Tissue carboxylesterase activity of rainbow trout
Abstract
The activity of carboxylesterase (CaE), a class of nonspecific serine hydrolases, was evaluated in vitro in tissues and microsomes of rainbow trout and compared to esterase activity in rats, other fish species, and embryo to adult life stages of trout. Trout gill and liver microsomes exhibited substantial CaE activity and limited variation over the range of 2 to 40°C, with a temperature optimum of approximately 22°C. Trout sera and rat liver microsomes exhibited a temperature optimum of approximately 35 to 40°C. The CaE of trout liver (maximum reaction rate [Vmax] = 672 nmol/min/mg microsomal protein) was four times less than in rats. Apparent Michaelis constant (Km) values ranged from 28 (trout liver) to 214 (trout sera) μM. Values of Vmax/Km suggested that in vivo CaE activity of trout liver would be about three times higher than serum, 135 times higher than gill, and three times lower than rat liver. The CaE activity in whole rainbow trout homogenates significantly increased 300% per gram of tissue to 1,200% per milligram of protein between the yolk-sac and juvenile stages. The CaE activity of whole fish homogenates was not significantly different in juvenile rainbow trout, channel catfish, fathead minnows, and bluegill. The results demonstrate that rainbow trout had high esterase activity over a broad range of temperatures, that CaE activity significantly increased between the yolk-sac and juvenile life stages, and that variation between the CaE activity in trout and three other families of freshwater fish was limited. The CaE activity in fish is expected to substantially influence the accumulation and toxicity of pesticides and other esters entering the aquatic environment.
INTRODUCTION
Carboxylesterases (EC 3.1.1.1) (CaEs) are a class of serine hydrolases that catalyze the cleavage of an alcohol from a variety of ester substrates. Carboxylesterases seem to belong to the B esterase class, which exhibits nearly irreversible phosphorylation in the presence of organophosphate cholinesterase inhibitors. Carboxylesterase activity is present in a variety of tissues in vertebrate and invertebrate species [1]. A number of CaE isozymes have been isolated that even in purified form display broad substrate specificity [2, 3].
Investigation of CaE activity in mammalian species has been primarily concerned with the metabolism of prodrugs and aryl ester toxicants. Investigations of biotransformation systems in aquatic animals have primarily focused on oxidation and conjugation pathways, rather than dealkylation activities of CaEs. Characterization of CaE activity in aquatic animals is needed to evaluate species differences in xenobiotic disposition and to evaluate the environmental fate of ester contaminants. Only limited investigation of CaE activity in fish has been conducted, despite the large number of esters present in the aquatic environment. Herbicide and phthalate esters represent important classes of chemical esters that are extensively biotransformed by CaEs [4-7]. Research on a limited number of ester substrates indicates that fish have high esterase activity, and ester hydrolysis can significantly reduce the bioaccumulation of hydrophobic contaminants [4-7]. In addition, recent work has demonstrated that CaEs protect against the toxicity of organophosphate and organophosphorothioate insecticides (OPs) in both mammals and fish [8, 9]. For example, inhibition of CaE activity potentiated the toxicity of OPs and lower hepatic CaE activity in juvenile rats contributed to the greater sensitivity to OP toxicity than adult rats [10, 11].
The objective of this study was to quantify CaE activity in rainbow trout (Oncorhynchus mykiss) because this fish species is important in aquatic toxicology and carcinogenicity investigations, and is an important ecological receptor for ester contaminants in the environment. The enzyme kinetics (Michaelis constant [Km], maximum reaction rate [Vmax]) and temperature dependence of CaE activity of trout gill and liver microsomes and sera were compared to concurrently measured CaE activity in rat hepatic microsomes. Additionally, the CaE activity in whole trout homogenates was compared to activity in channel catfish (Ictalurus punctatus), bluegill (Lepomis macrochirus), and fathead minnows (Pimephales promelas). Homogenates of whole trout embryos, yolk-sac fry, juveniles, and adults were assayed to provide insight into the development of CaE activity in this species. In all experiments, CaE activity was determined using the model substrate 4-nitrophenyl acetate because it is a substrate for multiple CaE isozymes [2]. The results of this study should have general applicability to a variety of xenobiotic esters.
MATERIALS AND METHODS
Animals and acclimation conditions
Rainbow trout were obtained as eyed embryos from Mt. Lassen Trout Farms (Mt. Lassen, CA, USA) and maintained at 12 ± 1°C in a salmonid hatcher until they were free swimming. Trout were then transferred to flow-through aerated raceways maintained at 12 ± 1°C. Channel catfish and bluegill were obtained as juveniles from Ossage Catfisheries (Ossage Beach, MO, USA) and acclimated to 22 ± 1°C for a minimum of 2 weeks in flow-through aerated holding tanks. Fathead minnows were produced from laboratory cultures (Dow Chemical, Midland, MI, USA) and acclimated to 22 ± 1°C for a minimum of 2 weeks in flow-through aerated aquaria. Juvenile rainbow trout (1.64 ± 0.07 g; wet weight ± SE), channel catfish (7.89 ± 0.74 g), fathead minnows (1.02 ± 0.13 g), and bluegill (1.89 ± 0.25 g) were less than one year old when used. The laboratory water was softened Lake Huron water that had been sand-filtered, pH adjusted with CO2, carbonfiltered, and ultraviolet irradiated. Laboratory water was monitored weekly for pH, alkalinity, conductivity, and hardness; and quarterly for selected inorganics, pesticides, and polychlorinated biphenyls. Typical water quality values were pH of 7.5, alkalinity of 43 mg/L, hardness of 70 mg/L (as CaCO3), and conductivity of 140 mhos/cm. Fish were killed by a blow to the head and placed immediately on ice before tissue preparation.
Preparation of microsomes
Rat (n = 3; ∼0.25 kg) and rainbow trout (n = 4; ∼2 kg) tissue microsomes (one animal per sample) were prepared according to the procedures of Guengerich [12]. Tissues were immediately excised, rinsed, and weighed in ice-cold Tris buffer. Gills were trimmed from the branchial arches before weighing. The Tris buffer consisted of 0.1 M Tris (pH 7.4), 0.1 mM KCl, 1 mM ethylenediaminetetraacetic acid, and 20 M of the antioxidant buterated hydroxy toluene. Blood was obtained from the caudal vein of two rainbow trout, and serum was mixed with buffer (1:5; v/v). Tissues were homogenized icecold in glass tubes with a motor-driven Teflon® homogenizer; gills were first processed with a high-speed tissue grinder-sonicator. Tissue:buffer (w/v) dilutions were 1:5 for fish and 1:10 for rats. Homogenates were filtered through cheesecloth, and the filtrate was centrifuged at 12,000 g for 60 min. The supernatant was removed and either assayed immediately for residual CaE activity or centrifuged at 100,000 g for 60 min to produce a microsomal pellet [12]. Microsomes were resuspended in buffer and stored at −80°C until assayed. Protein was assayed by the method of Lowry et al. [13].
Preparation of fish homogenates
Homogenates of juvenile fish (n = 3 for each species), rainbow trout embryos (n = 3; each sample consisted of 13–15 eggs, including the chorion), trout yolk-sac fry (n = 3; each sample consisted of 18 or 19 fish), and adult rainbow trout (n = 3) were prepared using identical methods, with the exception that adult rainbow trout were initially ground to homogeneity using a motorized tissue grinder and then subsampled for further analysis. Samples of all species and life stages were rinsed and weighed in ice-cold Tris buffer, and then homogenized on ice using a high-speed tissue grinder-sonicator. Tissue:buffer (w/v) dilutions were 1:5 for all homogenates. Homogenates were filtered through cheesecloth, and then the filtrate was centrifuged at 1,000 g for 20 min. The supernatant was immediately assayed for CaE activity to prevent loss of activity with storage [14].
Assay of CaE activity
Enzyme activity was assayed by quantifying the formation of 4-nitrophenol from 4-nitrophenyl acetate using the spectrometric method of Heymann and Mentlein [15]. Incubations were carried out in a Tris(hydroxymethyl)aminomethane hydrochloride (Tris-HCL) buffer (Sigma, St. Louis, MO, USA) (0.1 mM, pH 8). Assays were run for 1.25 min and product formation was linear during all runs. The CaE activity of whole fish and embryo homogenates were assayed under saturating conditions (440 μM 4-nitrophenyl acetate) at a constant temperature of 22°C. The temperature optima of CaE in rat liver and trout gill and liver microsomes were determined under saturating conditions (440 μM4-nitrophenyl acetate) and varying temperature (2–40°C, depending on the tissue). The Vmax and Km values were determined by varying the substrate concentration (3.8–769 μM 4-nitrophenyl acetate; Heymann and Mentlein [15]) at a constant temperature of 25°C (trout tissues) or 37°C (rat liver). The detection limit of the assay was approximately 1 nmol/min/mg protein.
Determination of Km and Vmax and statistical analyses
The values of Km (slope) and Vmax (intercept) were determined by linear regression analysis of an Eadie-Hofstee plot of velocity/substrate concentration (i.e., v/[S] versus velocity [16]). The value Vmax is the maximum reaction rate, and Km (Michaelis constant) is the substrate concentration at one half of Vmax. The values of Km and Vmax were also determined using the Michaelis–Menton equation using nonlinear modeling with simple weighting and the Levenberg-Marquart algorithm. Significant differences in tissue levels of CaE activity were determined by a two-sided Student's t test (alpha = 0.02).
RESULTS
CaE kinetics and temperature optima
The CaE activity exhibited Michaelis-Menton kinetics as evidenced by a curvilinear approach to Vmax on Cartesian coordinates (data not shown). Eadie-Hofstee linear regression plots of CaE activity (velocity [v]) and v/[S] were used to estimate Km (slope of regression) and Vmax (intercept) (Fig. 1). Gill and sera data were linear on Eadie-Hofstee coordinates, whereas both fish and rat liver exhibited a curvilinear response (Fig. 1). The CaE activity of trout liver (Vmax = 672 nmol/min/mg microsomal protein) was about four times less than in rat liver (Table 1). Apparent Km values ranged from 28 (trout liver) to 214 (trout sera) μM (Table 1). Values of Vmax (nmol/min/mg microsomal protein ± SE) determined by nonlinear modeling were nearly identical to the values derived from the Eadie-Hofstee method (Table 1): rat liver (2,683 ± 200); trout liver (678 ± 66); gill (16.1 ± 1.7); trout sera (1,737 ± 35). Values of Km (μM, ± SE) were also nearly identical to the values derived from the Eadie-Hofstee method (Table 1): rat liver (38.5 ± 9.5); trout liver (29.0 ± 12); gill (92 ± 27); trout sera (243 ± 12). The CaE activity of trout and rat tissues were also assayed at various temperatures under saturating conditions of substrate (Fig. 2). Trout gill and liver microsomes exhibited substantial CaE activity and limited variation over the range of 2 to 40±C, with a temperature optimum of approximately 22°C (Fig. 2). Trout sera and rat liver microsomes exhibited a temperature optimum of 35 to 40°C (Fig. 2).
The CaE activity in tissues (per gram or whole tissue) was calculated from the CaE activity per milligram of protein and the protein content of the tissue (Table 1), and tissue weight (Table 2). Rainbow trout sera and rat liver exhibited the highest activity (sera = 87 μmol/min/g; rat liver = 76 μmol/min/g). The CaE in sera and liver were estimated to constitute 57% and 10%, respectively, of the CaE in the whole rainbow trout (Table 2).
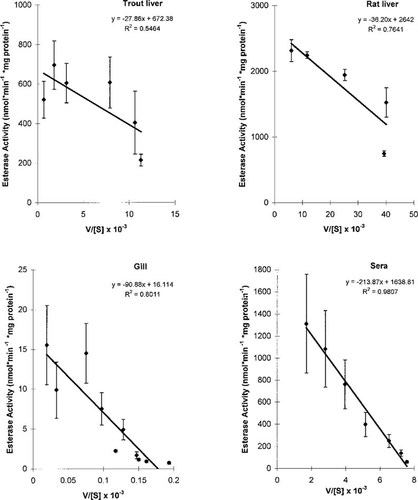
Eadie-Hofstee plot of the kinetics of carboxylesterase activity (nmol/min/mg protein) in rainbow trout sera, gill microsomes, and hepatic microsomes, and rat hepatic microsomes. Line shown is a linear regression plot (Michaelis constant [Km], slope; maximum reaction rate [Vmax], intercept). The detection limit of the assay was 1 nmol/min/mg protein.
CaE activity in fish homogenates
The CaE activity (as nmol/min/g wet weight tissue and nmol/min/mg tissue protein) was determined in whole homogenates of rainbow trout embryos, yolk-sac fry, juveniles, and adults (Fig. 3); and in juvenile channel catfish, fathead minnows, and bluegill (Fig. 4). The CaE activity in rainbow trout homogenates increased 300% per gram of tissue to 1,200% per milligram of protein from the yolk-sac to the juvenile life stage (Fig. 3). The CaE activities in rainbow trout eggs and yolk-sac fry were significantly different (p < 0.02) than CaE activity (expressed as activity per gram or milligram of protein) in juvenile and adult trout. The CaE activity in the four species of juvenile fish ranged from a mean of 2,000 (channel catfish) to 4,400 (fathead minnow) nmol/min/g tissue, and 59 (bluegill) to 84 (channel catfish) nmol/min/mg protein (Fig. 4). The CaE activity was more similar on a milligram protein basis, but was not significantly different (p > 0.03) between the four species whether activity was expressed per gram of tissue or per milligram of tissue protein.
DISCUSSION
The activity of CaEs, a class of nonspecific serine hydrolases, was evaluated in vitro in tissues and microsomes of rainbow trout and compared to esterase activity measured in rats and other fish species. Rainbow trout had substantial CaE activity at the embryo stage of development (718 nmol/min/g egg) and exhibited a 300% increase in CaE activity between the yolk-sac and juvenile life stages. The increase in activity was even more dramatic when expressed per milligram of protein (1,200%). The physiologic functions of CaEs are uncertain and may include aspects of the storage and mobilization of specific lipids, assimilation of biological and xenobiotic esters, and regulatory functions such as bioinactivation of specific hormones [1]. The increase in CaE activity between the yolk-sac and juvenile life stages may be related to the fish's transition from feeding on an endogenous to an exogenous food source, or to differences in the tissue composition of different life stages (e.g., effects of lipid content or composition on in vitro activity). However, this latter possibility seems to be less likely because of the nearly identical activity in eggs and yolk-sac fry, life stages that have substantial differences in tissue composition. Additionally, CaE activity is extremely stable over a range of pHs (6–8.8) and temperatures [17] and thus any differences in tissue composition or preparation are expected to have a negligible effect on the measured activity. For example, CaE activity in mammalian species exhibited a negligible loss with prolonged storage at −15°C and repeated freezing and thawing [2]; 60% of CaE activity remained even after a 56°C incubation for 1 h [17].
| Rat | Trout | |||
|---|---|---|---|---|
| Parameter | Left lobe of liver | Whole liver | Gill arches | Serum |
| Protein (mg/g) | 28.8 (1.76)* | 25.1 (7.14) | 6.77 (1.17) | 52.9 (24.1) |
| Apparent Km (μM) | 36.2 (11.6) | 27.9 (12.7) | 90.9 (16.0) | 214 (13.4) |
| Vmax (nmol/min/mg) | 2,642 (327) | 672 (92.1) | 16.1 (1.99) | 1,639 (72.7) |
| Approximate temperature optima (°C) Vmax/Km (ml/min/mg) | 40 73.0 | 22 24.1 | 22 0.18 | 35 7.66 |
- a Standard error of mean (protein) and of the linear regression (Km, Vmax) shown in parentheses.
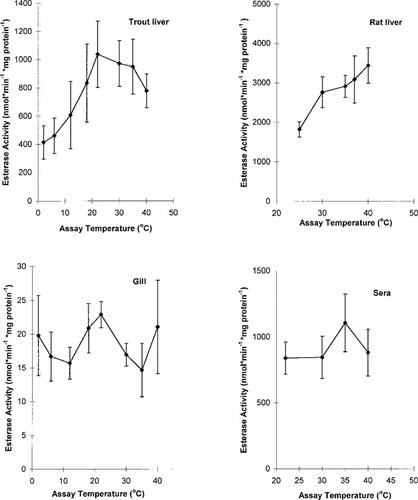
Temperature-dependence of carboxylesterase activity (nmol/min/mg protein) under saturating conditions in rainbow trout sera, gill microsomes, and hepatic microsomes, and rat hepatic microsomes. The detection limit of the assay was 1 nmol/min/mg protein.
| Tissue | Activity per g tissue (μmol/min/g) | Tissue weight (g) | Activity per whole tissue (μmol/min) | Percent of whole body activitya |
|---|---|---|---|---|
| Trout gill | 0.13 (0.05) | 14.1 (4.4) | 1.88 (1.00) | < 0.1 |
| Trout sera | 86.6 (27.0) | 65.7b (4.5) | 2,690 (1,580) | 57.7 (37.5) |
| Trout liver | 16.9 (7.4) | 20.8 (4.6) | 378 (73) | 10.2 (2.5) |
| Rat liver | 76.2 (21.7) | —c | — | — |
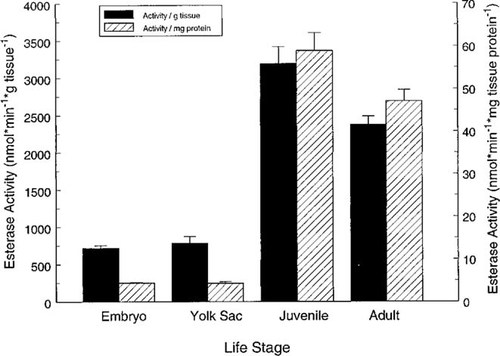
Carboxylesterase activity (left axis: nmol/min/g tissue; right axis: nmol/min/mg protein) in homogenates of four developmental stages of rainbow trout: eggs (including chorion and eyed embryo; 0.078 ± 0.003 g; wet weight ± SE), yolk-sac fry (0.055 ± 0.002 g), juvenile (1.64 ± 0.07 g), and adult (428 ± 52 g).
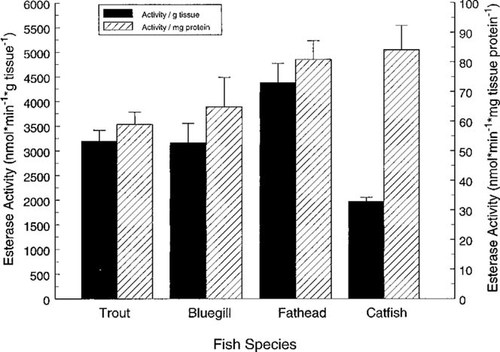
Carboxylesterase activity (left axis: nmol min g tissue; right axis: nmol/min/mg protein) in whole homogenates of juvenile rainbow trout (1.64 ± 0.07 g; wet weight ± SE), channel catfish (7.89 ± 0.74 g), fathead minnows (1.02 ± 0.13 g), and bluegill (1.89 ± 0.25 g).
Whole-body CaE activity (normalized for either body weight or protein content) was not significantly different in juvenile and adult life stages of rainbow trout, despite a 250-fold increase in body weight. Although allometric theory predicts a decrease in metabolic parameters with increasing animal size, the activity of biotransformation processes is expected to be relatively independent of body size [18]. The CaE activity of juvenile rainbow trout was not significantly different than activity in juvenile channel catfish, fathead minnows, and bluegill. Similar CaE activity in fish representing four freshwater families (Salmonidae, Ictaluridae, Cyprinidae, and Centrachidae) suggests that on a whole-body basis CaE activity may be generally similar among many fish species.
The CaE activity in rainbow trout sera, gill and liver, and rat liver exhibited Michaelis-Menton kinetics. Eadie-Hofstee plots were used to evaluate and compare the kinetics of CaE activity in specific rainbow trout and rat tissues. The Vmax and Km were determined on Eadie-Hofstee plots because this approach linearizes the Michaelis-Menton equation and allows a graphical assessment of the presence of multiple isozymes [19]. Rainbow trout gill and sera exhibited a linear relationship between CaE activity and v/[s] (activity divided by substrate concentration) on Eadie-Hofstee coordinates, whereas both fish and rat liver exhibited a curvilinear response. A curvilinear response is indicative of multiple isozymes acting on a single substrate [16, 19], which is consistent with multiple CaE isozymes reported in mammalian species. For example, rat liver contains at least five different CaE isozymes and may contain as many as 20 [2]. Choudhury [20] reported that whole serum of rats exhibited Michaelis-Menton kinetics, but the purified isozymes did not. Declining activity at the highest substrate concentrations for CaE activity in rainbow trout tissues and rat liver may be caused by substrate inhibition, as has been reported previously for mammalian species [2].
The Km and Vmax have not been previously reported for CaE activity in rainbow trout. The maximal CaE activity of trout liver (Vmax = 672 nmol/min/mg microsomal protein) was about four times less than in rats, and Km values were similar. These data were consistent with CaE activity measured in mosquito fish liver (230 nmol/min/mg protein; [8]) and in rat liver (2,700 nmol/min/mg protein; [15]). Rainbow trout sera had high CaE activity (Vmax = 1,639 nmol/min/mg protein), but the lowest affinity (1 Km). Gill tissue exhibited a very low Vmax (16 nmol/min/mg protein) and moderate affinity relative to liver. At physiologically relevant concentrations of substrate, the rate of reaction by an enzyme will be more likely determined by the ratio of Vmax to Km, rather than its maximum velocity [16]. The Vmax/Km values suggest that in vivo CaE activity of trout liver would be about three times higher than serum, 135 times higher than gill, and three times lower than rat liver. However, these comparisons should be viewed with caution because an endogenous substrate for CaE has not been determined and Vmax and Km can be dependent on the alkyl chain length of the ester substrate [18, 20; see below].
The low CaE activity of the gill relative to that of the liver was not consistent with the previously reported high activity of whole gill homogenates and isolated gill arches of rainbow trout toward the large hydrophobic ester di-2-ethylhexyl phthalate [5]. Gill activity possibly was not concentrated in microsomes; however, cytosol had negligible activity (data not shown). Tissuespecific dependence on the size (e.g., chain length and branching) and hydrophobicity of an ester may also determine Km and Vmax in rainbow trout, as it does in mammalian species [20]. For example, Vmax of rat liver increased 10 to 35 times with increasing chain length for a series of branched fatty acid esters (methyl formiate to methyl octanoate) [2]. Also, Vmax in rat serum increased and Km decreased with increasing carbon length of a homologous series of naphthol esters [19], and the affinity (1/Km) of a CaE from rat serum increased more than 100-fold with increasing ester chain length for a homologous series of 4-nitrophenyl esters [20]. Thus, the low CaE activity in the gill determined in vitro may be explained by the relatively small size (two carbon ester chain) of the substrate, despite the generally broad specificity of 4-nitrophenyl acetate (metabolized by most mammalian CaE isozymes; [15]). If the gill exhibited a substantially higher Vmax and lower Km with the eight carbon di-ester di-2-ethylhexyl phthalate, CaE activity would be expected to be exponentially higher, consistent with values reported by Barron et al. [5]. The CaE activity for a homologous series of esters remains to be investigated in fish.
The CaE activity in both gill and liver microsomes was very high relative to many other xenobiotic biotransformation pathways. For example, the activity of several mixed-function oxygenases (e.g., 7-ethyoxycoumarin-O-deethylase; 3,4-benzpyrene hydroxylase) and UDP-glucuronyltransferase in rainbow trout hepatic microsomes is typically less than 1 nmol/min/mg protein [21, 22]. The CaE activity in rainbow trout hepatic microsomes was similar to the activity of glutathiones-transferase in rainbow trout [23]. Trout gill and liver microsomes exhibited substantial CaE activity with limited variation over the range of 2 to 40°C, with a temperature optimum of approximately 22°C. Trout sera and rat liver microsomes exhibited a temperature optimum of 35 to 40°C, similar to temperature optima of 30 to 37°C reported for epoxide hydratase and glutathione-s-transferase activity in rainbow trout liver, kidney, and gill tissues [23]. Appreciable CaE activity over a broad temperature range may be a favorable adaptation for fish such as rainbow trout that experience ranges in environmental temperatures of 2 to 20°C.
Rainbow trout sera and rat liver exhibited the highest activity (sera = 86 μmol/min/g; rat liver = 76.2 μmol/min/g) on a whole-tissue basis. The CaE in sera was estimated to comprise 57% of the activity in the whole rainbow trout (Table 2). The CaE activity in tissues (per gram or whole tissue) were extrapolated from microsomal activity (gill and liver) or from blood protein content, and should be viewed as approximations. Definitive estimates of the relative contribution of individual tissues to whole-body CaE activity would require whole-tissue preparations and larger sample sizes.
Rapid biotransformation of a variety of xenobiotic substrates is known to reduce the bioconcentration of environmental contaminants [24, 25]. Previous work on a limited number of ester substrates indicated that fish have high CaE activity in both sera and liver [26], and ester hydrolysis significantly reduced accumulation of several hydrophobic esters [4-6]. Inhibition of CaE activity with the selective active-site inhibitor bis-nitrophenyl phosphate caused an estimated sevenfold increase in the bioconcentration of triclopyr butoxyethyl ester and an eightfold increase in di-2-ethylhexyl phthalate bioconcentration [5, 27]. Presystemic metabolism in the gill has also been shown to lower the amount of unhydrolyzed di-2-ethylhexyl phthalate entering rainbow trout [5]. This may have favorable toxicologic consequences because the hydrolysis product should be less persistent in the fish. The CaE activity is also protective against OP toxicity in both fish and mammals [9, 11]. Atterberry et al. [11] concluded that age-related changes in CaE seemed to contribute to the age-dependent toxicity of the acetylcholinesteraseinhibiting insecticide chlorpyrifos in rats. Abas and Hayton [9], using computer simulations, concluded that CaE was an important detoxication pathway for the acetylcholinesterase inhibitor paroxon in rainbow trout. Carboxylesterases detoxify OPs by stoichiometrically reducing the amount of OP compound available to inhibit acetylcholinesterase [10].
The results of this study demonstrated that rainbow trout had high esterase activity over a broad range of temperatures, that CaE activity significantly increased between the yolk-sac and juvenile life stages, and that variation between the CaE activity in trout and three other families of freshwater fish was limited. The CaE activity of fish is expected to substantially influence the accumulation and toxicity of pesticides and other esters entering the aquatic environment. The tissue-specific biotransformation results reported here may be useful in understanding both the toxicity and bioaccumulation of ester contaminants. Our results should have general applicability to a variety of xenobiotic esters and may be useful in developing physiologically based pharmacokinetic models and assessing risks of ester contaminants. However, the results should be applied with the recognition that CaE activity may be dependent on the chain length and hydrophobicity of the ester. Additional research with fish is needed to elucidate substrate-specific biotransformation for a homologous series of environmental esters.
Acknowledgements
We thank Elizabeth Fergeson for Michaelis-Menton analyses; T. Ball, P. Wilga, and S. Gorzinski for technical assistance; and M.A. Mayes, P.J. Gering, and P.G. Watanabe for advice and review.




