to adjustment of the ERL.
The number of toxicity test data used to derive ERLs for the individual congeners varied between two and seven (Table 1). If only two toxicity data were available, those data involved the relatively vulnerable mammals and/or birds.
Comparison with effects found in the field
Effects found in field-exposed fish-eating birds, otters, and minks. The ERLs as given in Table 6 can be compared with levels of PCBs associated with adverse effects in field studies. Several studies have been performed in which effects in wildlife top predators were related to the internal concentration of PCBs. This can only be done in a correlative way. Deriving a causal relationship between concentrations of (a group of) chemicals and an effect is not possible since the total composition of the mixture of chemicals in the field is unknown and other substances in the mixture may have attributed to the effects observed. We focused on field studies from The Netherlands since PCB congener patterns and the corresponding effects may be region specific.
Fish-eating birds are top predators, known to accumulate high concentrations of PCBs and related chemicals [30]. In a study by Bosveld et al. [3], eggs from the common tern were taken from seven colonies in The Netherlands. Concentrations of PCBs (including the planar PCBs), polychlorinated dibenzodioxins, and polychlorinated dibenzofurans were analyzed in the yolk, and the eggs were artificially incubated. If the concentration of PCBs was higher than or equal to 3.5 ng toxic equivalents (TEQ)/g lipid (chicken toxic equivalency factors [TEFs] were used, [3]), a significantly longer incubation period was observed. A longer incubation period in the laboratory translates into an even longer incubation period in the field [49]. The study by Bosveld et al. [3], showing a sensitive effect that is presumably relevant to population growth, was used to validate the ERLs.
In addition, an EC50 for PCBs exerting effects on reproduction in mink was derived based on a series of literature data [6]. Results were expressed as TEQ using Safe TEFs [5] and were based on whole-body concentrations calculated with a one-compartment bioaccumulation model. The lipid-normalized EC50s are 5 to 10 ng TEQ/g lipid for litter size and kit survival. It was shown that above a level of 4 ng Safe TEQ/g lipid, disease incidences were increased in Danish otters [50].
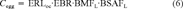 (6)
(6)The resulting concentrations in the eggs are expressed in TEQs using chicken TEFs and are summed. Concentrations for PCB congeners 77, 105, 118, 126, 156, 157, and 169 in the sediment at ERL level result in a chicken TEQ of 1.9 ng/ g lipid in the egg. This is approximately half the lowest effect concentration observed by Bosveld et al. [3].
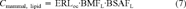 (7)
(7)| PCB | Mean ± SD of combined distribution |
|---|---|
| 77 | 0.76 ± 0.64 |
| 105 | 1.85 ± 2.02 |
| 118 | −0.01 ± 1.44 |
| 126 | −2.89 ± 0.82 |
| 153 | 2.34 ± 0.88 |
| 156 | 0.42 ± 1.19 |
| 157 | −0.76 ± 0.65 |
| 169 | −1.25 ± 1.17 |
Concentrations for PCB congeners 77, 105, 118, 126, 156, 157, and 169 in the sediment at ERL level result in a Safe TEQ of 34 ng/g lipid. This is approximately seven times the critical level deduced from mixture and field studies [6, 50].
It is concluded that the concentrations expressed in TEQ that are associated with adverse effects in field studies are comparable with the TEQ concentrations that would result if all planar congeners were present at the ERL level.
Potency rating of the different congeners: Comparison of toxicological effects with biochemical and histopathological effects
The relative potencies of the different congeners based on toxicological effects were compared with the relative potencies based on biochemical and histopathological effect data. For biochemical or histopathological effects from exposure to PCBs, more literature data are available than for effects on growth, reproduction, and survival. As toxicological and biochemical/histopathological effects are exerted via the same mechanism of action, the relative potencies are expected to be comparable for both types of effects.
The types of biochemical and histopathological effects studied are, e.g., splenic immunosupression in the mouse, ethoxyresorufin O-deethylase activity in the rat or mouse, free thyroxin levels in the monkey or in rat pups, liver cell abnormalities in the rat, etc. (see [24] for detailed information). Probability distributions of concentrations in the organic carbon associated with biochemical or histopathological effects were derived following methods identical to those used for toxicological effects. Again, combined distributions were fitted over all data for each congener. Statistics of these combined distributions are given in Table 8. Means of the distribution for biochemical/histopathological effects are 1.5 to 3 log units less than means of the distributions for the toxicological effects that formed the basis of the ERLs (Table 6). So, in general, biochemical or histopathological effects of PCB congeners will occur at concentrations that are 100 to 1,000 times lower than concentrations at which toxicological effects occur. Exceptions were found for congener 157, where the difference was 3.7 log units, and for congener 105, where there was no difference. The latter may be due to the fact that the distribution for toxicological effects for congener 105 was based on the most sensitive toxicity value (see Table 6).
A comparison of the relative potencies normalized to PCB 126 is shown in Table 9. The data were normalized to congener 126 since this is the most potent congener. Again, means of the distributions (Tables 6 and 8) were used. The difference between relative effect levels for toxicological and biochemical/histopathological effects were within an order of magnitude except for congener 105, where a difference of a factor 1,000 can be observed. This may again be explained by the fact that the ERL for this congener was based on the most sensitive individual probability distribution. The fact that relative potencies were comparable for toxicological effects on the one hand and biochemical or histopathological effects on the other leads to the conclusion that, although there was a paucity of toxicity data for some congeners, the dataset used is a good predictor of the toxicity.
| PCB | Normalized means of distribution for toxicological effect | Normalized means of distribution for biochemical/histopathological effect |
|---|---|---|
| 77 | 0.0001 | 0.0002 |
| 105 | 0.02 | 0.00002 |
| 118 | 0.003 | 0.001 |
| 126 | 1 | 1 |
| 156 | 0.0002 | 0.0005 |
| 157 | 0.001 | 0.007 |
| 169 | 0.1 | 0.02 |
Derivation of mixture ERL
Information on the toxicity and environmental chemistry of the individual congeners was used to derive ERLs, and as a second step, a mixture ERL was derived based on this information. Toxic equivalency factors were not used in order to prevent building in circular arguments in the derivation of a (mixture) ERL due to the fact that TEFs are also based on toxicity information [5, 51]. Congeners other than those listed in Table 7 were not taken into account. Multiple-ortho-substituted congeners show CYP1A1 induction, which points to AhR binding, although with EC50s that are 1,000 to 10,000 times less potent than PCB 126 [52]. Given their high environmental residues, these multiple-ortho-substituted congeners will probably contribute to AhR-mediated toxicity. Their toxicity will probably be exerted mainly via other mechanisms. Unfortunately, the lack of toxicity information prevented the inclusion of these congeners in a mixture ERL. Other types of halogenated aromatics that will have an additive effect with the AhR-binding PCBs were also not incorporated in the mixture ERL.
CONCLUSION
In the present study, toxicity data for aquatic organisms, mammals, and birds were transformed into equivalent toxic concentrations in the organic carbon of sediment or soil using bioconcentration factors, biota-to-sediment concentration factors, and biomagnification factors. This allowed data from different studies to be readily compared and integrated into one ERL. The ERLs were derived for the individual congeners 77, 105, 118, 126, 153, 156, 157, and 169 (Table 6). Parameters such as BCFL and Koc that are difficult to determine for these hydrophobic compounds were avoided where possible. The use of BCFL/BSAFL instead of the organic carbon normalized sediment/water or soil/water partition coefficient (Koc) also has the advantage that possible biotransformation of the compound is taken into account. We used data from field studies for incorporation of biota-to-sediment accumulation and biomagnification processes.
A mixture ERL addressing the toxicity of the mixture of congeners 77, 105, 118, 126, 156, 167, and 169 was derived. The mixture ERL for congener 118 of 5 μg/kg OC aims to protect the ecosystem for the total mixture of planar PCB congeners.
The probabilistic approach as sketched requires a sufficiently large dataset on toxicity and on the environmental behavior of the compounds studied.
Acknowledgements
This work was done under the authorization of the Directorate-General for Environmental Protection, Directorate for Chemicals, External Safety, and Radiation, in the context of the project ‘Setting Integrated Environmental Risk Limits.’ We thank M. Polder, R. Posthumus, P. van Vlaardingen, E. van de Plassche, as well as R. Luttik, J. Boon, B. Bosveld, P. de Voogt, J. Hendriks, P. Leonards, T. Murk, J. de Boer, M. van den Berg, and B. van Hattum.




