Technical basis for narcotic chemicals and polycyclic aromatic hydrocarbon criteria. II. Mixtures and sediments
Abstract
Abstract—A method is presented for developing sediment quality guidelines (SQG) for narcotic chemicals in general and polycyclic aromatic hydrocarbons (PAHs) in particular. The guidelines can be applied to any individual or mixture of narcotic chemicals including PAHs using only the chemical's octanol/water partition coefficient. They are derived using the final chronic values for type I narcotics developed from a database consisting of LC50s for 145 chemicals and 33 species, including fish, amphibians, arthropods, mollusks, polychaetes, coelenterates, and protozoans. The target lipid model is used that accounts for the variations in toxicity due to differing species sensitivities as well as chemical differences. The SQGs are derived using the equilibrium partitioning model (EqP), and the results are compared to other sediment quality guidelines. The criterion for a mixture of PAHs is based on the known additivity of narcotic chemicals. The toxic unit concentration corrected for solubility is calculated for each chemical in the mixture and then summed. A total toxic unit greater than one indicates a toxic mixture. The prediction is compared to the results from an experiment using sediment spiked singly and with a mixture of PAHs. The toxicity and amphipod abundance of field-collected sediments are also examined.
INTRODUCTION
The model used below to develop sediment quality guidelines (SQGs) is presented in the companion paper [1]. Equilibrium partitioning (EqP) [2] is used to convert water quality criteria (WQC) into the equivalent SQG. The model is similar to an amphipod toxicity model for a mixture of 13 polycyclic aromatic hydrocarbons (PAHs) presented by Swartz et al. [3] that relies on additivity, a QSAR that relates toxicity to the octanol/water partition coefficient, and EqP. The model is parameterized using data from amphipod sediment toxicity tests.
For narcotic chemicals as a class, there exist data for many more chemicals and many other species. Thus, a model that can describe the toxicity for type I narcotics including PAHs and that considers the species sensitivity explicitly can be used to develop more robust WQCs following the U.S. Environmental Protection Agency (U.S. EPA) technical guidelines [4] and sediment quality guidelines using EqP [2]. The water quality and tissue criteria are presented in the companion paper [1]. The application to sediments, PAHs, and mixtures is presented below.
PRELIMINARIES
Mixtures and additivity
 (1)
(1) (2)
(2) (3)
(3) (4)
(4)The additivity of the toxicity of narcotic chemicals has been demonstrated by a number of investigators. Figure 1, adopted from Hermens [6], presents the results of mixture experiments that employed a large enough number of narcotic chemicals so that nonadditive behavior would be easily detected. Three of the four experiments demonstrated essentially additive behavior, and the fourth, a chronic exposure, was almost additive. Thus, the correct use of the water and sediment quality guidelines for narcotic chemicals in general, and PAHs in particular, is as a mixture guideline.
Aqueous solubility constraint
The highest dissolved concentration in water that can be achieved by a chemical is its aqueous solubility S. Therefore, the maximum lipid concentration that can be achieved is limited as well. It is for this reason that the acute LC50 database that was used to generate the water quality criteria for narcotic chemicals was screened initially for LC50s < S [1]. This is also the reason that the LC50 database is limited to chemicals with log(KOW) ≤ 5.3. It appears that, for chemicals with higher KOWs, the aqueous solubilities are too low to result in the lipid concentration necessary to cause mortality of even the most sensitive species. The existence of a solubility cutoff was suggested by Veith et al. [7] based on data for fathead minnows and guppies.
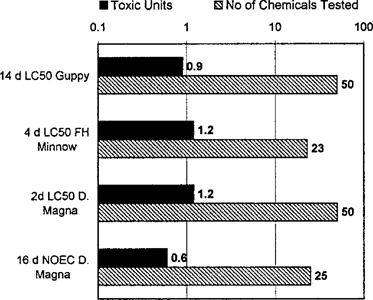
Additivity of type I narcosis toxicity (adopted from Hermens [6]). Comparison of the observed toxic unit (TU) concentration to the predicted TU = 1. Number of chemicals in the tested mixture is as indicated.
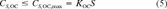 (5)
(5)The question of whether highly insoluble chemicals, e.g., heavy PAHs, contribute to toxicity when present as mixtures is currently being examined experimentally [8]. Narcosis theory suggests that they, in fact, should contribute fractional toxic units, limited by the solubility constraint (Eqn. 5). This observation points out the importance of knowing the aqueous solubility S of these chemicals so that Equation 5 can be applied reliably.
SEDIMENT QUALITY GUIDELINES
The EqP model can be used to predict sediment toxicity by applying water-only effects concentrations to pore-water concentrations [9]. For chemicals for which the pore-water-sediment partitioning is understood, a sediment effects concentration can also be derived [2]. For nonionic chemicals, partitioning is primarily determined by the organic carbon concentration of the sediment. Although there are some known exceptions, e.g., if the organic carbon fraction fOC < 0.1 to 0.2% or if equilibrium between pore water and the solid phase is not achieved, it is possible to predict the sediment effect concentrations from the effects concentration in water-only exposures. Since the target lipid model provides the water effects concentration, it can be used to establish sediment quality guidelines for narcosis chemicals in general and PAHs in particular.
 (6)
(6)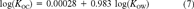 (7)
(7)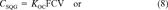 (8)
(8)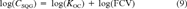 (9)
(9)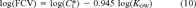 (10)
(10)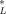 is the critical body burden corresponding to the FCV [1]. Using Equation 10 for the final chronic value and substituting Equation 7 for KOC yields
is the critical body burden corresponding to the FCV [1]. Using Equation 10 for the final chronic value and substituting Equation 7 for KOC yields
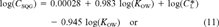 (11)
(11)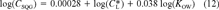 (12)
(12)This is the desired equation. For baseline narcotic chemicals, the critical body burden corresponding to the FCV is C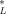 = 6.94 (μmol/g octanol and CSQG ranges from 6.94 to 10.8 (μmol/g OC over a range of log KOW of 0 to 5. For PAHs, C
= 6.94 (μmol/g octanol and CSQG ranges from 6.94 to 10.8 (μmol/g OC over a range of log KOW of 0 to 5. For PAHs, C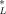 = 3.79 (μmol/g octanol and CSQG ranges from 4.9 to 5.9 μmol/g OC over a range of log KOW of 3 to 5. These results are listed in Table 1. Table 2 presents SQGs for the 23 PAHs that are quantified in the EMAP [11, 12] and NOAA National Status and Trends [13] data sets. Table 3 presents SQGs for type I narcotic chemicals that are reported to be found in sediments in the EPA STORET database. These are based on Equation 12 and the critical body burdens and chemical class corrections discussed above and in the companion paper [1].
= 3.79 (μmol/g octanol and CSQG ranges from 4.9 to 5.9 μmol/g OC over a range of log KOW of 3 to 5. These results are listed in Table 1. Table 2 presents SQGs for the 23 PAHs that are quantified in the EMAP [11, 12] and NOAA National Status and Trends [13] data sets. Table 3 presents SQGs for type I narcotic chemicals that are reported to be found in sediments in the EPA STORET database. These are based on Equation 12 and the critical body burdens and chemical class corrections discussed above and in the companion paper [1].
It should be pointed out that, for the PAHs in Table 2 with log(KOW) ≳ 5, these criteria are an extrapolation beyond the limits of the data set used to validate the target lipid model. Their validity will be examined below by comparison to laboratory and field data sets.
Multiple phase equilibrium
 (13)
(13) (14)
(14) (15)
(15)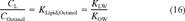 (16)
(16) (17)
(17) (18)
(18)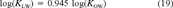 (19)
(19)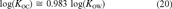 (20)
(20)| Octanol/water partition coefficient | KOW | 100 | 103 | 105 | |
|---|---|---|---|---|---|
| Baseline narcotics | Tissue | C (μmol/g octanol)a (μmol/g octanol)a |
6.94 | 6.94 | 6.94 |
| Sediment | CSQG (μmol/g OC) | 6.94 | 9.03 | 10.8 | |
| PAHs | Tissue | C (μmol/g octanol) (μmol/g octanol) |
3.79 | 3.79 | 3.79 |
| Sediment | CSQG (μmol/g OC) | 3.79 | 4.93 | 5.87 |
-
a The units μmol/g octanol reflect the fact that these concentrations are obtained from the y-intercept of the LC50-KOW relationship. The experimental finding that measured tissue lipid concentrations (μmol/g lipid) are equal to the predicted C
 supports their use as tissue criteria.
supports their use as tissue criteria.
For KOW > 1, narcotic chemical concentrations are lower in lipid than they are in octanol and the ratio decreases as the KOW increases (Table 4, line (a)). For organic carbon, the concentration is also lower than in octanol but less so than in lipid (Table 4, line (b)). As a consequence, the concentration in organic carbon is slightly higher than in lipid (Table 4, line (c)). Hence, a slightly larger concentration of chemical in organic carbon is necessary to achieve a prescribed concentration in lipid. This is the reason that the organic carbon normalized guidelines for PAHs in sediments are slightly higher than the critical body burden. The CSQG ranges from 4.9 to 5.9 μmol/g OC over a range of log KOW of 3 to 5 for a critical body burden of C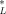 = 3.79 μmol/g lipid.
= 3.79 μmol/g lipid.
| Cs,OC | Cs,OC,max | |||||
|---|---|---|---|---|---|---|
| Chemical | CAS number | MW (g/mol) | log(KOW) | log(S)b (mol/L) | (μmol/g OC) | |
| Acenaphthylenec | 208968 | 152.2 | 3.22 | −3.97 | 5.03 | 158 |
| Naphthalenec | 91203 | 128.19 | 3.36 | −3.62 | 5.09 | 482 |
| 1-Methylnaphthalene | 90120 | 142.2 | 3.84 | −3.71 | 5.31 | 1,160 |
| 2-Methylnaphthalene | 91576 | 142.2 | 3.86 | −3.75 | 5.32 | 1,088 |
| Acenaphthenec | 83329 | 154.21 | 4.01 | −4.61 | 5.39 | 217 |
| Fluorenec | 86737 | 166.2 | 4.21 | −4.94 | 5.48 | 157 |
| 2,6-Dimethylnaphthalene | 581420 | 156.23 | 4.37 | −4.96 | 5.56 | 217 |
| Anthracenec | 120127 | 178.2 | 4.53 | −6.60 | 5.64 | 7.25 |
| Phenanthrenec | 85018 | 178.2 | 4.57 | −5.21 | 5.66 | 192 |
| 2,3,5-Trimethylnaphthalene | 2245387 | 170.26 | 4.86 | −4.43d | 5.80 | 2,230 |
| Pyrenec | 129000 | 202.26 | 4.92 | −6.19 | 5.83 | 45.0 |
| 1-Methylphenanthrene | 832699 | 192.26 | 5.04 | −5.85 | 5.89 | 127 |
| Fluoranthenec | 206440 | 202.26 | 5.08 | −5.93 | 5.92 | 118 |
| Benzo[a]anthracenec | 56553 | 228.29 | 5.67 | −7.32 | 6.23 | 18.2 |
| Chrysenec | 218019 | 228.29 | 5.71 | −8.06 | 6.25 | 3.62 |
| Benzo[a]pyrenec | 50328 | 252.31 | 6.11 | −7.82 | 6.47 | 15.2 |
| Perylene | 198550 | 252.31 | 6.14 | −8.80 | 6.49 | 1.71 |
| Benzo[e]pyrene | 192972 | 252.32 | 6.14 | −7.80 | 6.49 | 17.1 |
| Benzo[b]fluoranthenec | 205992 | 252.32 | 6.27 | −8.23 | 6.56 | 8.60 |
| Benzo[k]fluoranthenec | 207089 | 252.32 | 6.29 | −8.50 | 6.58 | 4.85 |
| Benzo[ghi]perylene | 191242 | 276.34 | 6.51 | −9.03 | 6.70 | 2.35 |
| Dibenz[a,h]anthracene | 53703 | 278.35 | 6.71 | −8.67 | 6.82 | 8.58 |
| Indeno[1,2,3-cd]pyrene | 193395 | 276.34 | 6.72 | −8.37d | 6.83 | 17.2 |
| Chemical | Rankb | CAS number | MW (g/mol) | log(KOW) | log(S) (mol/L) | C S,OC (μmol/gOC) |
|---|---|---|---|---|---|---|
| Acetone | 175 | 67641 | 58.08 | −0.16 | 1.14 | 3.90 |
| 2-Butanone | 188 | 78933 | 72.11 | 0.32 | 0.45 | 4.06 |
| Chloromethane | 132 | 74873 | 50.49 | 0.68 | −1.18 | 4.20 |
| 4-Methyl-2-pentanone | 186 | 108101 | 100.16 | 1.17 | −0.06 | 4.38 |
| Methylene chloride | 107 | 75092 | 84.93 | 1.18 | −0.68 | 4.39 |
| Benzyl alcohol | 147 | 100516 | 108.14 | 1.20 | −0.73 | 7.71 |
| 2-Hexanone | 182 | 591786 | 100.16 | 1.29 | −0.22 | 4.42 |
| 1,2-Dichloroethane | 115 | 107062 | 98.96 | 1.40 | −0.94 | 4.48 |
| Chloroethane | 111 | 75003 | 64.52 | 1.42 | −1.07 | 4.48 |
| 1,1-Dichloroethane | 121 | 75343 | 98.96 | 1.82 | −1.30 | 4.64 |
| 1,3-Dichloropropane | 165 | 142289 | 112.99 | 1.84 | −1.44 | 4.65 |
| 1,2-Dichloropropane | 124 | 78875 | 112.99 | 1.86 | −1.47 | 4.66 |
| Chloroform | 100 | 67663 | 119.38 | 1.91 | −1.50 | 4.68 |
| 1,1,2-Trichloroethane | 109 | 79005 | 133.40 | 1.91 | −1.43 | 4.68 |
| Bis(chloromethyl) ether | 145 | 542881 | 114.96 | 1.92 | −4.63 | 4.68 |
| Bromodichloromethane | 123 | 75274 | 163.83 | 1.97 | −1.51 | 4.70 |
| Benzene | 42 | 71432 | 78.11 | 2.00 | −1.59 | 8.27 |
| trans-1,2-Dichloroethylene | 119 | 156605 | 96.94 | 2.10 | −1.69 | 4.76 |
| Chlorodibromomethane | 120 | 124481 | 208.29 | 2.12 | 4.77 | |
| Bromoform | 110 | 75252 | 252.73 | 2.25 | −2.19 | 4.82 |
| 1,1,2,2-Tetrachloroethane | 108 | 79345 | 167.85 | 2.31 | −1.74 | 4.85 |
| Dichlorodifluoromethane | 154 | 75718 | 120.91 | 2.31 | −2.67 | 4.85 |
| 1,1,1-Trichloroethane | 99 | 71556 | 133.40 | 2.38 | −2.18 | 4.88 |
| Trichlorofluoromethane | 129 | 75694 | 137.37 | 2.53 | −2.12 | 4.94 |
| Chlorobenzene | 112 | 108907 | 112.56 | 2.58 | −2.49 | 4.96 |
| Toluene | 103 | 108883 | 92.14 | 2.62 | −2.22 | 8.73 |
| Styrene | 178 | 100425 | 104.15 | 2.72 | −2.26 | 8.81 |
| Carbontetrachloride | 116 | 56235 | 153.82 | 2.73 | −2.61 | 5.03 |
| Trichloroethylene | 98 | 79016 | 131.39 | 2.81 | −2.44 | 5.06 |
| Ethylbenzene | 106 | 100414 | 106.17 | 3.06 | −2.66 | 9.08 |
| Xylene | 166 | 106.17 | 3.20 | −2.79 | 9.19 | |
| 1,4-Dichlorobenzene | 72 | 106467 | 147.00 | 3.24 | −3.24 | 5.26 |
| 1,2-Dichlorobenzene | 73 | 95501 | 147.00 | 3.31 | −3.29 | 5.29 |
| 1,3-Dichlorobenzene | 77 | 541731 | 147.00 | 3.31 | −3.28 | 5.29 |
| Tetrachloroethylene | 105 | 127184 | 165.83 | 3.38 | −3.15 | 5.31 |
| Hexachloroethane | 82 | 67721 | 236.74 | 3.58 | −4.85 | 5.42 |
| 2-Chloronaphthalene | 91 | 91587 | 162.62 | 3.88 | −4.00 | 3.03 |
| Dibenzofuran | 138 | 132649 | 168.20 | 3.95 | −4.63 | 9.81 |
| 1,2,4-Trichlorobenzene | 78 | 120821 | 181.45 | 4.00 | −4.05 | 5.62 |
| 4-Chloro diphenyl ether | 96 | 7005723 | 204.66 | 4.77 | −5.01 | 6.01 |
| 4-Bromo diphenyl ether | 93 | 101553 | 249.11 | 5.06 | −5.39 | 6.17 |
- a CAS = chemical abstract number; MW = molecular weight.
- b Ranking is based on the number of times the chemical is reported to be found in sediments in the STORET database.
It may be that these small differences (Table 4) are due to kinetic effects, i.e., are the result of incomplete equilibration, and that it is actually the case that lipid = organic carbon = octanol. However, regression analyses restricted to log(K OW) < 3.5, 4.0, etc., gave essentially the same slopes, indicating that the slope in Equation 19 is not determined by the more hydrophobic chemicals [1]. Furthermore, for applications where the exposure time is similar to that for which the data were generated, e.g., analyzing 96-h tests, these differences are real and need to be considered.
| KOctanol, Water | KOW | 100 | 101 | 103 | 105 | 107 |
|---|---|---|---|---|---|---|
| KLipid, Octanol | K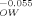 |
1.00 | 0.881 | 0.684 | 0.531 | 0.412 (a) |
| KOrganic carbon, Octanol | K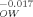 |
1.00 | 0.962 | 0.890 | 0.823 | 0.761 (b) |
| KLipid, Organic carbon | K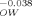 |
1.00 | 0.916 | 0.769 | 0.645 | 0.542 (c) |
Biota sediment accumulation factor and metabolism
 (21)
(21)The reason for this wide range of BSAFs is that PAHs are metabolized by certain organisms [16]. Since the data upon which the target lipid model narcotic criteria are based come from both short- and long-term exposures—the acute and chronic databases—the effect of metabolism is implicitly included. The question is why the target lipid model, which ignores metabolism entirely, appears to describe narcosis toxicity. The only plausible explanation is that a large portion of the metabolic end products remain in the organism. Since narcosis toxicity is additive (Fig. 1), these metabolites exert an equivalent toxicity. The decrease in the parent compound is compensated for by an increase in metabolites. The BSAF for the parent compound reflects the reduction due to metabolism, but the toxicity is much less affected.
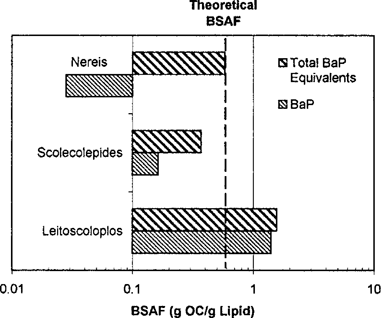
Polychaete bioaccumulation experiments by Driscoll and McElroy [17] utilizing radiolabeled benzo[a]pyrene (BaP) for a 9-d exposure clearly demonstrate this effect (Fig. 2). The BaP BSAFs for three polychaetes range from 0.028 to 1.40, which illustrates the wide variation in metabolism that can occur. However, if total BaP equivalents based on the radioactivity are used, then the BSAFs range from 0.58 to 1.56, a much smaller variation [17]. Figure 2 also includes the theoretical BSAF for BaP (Table 4, line (c)) that is similar to the observed total BaP data. Some reduction in toxicity due to metabolic activity and excretion of the metabolites must occur, but it appears to be of secondary importance, although it may be a reason for the observed range in species sensitivity and the occasional outlier in the toxicity data [1].
APPLICATION TO POLYAROMATIC HYDROCARBONS
The motivation for the development of the target lipid model was to apply it to mixtures of PAHs and other persistent narcotic chemicals in sediments. The narcosis database used to determine the universal narcosis slope and the critical body burdens consists of 145 chemicals, of which 10 are unsubstituted and substituted PAHs [1]. A comparison of the LC50 data for just these chemicals and the target lipid model is shown in Figure 3. The solid log(LC50)–log(KOW) lines are computed using the universal narcosis slope and the appropriate body burdens for PAHs for each organism listed. The dotted lines apply to the chloronaphthalenes that have a slightly lower critical body burden due to the halogen substitution [1]. The lines are an adequate fit of the data, although the scatter in the Daphnia data is larger than some of the other species with multiple sources of data and there is a clear outlier for Mysidopsis. The 10-d Rhepoxynius data are discussed below.
It is clear from this plot that fitting a narcosis model to just the PAH data would yield less certain estimates for the slopes and critical body burdens. In contrast to this, the target lipid model is fit to the entire narcosis data set that produces more reliable estimates of the universal slope, critical body burdens, and chemical class corrections.
Mixture experiment
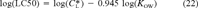 (22)
(22)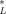 = 12.2 μmol/g octanol. This relationship is shown in Figure 3 (lower right-hand side). With the exception of the one data point at log(KOW) = 3.3, the narcosis slope appears to be representative. Due to its deviation from the universal narcosis slope, this data point is considered an outlier and has been excluded from the analysis.
= 12.2 μmol/g octanol. This relationship is shown in Figure 3 (lower right-hand side). With the exception of the one data point at log(KOW) = 3.3, the narcosis slope appears to be representative. Due to its deviation from the universal narcosis slope, this data point is considered an outlier and has been excluded from the analysis.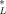 = 12.2 μmol/g octanol replacing the criteria value in Equation 12.
= 12.2 μmol/g octanol replacing the criteria value in Equation 12.
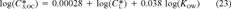 (23)
(23)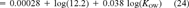 (24)
(24)The resulting C*S,OC are the predicted organic carbon normalized LC50s. For acenaphthene, phenanthrene, fluoranthene, and pyrene, they range from 17.3 to 19.0 μmol/g OC, with an average of 18.3 μmol/g OC.
 (25)
(25)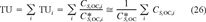 (26)
(26)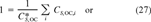 (27)
(27) (28)
(28)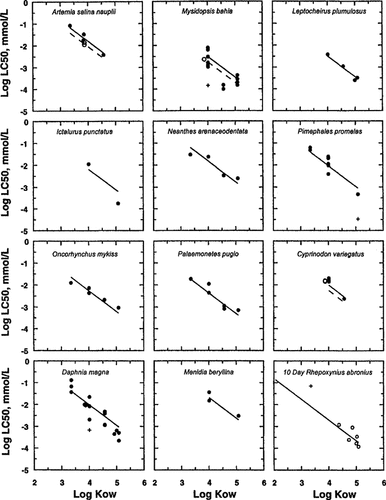
Comparison of target lipid model fit and observed LC50 data for polycyclic aromatic hydrocarbons (PAHs) and the indicated species. The PAHs included are naphthalene (3.36), 1-methylnaphthalene (3.84), 2-methylnaphthalene (3.86), 2-chloronaphthalene (3.88), 1-chlorona-phthalene (3.88), acenaphthene (4.01), phenanthrene (4.57), pyrene (4.92), 9-methylanthracene (5.01), fluoranthene (5.08). Number in parentheses is log(KOW). Solid line and filled symbols for nonhalogenated PAHs. Dotted line and unfilled symbols for the halogenated (i.e., chlorinated) PAHs: + denotes outliers (see [1] for criteria).
This comparison is made in Figure 4A. The line is the predicted LC50. The two parallel lines represent ± a factor of two, which is the approximate uncertainty of EqP-derived sediment organic carbon normalized LC50s. As can be seen, the model reproduces the observed dose–response for both the single chemical and mixture spiked sediment data.
PAHs with log(KOW) > 5
The PAHs employed in the mixture tested in the experiment analyzed above all have log(KOW) < 5. The remaining question is whether the highly insoluble PAHs with log(KOW) > 5 (and, by implication, all the other insoluble chemicals) contribute to narcotic toxicity, each limited by their aqueous solubility. Narcosis theory suggests that all the organic chemicals in a sediment should contribute additively to the toxicity exhibited by that sediment. This conclusion follows from the additivity of narcotic chemicals. However, the bioavailability of chemicals is constrained by their aqueous solubility. This is the explanation for the lack of any reported toxicity for single chemicals with log(KOW)≳ 5.
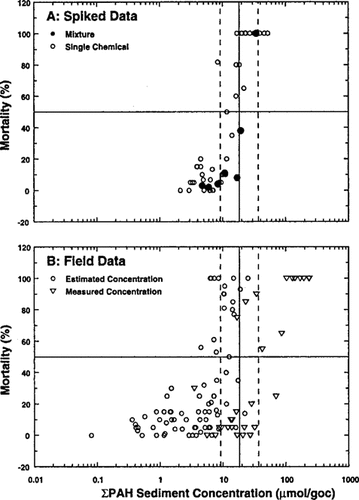
Comparison of observed morality in Rhepoxynius abronius 10-d sediment toxicity tests. (A) Data from the spiked mixture experiment of Swartz et al. [18] for either the individual PAHs (º) or the sum of the PAH concentrations (•). (B) Data from Swartz et al. [3] using the estimated total of 34 PAHs from the measured 13 PAHs (º) or the measured 34 PAHs from the Ozretich et al. [19] Elliot Bay data set (▽).
An experiment has been performed by Spehar et al. [8] that directly addresses this issue. A mixture of 13 PAHs was employed, with each log(KOW) ranging from 5.36 to 6.76, of which seven had log(KOW) > 6. The concentration of each chemical was limited either to 0.5 TUs or less if limited by its solubility. The TUs were computed from an estimate of the critical body burden for Hyalella azteca, the test animal employed. The test duration was 28 and 10 d for flow-through and static exposures, respectively, with survival, growth, and reproduction measured. In addition, Leptocheirus plumulosus were also tested using the same mixture of PAHs. Significant mortality and growth effects occurred at a total PAH tissue concentration of 20 to 35 μmol/g lipid. This experiment clearly shows that high KOW PAHs can collectively cause toxicity by additive narcosis at tissue concentrations predicted from the target lipid model.
Thus, the available experimental information supports the extension of the narcosis model beyond the limit of log(KOW) ≲ 5, which is the limit of the single chemical data employed in its derivation.
FIELD DATA
The ultimate test of validity of sediment guidelines is their predictive ability, i.e., can they be used to predict effects seen in field-collected samples. Unfortunately, the problem of validation using field-collected samples has no straightforward solution. It is extremely difficult to separate actual cause and effect from simple correlation. The primary reason is the presence of covariation of many chemical contaminants in field-collected sediments, some of which may be unmeasured. Therefore, it cannot be presumed that the response is due to only the chemical(s) being investigated.
However, one fact is unequivocal. If the guideline predicts an effect, e.g., 50% mortality of a test organism, at a certain chemical concentration and the organism survives exposures above that concentration, then the criterion is invalidated. No other comparison is definitive. Mortality at concentrations below the predicted effects concentration may be due to other causes and provides no evidence for the validity or invalidity of the prediction.
Toxicity tests
A set of toxicity data has been assembled by Swartz et al. [3] from locations for which PAHs are suspected to be the primary cause of toxicity. In addition, a set of data obtained by Ozretich et al. [19] from Elliott Bay is also available. For these data sets, the number of PAHs included in the total PAH concentration depends on (1) the number that were explicitly measured and (2) the number that would be expected to be present.
This issue is discussed at some length in the U.S. EPA's ESG (Equilibrium Partitioning Sediment Guidelines) [20]. The most complete data sets comprise 34 PAHs that include the commonly measured parent, i.e., unsubstituted, PAHs as well as some alkylated homologous series that are less frequently measured. It is shown that the toxicity of sediment-associated PAHs can be severely underestimated if these alkylated PAHs are not considered. A method for estimating the total of the 34 PAH concentrations using either a subset of 13 or 23 commonly measured PAHs is provided in the document. The median ratio of the concentration of 34 PAHs to the concentration of the 13 PAHs is 2.75.
The Ozretich et al. [19] Elliott Bay data set had measurements for 33 PAHs that included some alkylated homologous series. The alkylated PAHs were well represented. Thus, the reported total concentrations were used without any correction factor. The Swartz et al. [3] data set had measurements for only 13 PAHs. The estimated total concentration of the 34 PAHs is obtained by multiplying the reported total concentration of the 13 PAHs by 2.75 [20].
Figure 4B is a comparison of observed mortality of Rhepoxynius abronius in the standard 10-d sediment test to the total PAH concentration for both data sets. The line is the predicted LC50 = 18.3 μmol/g OC obtained from the pore-water data [19] analyzed above. The measured and estimated data refer to the Elliott Bay and the Swartz et al. [3] data sets, respectively.
Consider first the data for which the total PAH concentration is measured. There is only one point below 50% mortality. All the rest exhibited >50% mortality, consistent with the prediction. Strictly speaking, that one observation below 50% mortality invalidates the guideline since less than 50% of the test organisms died. However, since it is only one test, we regard it as a statistical fluctuation. However, if many of the tests exhibited that behavior—surviving when the observed total PAH concentration exceeded the predicted LC50—then these observations would invalidate the guideline.
For the remaining data, the total PAH concentrations are estimated from the 13 measured PAHs. A number of sediments exceeded 50% mortality at concentrations below ˜10 μmol/g OC, the lower bound of the predicted LC50 in Figure 4B. The total PAH concentration is estimated using the median ratio of the observed total of 34 PAHs to the total of 13 PAHs. This ratio has 90% confidence limits of 1.57 to 16.9 [20]. Thus, the deviations are well within the probable error of this estimate.
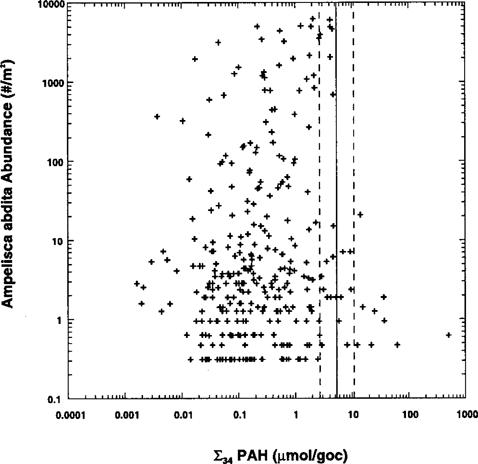
Organism abundance
Another test of the guidelines is observations of the organism density of sensitive animals infield-collected samples. Figure 5 presents the observed Ampelisca abdita abundance versus estimated total PAH. The data are from sediments collected as part of the Virginian and Louisianian province EMAP [11, 12] and the New York/New Jersey Harbor REMAP [13] sediment sampling programs. The total PAH estimate is obtained using the sum of the 23 measured PAHs and the median ratio of 1.64 (90% confidence limits: 1.12–4.14) [20] between the 23 PAHs and the 34 PAHs found in the EMAP data sets. The identity of the measured PAHs are listed in Table 2. The vertical lines are the averages of the FCV sediment quality guidelines listed in Table 2. The results are very encouraging. All the sediments that exceed the FCV guideline have significantly lower amphipod abundance, consistent with the narcosis prediction.
It is tempting to conclude from the coincidence of the predicted effect concentration and the drop in amphipod abundance that in fact these data support the validity of the guidelines. However, it should be pointed out again that these data can only be used to demonstrate that the guidelines are not in conflict with observations. They cannot be used to validate the guidelines. For example, a very much larger effects concentration would also fail to be invalidated. However, these data would invalidate a much lower concentration since an effect would be predicted and none would be observed. We return to this observation later.
The validation procedure requires sediments for which the nature of all the biologically active chemicals are known and quantified. This is only satisfied with laboratory-spiked sediments where the identity of the toxic components is certain, hence, the importance of the experimental demonstration of the validity of the narcosis mixture guidelines (Fig. 4A).
Resistant PAH phases and soot carbon sorption
The comparison of the target lipid model narcotic SQG using field-contaminated sediments rather than laboratory-spiked sediments is important because there is concern that solid phase PAHs might be present in field-contaminated sediments that are not in equilibrium with the pore water, thereby invalidating Equation 8 and, presumably, the sediment quality guidelines. Both chemical [21, 22] and biological [23] data have been used to suggest that this may be occurring in certain situations. The idea is that particles of coal or tar-like materials may be present. They would be measured as part of the sediment PAH concentration, but if they are kinetically inhibited from dissolving, then Equation 8 would not apply.
The toxicological data presented by Paine et al. [23] for Rhepoxynius abronius 10-d mortality tests indicates 75% survival at total PAH concentrations greater than the 10-d LC50 of 18.3 μmol/g OC ⋍ 3,200 μg/g OC ⋍ 32.0 μg/g for a sediment with fOC = 1% OC. The sediments are reported to contain visible pitch and/or coke particles or globules. The organic carbon content of many samples with high PAH concentrations was also elevated, suggesting significant quantities of pitch and/or coke [23].
 (29)
(29)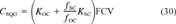 (30)
(30)It is important to note that both of these phenomena, the lack of equilibrium and the presence of a more highly sorptive phase, would result in higher sediment quality guideline concentrations. Thus, at a minimum, the proposed criteria without the modification in Equation 30 or a method to quantify lack of equilibrium can still be used reliably to assess sediments for the lack of toxicity. If the guideline is exceeded, then the other tenet of the EqP model, that pore-water concentrations can be used to predict the toxicity of sediments [2], can be used together with the FCVs (Table 6 in [1]) to make an assessment.
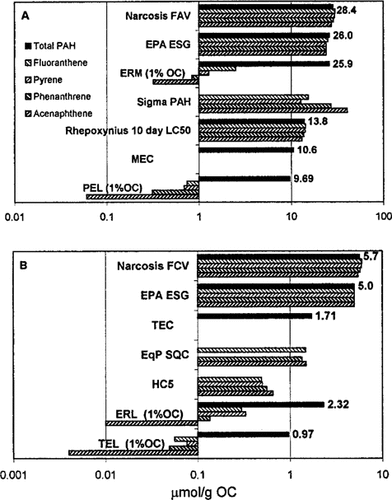
Comparison of various estimates of sediment effects concentrations. The effects range low (ERLs) and effects range median (ERMs) from Long and Morgan [25]; threshold effects level (TELs) and probable effects level (PELs) from MacDonald et al. [26]; HC5 from Van Leeuwen et al. [27]; ΣPAH from Swartz et al. [3]: EPA SQC from [28-30]; threshold effects concentration (TEC) and median effects concentration (MEC) from Swartz [31]; Rhepoxynius 10-d LC50 from Swartz et al. [18]; EPA ESG from [20]; narcosis final acute value (FAV) and final chronic value (FCV) from this work.
However, the comparisons presented in Figures Fig. 5., Fig. 6. suggest that this problem may not be a common occurrence since there are no sediments in these comparisons with predicted but not observed effects, which would be the consequence of significant soot carbon partitioning and lack of pore-water equilibrium.
Other PAH effects
The basis for the sediment quality guidelines presented in this paper are the acute and chronic toxicity data included in the analysis. The endpoints considered are mortality, growth, and reproduction. Other endpoints associated with PAHs, e.g., tumors and immunological and endocrine disruption, are not specifically addressed. To the extent that these affect the ability of organisms to survive and reproduce, which are the endpoints that are considered, their effects are implicitly included in the guidelines. A more detailed analysis of these effects is available as part of the U.S. EPA Equilibrium Partitioning Sediment Guidelines (ESG) for PAHs [20].
COMPARISON TO OTHER APPROACHES
There are many other sediment criteria and guidelines that have been suggested for individual and for total PAHs as mixtures. Table 5 lists a representative sample. The effects range low and effects range median [25] and the threshold effects level and probable effects level [26] are estimates of threshold and definite effects levels derived from an analysis of field data sets. They are based on dry weight concentrations and therefore ignore the effect of organic carbon on bioavailability of nonionic hydrophobic organic chemicals in sediments [2].
The HC5 concentrations [27] are based on an analysis similar to the multilinear regression analysis used in the target lipid model [1]. The QSARs for the effects of narcotic chemicals and KOWs were developed for 19 species where species-specific slopes and y-intercepts were computed. The QSARs represent chronic toxicity and were expressed on a NOEC (no-observed-effect concentration) basis. If the data were acute, an acute-to-chronic ratio (ACR) was applied to convert the data to chronic values. From the individual QSARs, concentrations were extrapolated to a level that protects 95% of the aquatic community. These levels were denoted as HC5, the hazardous concentration for 5% of the species.
The EqP SQG estimates are derived from the final chronic values computed from aquatic toxicological data for the individual chemicals [28-30] following the U.S. EPA technical guidelines [4]. Equilibrium partitioning is used to derive the sediment quality guidelines.
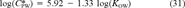 (31)
(31)The threshold effects concentration and median effects concentration are threshold and median consensus values derived by Swartz [31] from an analysis of various suggested guidelines, including the results of the ΣPAH model [3].
The U.S. EPA's ESG (Equilibrium Partitioning Sediment Guidelines) [20] are derived following the National Water Quality Criteria Guidelines [4]. The acute toxicity data base included 104 (77) freshwater (saltwater) water-only tests employing 12 (8) different PAHs and 24 (30) species from 20 (29) genera. The narcosis model was used to translate these water-only LC50s to critical body burdens using the universal narcosis slope (Eqn. 22). This is a much larger database than the PAHs included in the narcosis database (see Fig. 3).
A comparison of these guidelines is facilitated by a graphical presentation (Fig. 6). The guidelines that are expressed on a dry weight basis are converted to molar concentrations using the molecular weight (mol wt) for each chemical, or an average mol wt = 173 g/mol for total PAH, and an assumed organic carbon fraction of one percent, i.e., fOC = 0.01. For criteria that apply to individual PAHs only, the total PAH criteria are computed from the average of five PAHs as indicated.
It should be pointed out that there are substantial variations in sediment organic carbon. For the EMAP data sets, the median fOC is 1% but the 68% range (the log mean ± 1 log standard deviation) is from 0.3 to 3% organic carbon, an order of magnitude. Therefore, the dry weight normalized guidelines have a one order of magnitude uncertainty when the bioavailable chemical concentration is considered. Therefore, the dry weight guidelines will be denoted as median effects range median, etc.
| Acenaphthene | Phenanthrene | Pyrene | Fluoranthene | Total PAH | |
|---|---|---|---|---|---|
| (μmol/g OC) | |||||
| TEL (a)c | 0.004 | 0.049 | 0.076 | 0.056 | 0.97d |
| ERL (b)c | 0.010 | 0.135 | 0.329 | 0.297 | 2.32d |
| HC5 (c) | 0.65 | 0.56 | 0.50 | 0.49 | |
| EqP SQC (d) | 1.49 | 1.35 | 1.48 | ||
| TEC (e) | 1.71 | ||||
| U.S. EPA ESG (f) | 3.17 | 3.33 | 3.43 | 3.48 | 3.35e |
| Narcosis FCV (i) | 5.39 | 5.66 | 5.83 | 5.92 | 5.70e |
| PEL (a)c | 0.06 | 0.31 | 0.69 | 0.74 | 9.69d |
| MEC (e) | 10.6 | ||||
| Rhepoxynius | |||||
| 10-d LC50 (g) | 13.1 | 13.7 | 14.1 | 14.3 | 13.8 |
| EPA ESG (f) | 13.2 | 13.8 | 14.3 | 14.5 | 13.9e |
| X PAH (h) | 40.6 | 27.0 | 12.8 | 15.3 | |
| ERM (b)c | 0.32 | 0.84 | 1.29 | 2.52 | 25.9d |
| Narcosis FAV (i) | 27.4 | 28.8 | 29.7 | 30.1 | 29.0e |
- a TEL = threshold effects level; ERL = effects range low; HC5 = hazardous concentration for 5% of species; EqP SQC = equilibrium partitioning sediment quality criteria; TEC = threshold effects concentration; U.S. EPA ESG = U.S Environmental Protection Agency equilibrium partitioning sediment guidelines; FCV = final chronic value; PEL = probable effects level; MEC = median effects concentration; ERM = effects range median.
- b In the first column, (a) = MacDonald et al. [26]; (b) = Long et al. [35]; (c) = Van Leeuwen et al. [27]; (d) = U.S. EPA [28-30]; (e) = Swartz [31]; (f) = U.S. EPA [20]; (g) = Swartz et al. [18]; (h) = Swartz et al. [3]; (i) = this work.
- c Computed assuming fOC = 1.0%.
- d Computed using average molecular weight, MW = 173.
- e Average of acenaphthene, anthracene, phenanthrene, pyrene, and fluoranthene.
For total PAHs, the acute guidelines based on the narcosis final acute value (FAV) is the largest of the suggested concentrations (Fig. 6A). The U.S. EPA ESG is essentially the same. The median effects range median is remarkably close, and the median effects concentration and median probable effects level are approximately a factor of three lower. The narcosis FAV concentrations for each individual PAH are also essentially constant at 27 to 30 μmol/g OC. This is in sharp contrast to the median effects range median and probable effects level, which are a factor of 10 to 100 smaller for the individual PAHs. Since PAHs are all narcotic chemicals, it is virtually impossible that these low concentrations would, by themselves, produce mortality, growth, or reproduction effects. The Rhepoxynius abronius 10-d LC50s (Table 5) are included to support this observation. These median effects range median and probable effects level concentrations are more a reflection of the composition of PAHs in toxic sediments, since they are derived by analyzing the chemical concentrations in toxic sediments [32], rather than a reflection of the toxicity of the individual chemicals. Swartz [31] has examined these relationships more closely. The SQGs derived from the ΣPAH model have an inverse relationship to KOW due to the large negative slope (Eqn. 31).
The final chronic value (FCV) narcotic guidelines for total PAH are also the largest concentrations of the threshold values (Fig. 6B). Again, the U.S. EPA ESG is essentially the same. The other total PAH concentrations are a factor of three lower. The individual PAH concentrations for the median effects range low and threshold effects level are one to two orders of magnitude lower. The explanation is the same as given above.
The HC5 values (˜0.5 μmol/g OC) [27] are approximately an order of magnitude lower than the FCV narcotic estimate (5.70 μmol/g OC). The reason is that the extrapolation used to compute the fifth percentiles for these guidelines considers not only the variation in the intercepts of the regressions but is also sensitive to the variations in the species-specific slopes. The target lipid model considers only the variations in the intercepts. The statistical test applied to the slopes of the narcosis database failed to reject the hypothesis of a single universal narcosis slope [1]. Therefore, the variations in slopes, which are amplified as the lines are extrapolated beyond the range of data for each line, are more a reflection of the statistical uncertainty of the slope estimate rather than a measure of species sensitivity. Nevertheless, these variations contribute variability, which produces a lower fifth percentile. Note that the resulting HC5 concentration of ˜0.5 μmol/g OC is considerably lower than the ˜3.0 μmol/g OC threshold limit above which Ampelisca abundance declines (Fig. 5). Assuming that this is a measure of the threshold of effects that the HC5 is protective of, the coexistence of apparently abundant populations and the HC5 concentration suggests that this concentration is too low.
Therefore, it appears that the SQGs, which are based on the target lipid model of narcosis toxicity, should be preferred over the other sediment guidelines. They properly account for mixtures of PAHs, which is always the situation encountered in contaminated sediments. They are based on a very large narcotic chemical database that results in more reliable estimates of the universal narcosis slope and the critical body burdens from which the criteria are derived. They are based on direct experimental determinations of acute and chronic effects of narcotic chemicals and PAHs, so causality is not in question. They do not predict effects when none are observed except as discussed above where the partitioning model breaks down. For these cases, the guidelines are conservative.
In contrast, the empirically based guidelines are based on dry weight normalized concentrations so that they make no correction for bioavailability. And since they are derived from tests and observations using field collected sediments, which contain many covarying chemicals, they have no cause and effect basis. This is the reason that the empirical guidelines for individual PAHs are unrealistically low.
CONCLUSIONS
The target lipid narcosis model has been used to derive sediment quality guidelines for narcotic chemicals in general and PAHs in particular. Guideline concentrations corresponding to the FAV and FCV levels of protection are almost constant over the range of KOWs characteristic of PAHs until solubility lowers the applicable concentrations. The total PAH guideline is evaluated using strict additivity. The predictions of the target lipid model are verified using the results of a spiked sediment mixture experiment.
The predictions are compared to the results of Rhepoxynius toxicity tests using field-collected sediments from locations where PAHs are suspected to be the primary cause of toxicity. The results are consistent with the predictions when 31 PAHs were measured. For the data when only 13 PAHs and no alkylated PAHs were quantified, the results are comparable if an estimate of the total PAH concentration is used.
Observations of Ampelisca abundance in field-collected sediments is consistent with the prediction of benthic effects when the narcosis FCV guideline is exceeded. The lower effects levels suggested by others are inconsistent with these data.
A situation has been reported where Rhepoxynius toxicity is predicted but not observed. This is attributed to the presence of visible pitch and/or coke particles or globules that are not in equilibrium with the pore water.
Empirically derived guidelines for total PAH are roughly equal to, or a factor of three lower, for a 1% organic carbon sediment. However, there is a one order of magnitude uncertainty due to the variation in organic carbon concentrations in sediments that they do not account for. The individual PAH empirical guidelines are one to two orders of magnitude smaller than the narcotic concentrations that are known to cause mortality, growth, or reproduction effects and therefore are not reflective of actual effects concentrations for these endpoints.
Acknowledgements
This work was performed for the U.S. Environmental Protection Agency (U.S. EPA), Office of Water, with additional support from a Cooperative Agreement with Manhattan College. We wish to thank our project officer Mary Reiley and Heidi Bell for their support and our colleagues Walter Berry, Rob Burgess, Dave Hansen, Dave Mount, Bob Ozretich, Bob Spehar, and Rick Swartz for generously sharing their data prior to publication and for their help and advice.




