Accumulation of polycyclic aromatic hydrocarbons in semipermeable membrane devices and caged mussels (Mytilus edulis L.) in relation to water column phase distribution
Abstract
Semipermeable membrane devices (SPMDs) and blue mussels (Mytilus edulis L.) were deployed at a site contaminated by discharges of polycyclic aromatic hydrocarbons (PAHs) from an aluminium reduction plant, and at a reference site. The accumulation of PAHs in SPMDs versus mussels, along with the ability of the two matrices to predict contaminant concentrations in the ambient environment, were evaluated through concurrent measurements of particulate, dissolved, and colloidal PAHs in the water column. Analysis of the results showed that blue mussels were more efficient at sequestering PAHs than were SPMDs. The PAH profile (i.e., the relative abundance of individual PAHs) in the two matrices were similar, but differed significantly from the profile in the dissolved phase. Further, back-calculation of the ambient dissolved concentrations from SPMDs indicated systematic overtrapping with increasing hydrophobicity. Calculation of in situ bioconcentration factors (BCFs) for the blue mussels at the smelter site indicated that uptake via particles (>0.7 μm) or from colloids dominated over direct uptake from the dissolved phase, as opposed to the reference site. The in situ BCFs differed markedly from literature values, which implies that the use of mussels to predict ambient concentrations would require that site-specific BCFs be applied.
INTRODUCTION
Bivalve molluscs have been used over the past 30 years to monitor contaminant levels in aquatic systems [1-4]. The levels in these organisms are generally considered to represent a time-integrated picture of the concentrations in the ambient environment. Therefore, the monitoring of contaminant levels in organisms is used as an alternative to water sampling, for which labor-intensive and costly programs would be needed to describe the most important trends.
The blue mussel (Mytilus edulis L.) is among the molluscs most commonly used in monitoring programs. In some areas, for example in Norway, the physical and chemical conditions are such that no single species is found along the entire coast. Although the choice of monitoring organisms might be standardized insofar as possible among the various areas investigated, the choice of organism is inevitably dependent on local availability and occurrence. Further, the detection of spatial trends may be hampered by the use of different organisms because they may differ in their contaminant accumulation characteristics. To counterbalance the limitations of using living organisms as measures of contaminant exposure, in situ passive sampling devices such as lipid-filled semipermeable membrane devices (SPMDs) have been developed and used extensively in recent years [5-8]. These devices are designed to sample the truly dissolved fraction exclusively, and thereby to mimic the bioconcentration process in aquatic organisms [5, 9, 10]. Therefore, use of man-made devices instead of living organisms might be prudent. However, before SPMDs can be used to replace living organisms, their performance must be tested relative to the organisms in question.
Mussels are used not only in temporal programs, but also as a tool to compare contaminant levels at different sites. This ability is predicated on the assumption that bioconcentration factors (BCFs), that is, the relationship between the concentrations in the mussel and in the ambient waters, do not differ significantly between sites subject to different environmental conditions. However, in field studies with mussels, the presence of the contaminants in the different phases in the water column is rarely determined. This study was on the accumulation of polycyclic aromatic hydrocarbons (PAHs) in SPMDs and blue mussels deployed at a contaminated site and at a reference site. The ability of the membrane devices and the mussels to predict contaminant concentrations in the ambient environment was evaluated through concurrent measurements of PAHs in the particulate, colloidal and dissolved fractions in the water column.
MATERIALS AND METHODS
Sampling
Sampling at a contaminated site was performed in the waters affected by discharges from the primary aluminium smelter at Lista in southern Norway (Fig. 1). The receiving water received PAH-carrying effluents from the seawater scrubbing of furnace off-gases and ventilation air from the plant. The effluent is piped into the receiving water, an open bay on the south coast of Norway. The reference site was located in coastal waters approximately 200 km from the contaminated site. The reference site is influenced by general human activities, but not by any point sources.
Caged blue mussels and SPMDs held in a stainless steel container were deployed in June 1996 at each site at a depth of 0.5 m. Both mussels and SPMDs were attached to the same deployment device and spaced closely together (1 m apart). The SPMDs consisted of commercially available 45.7 × 2.54 cm (surface area 232 cm2) flat polyethene tubes filled with 0.455 g of triolein. Twenty-five mussels ranging in size from 4 to 7 cm were put in a plastic mesh and transplanted from a clean site. Initial concentration total PAHs of in these mussels were low, approximately 10 ng/g (wet weight). For each site, two replicates of mussels and SPMDs were held for 34 and 26 d for the smelter and reference sites, respectively, and analyzed separately.
Map of the investigation area.
Water samples from a depth of 0.5 m were taken on three occasions from both sites during the deployment period. An on-line filtration system was used to collect PAHs. It consisted of a precleaned 142-mm borosilicate glass microfilter (Whatman GF/F, Whatman, Clifton, NJ, USA) in a stainless steel holder for the retention of particulate matter (nominal cut-off 0.7 μm). That was followed by two polyurethane foam (PUF) adsorbents (30-mm diameter, 45-mm length) in stainless steel or glass (effluent water) casing. The PUF is assumed to retain PAHs in the dissolved fraction. Some colloids and thereto sorbed PAHs are large enough to be collected on the GF/F-filter [11] and some of the PAHs sorbed to colloids may be desorbed during the passage through the PUF and trapped there. The sorption kinetics of PAHs on colloids smaller than 0.3 μm has been shown to be so fast that equilibrium is attained within a few minutes [12]. However, it is reasonable to assume that most of the PAHs going through both the filter and the PUF are sorbed to colloids. This fraction of PAHs therefore is denoted colloidal. To extract this fraction the water that passed the filter and PUF was collected in 10-L glass containers and immediately preserved with toluene (glass-distilled quality, Burdick and Jackson, Labora, Sweden). Water supply to the on-line filtration system was provided at a flow of 1 L/min by a modified Flojet® pump (Flojet, Irvine, CA, USA) equipped with a silicone hose. Thirty liters of smelter site water and 40 L of reference site water were pumped through the system at each sampling. The filters, casings, and containers were washed with toluene and heated to 480°C before use.
Water samples were kept dark in sealed containers. Immediately after collection, they were extracted with 50 ml toluene per liter of sample, and spiked with picene and D12-perylene to serve as internal standards. They were subsequently shaken for 24 h, then left to stand for another 24 h for phase separation. Most of the seawater was siphoned off and the remaining solution was kept cool (4°C) until analysis. Filters and PUF adsorbents were stored frozen at −20°C before chemical analysis, and filtrates were stored at 4°C.
Chemical analysis
The following PAHs were analyzed: phenanthrene, anthracene, 3-methylphenanthrene, 1-methylenephenanthrene, fluoranthene, pyrene, 2-methylpyrene, 1-methylpyrene, benzo[ghi]fluoranthene, benzo[a]anthracene, chrysene/triphenylene, benzo[k]fluoranthene, benzo[e]pyrene, benzo[a]pyrene, perylene, indeno[1,2,3-cd]pyrene, benzo[ghi]perylene, and coronene. Mussels, glass fiber filters, and PUF adsorbents were spiked with two PAHs (D12-perylene and picene), then Soxhlet extracted for 24 h in toluene with a Dean-Stark trap for water removal. The amount of extractable organics (lipids) in the mussels was determined by evaporating the solvent from the extracts and continually weighing the extract until a constant weight was obtained. An almost transparent film of biofouling was mechanically removed from SPMDs by means of facial tissues and rinsing in tap water. The contents of two SPMDs from each site were extracted three times in capped glass containers using 100 ml pentane for 24 h each. Water was removed from the liquid-liquid extracts with precombusted Na2SO4. The volume-reduced extract from the PUFs was first eluted on 100 × 10-mm columns containing deactivated SiO2 (10% water, w/w) with toluene as the mobile phase. The volume-reduced extracts of all the samples were then treated by a dimethylformamide cleanup procedure. The hexane solution containing the PAHs was then finally eluted on 100 × 10-mm columns containing deactivated SiO2 (10% water, w/w) with hexane as the mobile phase. Before analysis a recovery check standard (D12-chrysene) was added. The internal standard recovery was 60 to 90%. The extracts were analyzed on a gas chromatograph-mass spectrometer (Hewlett Packard 5890/5971A, Hewlett Packard, Avondale, PA, USA) equipped with a Chrompack CP-Sil-8, 25-m × 0.25-mm column (Chrompack, Bergen op Zoom, The Netherlands). The injector temperature was 110°C. Splitless injection and a constant 15 psi head-pressure was used. The mass spectrometer conditions were ionization mode: EI (70 eV), multiple ion recording, and scan frequency 4.10/s. Response factors and retention times were determined by analyzing a standard mixture containing all the PAH compounds analyzed, including the internal standards. This mixture was extracted and cleaned up following the same procedure as the samples. All the solvents used (toluene and hexane; Burdick & Jackson; pentane; Merck, Kebo, Sweden) were of glass-distilled quality. Nondetectable amounts of high molecular weight PAHs (MWh defined as MW > 250) PAHs and negligible amounts of low molecular weight PAHs (MWl defined as MW < 250) were found in PUF and SPMD blanks. Nondetectable amounts of PAHs were found in filter blanks. The detection limit for PAHs in the PUF was generally between 0.01 ng/L and 0.1 ng/L, depending on the site. For PAHs in the wet extraction, the detection limit was 0.01 to 0.3 ng/L. The detection limits were higher at the smelter site.
Statistical analyses
Principal component analysis (PCA) was used to examine PAH profile patterns between the different matrices, and to apportion the variance in the data. This was performed using double centered PCA on log-transformed data (i.e., eliminating the effects of scale, thus focusing on relative patterns). The PCA represents the patterns and trends by arranging the PAHs (variables) and sites along axes (principal components), which are assumed to represent basic factors or relationships. The first axis (PC1) illustrates the most prominent trend, whereas the successive axes (PC2, PC3, and so on) represent secondary trends in decreasing order of importance. The axes are uncorrelated. The amount of variance explained by each axis, stated as a percentage of the total variance, may be taken as a measure of the relative importance of the axis. The results are depicted in combined plots (biplots) of variables and samples, with the variables represented by arrows running from the origin of the plot to the position of the variable scores (loadings). The arrows point in the direction of increasing variable concentrations, whereas the length of the arrow represents the strength of the increase.
To test the significance of observed differences in PAH profiles between different matrices, the data were used in redundancy analyses (RDAs) applying the forward selection procedure and Monte Carlo permutation tests. The RDA is a technique related to PCA, but it includes an additional data set of explanatory variables. The axes represent linear combinations of the explanatory variables. Thus, the analysis will detect specific variation patterns among PAHs, which correlate with the explanatory variables. In the RDA, each sample was assigned to an appurtenant matrix, which were entered in the analyses as explanatory variables. The PCAs and RDAs were performed using the software CANOCO version 3.10 software [13, 14]. Plots were made using CanoDraw 3.0 [15].
RESULTS AND DISCUSSION
Characteristics of PAHs in the water phase
The average concentrations in the three sampled phases (particulates [Cpart], colloids [Ccol], and dissolved [Cdis]) at the two sites are presented in Table 1. In all phases and at both sites, phenanthrene, fluoranthene, pyrene, and chrysene were quantitatively most important. At the reference site, the filtered volume was too small to accurately quantify MWh PAHs. The water column phase distributions are described and discussed in detail elsewhere [16]. The inclusion of the soot-carbon partitioning model was necessary to explain the high in situ partition coefficients between the dissolved and particulate phases. The relative apparent distribution between particles, colloids and the dissolved phase differed between the smelter and reference site waters. At the smelter site most of the MWl PAHs and 10 to 30% of the MWh PAHs was found in the colloidal fraction, that is, passed both the filter and the PUF. Also, at the reference site a significant portion of both MWl and MWh PAHs was found in this fraction. At both sites most of the individual PAHs were in the sorbed form, either particulate or colloidal.
Accumulation in SPMDs and mussels
Both matrices reflected the considerable difference in concentrations between the reference and the smelter site. At the reference site, the total PAH concentration in the mussels was equal to or slightly higher than what has been denoted as a background level in uncontaminated areas, according to Norwegian criteria for the assessment of environmental quality [17]. Mussels from the smelter site were classified as extremely contaminated. As for the three water phases, phenanthrene, fluoranthene, pyrene, and chrysene were quantitatively most important in the membrane devices and in the mussels. However, their accumulation efficiency differed considerably with the highest concentrations in the blue mussels (Table 1). At the reference site, the ratio between the accumulation in mussels and in SPMDs calculated as total PAH was 2.2 to 2.4 on a lipid basis, and ranged from 3.6 to 6.7 in the receiving waters of the smelter site. The magnitude of the higher sequestering efficiency of the mussels compared with SPMDs coincides reasonably well with findings elsewhere [4, 8, 18].
A marked difference in concentrations was also observed between parallel samples of both mussels and SPMDs. Although PAHs in SPMDs at the smelter site differed by less than 10%, PAHs in SPMDs at the reference site varied with nearly a factor of two. The PAHs in mussel tissues at both sites varied by a factor two between the two replicates. For the SPMDs, this was unexpected because identical tubes were closely spaced in the same steel container. However, accumulation differences between parallel SPMDs have been reported previously, for instance, Moring and Rose [19] observed a coefficient of variation of up to 50% for many PAH compounds. One explanation for the difference in accumulation at the reference site may be related to differences in biofouling on the membranes. Biofouling is known to substantially diminish membrane permeability and lead to poor reproducibility [5, 7]. In contrast to the reference site, an excellent agreement was found between the parallel SPMDs at the smelter site.
The mussels were transplanted from the same uncontaminated site. Differences in body size and physiologic state have been shown to have a marked influence on the rate of contaminant uptake in the blue mussel [20]. However, an effort was made to minimize this by using mussels of roughly the same size. Further, algal food concentrations may influence contaminant accumulation in mussels [21]. The parallel mussel cages were closely spaced (approximately 1 m apart, so the food supply should have been the same). The mussels were caged in plastic meshes. This means that the food supply of the sample specimens that were closely spaced in the center of the mesh could theoretically have differed from that of the individuals on the outer part of the mesh that hence were more exposed to the water masses. Therefore, the results indicate substantial differences in PAH accumulation among individuals.
PAH profile comparison
The PCA performed to evaluate differences in relative accumulation revealed that the main trend in the data from the smelter site was the difference in PAH profile between the dissolved fraction sampled by PUF and by the other sample matrices (Fig. 2). The PAHs in the dissolved fraction were dominated by the compounds phenanthrene, metylphenanthrene, flouranthene, anthracene, and pyrene. This trend accounted for 93% of the total variance. The second most important trend (PC2) was represented by a difference in profile between the particulate and the colloidal fraction, which captured 5% of the variance in the data set. The PAH profiles from the SPMDs and blue mussels were quite similar, although they differed on PC3, but this accounted only for 1% of the total variance. Although considerable differences were observed in total concentrations for parallel samples from SPMDs and mussels, note that the PAH profiles within each matrix were very similar.
| Mean of three samplings (ng/L) | Reference site | Smelter | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Reference site | Smelter | ng/g triolein | ng/g lipid | ng/g triolein | ng/g lipid | |||||||||
| PAHa | Colloids | Dissolved | Particles | Colloids | Dissolved | Particles | SPMD 1 | SPMD 2 | Mytilus 1 | Mytilus 2 | SPMD 1 | SPMD 2 | Mytilus 1 | Mytilus 2 |
| Phe | 1.6 | 1.2 | 6.0 × 10−1 | 7.8 × 102 | 9.9 × 101 | 3.3 × 101 | 4.8 × 102 | 2.3 × 102 | 4.5 × 102 | 4.6 × 102 | 6.1 × 104 | 6.8 × 104 | 2.4 × 105 | 5.9 × 105 |
| Ant | 6.0 × 10−2 | 1.4 × 10−1 | 1.1 × 101 | 2.7 | 4.2 | 1.3 × 101 | 1.3 × 101 | 2.5 × 101 | 2.1 × 101 | 3.3 × 103 | 4.7 × 103 | 1.7 × 104 | 4.7 × 104 | |
| 3-mePhe | 3.0 × 10−1 | 4.0 × 10−1 | 2.9 × 10−1 | 7.7 × 101 | 1.1 × 101 | 8.4 | 1.9 × 102 | 1.0 × 102 | 2.0 × 102 | 1.9 × 102 | 1.2 × 104 | 1.2 × 104 | 6.4 × 104 | 1.7 × 105 |
| 1-mePhe | 2.4 × 10−1 | 2.8 × 10−1 | 2.0 × 10−1 | 2.8 × 101 | 3.6 | 3.9 | 1.1 × 102 | 5.3 × 101 | 1.0 × 102 | 1.1 × 102 | 4.8 × 103 | 4.6 × 103 | 2.2 × 104 | 4.9 × 104 |
| Flu | 5.8 × 10−1 | 6.3 × 10−1 | 8.6 × 10−1 | 1.0 × 103 | 1.2 × 102 | 2.8 × 102 | 6.5 × 102 | 2.4 × 102 | 1.2 × 103 | 4.5 × 102 | 4.8 × 105 | 4.5 × 105 | 8.8 × 105 | 1.6 × 106 |
| Pyr | 1.6 × 101 | 1.4 × 10−1 | 4.2 × 10−1 | 2.8 × 102 | 3.3 × 101 | 1.1 × 102 | 1.0 × 102 | 5.3 × 101 | 3.5 × 102 | 1.3 × 102 | 7.2 × 104 | 7.1 × 104 | 3.6 × 105 | 8.9 × 105 |
| 2-mePyr | 2.0 × 10−2 | 6.2 | 7.2 × 10−1 | 1.1 × 101 | 6.1 | 1.3 | 3.7 × 101 | 7.8 | 2.8 × 103 | 2.5 × 103 | 3.9 × 104 | 9.9 × 104 | ||
| 1-mePyr | 6.1 | 6.9 × 10−1 | 8.0 | 5.8 | 1.6 | 2.8 × 101 | 1.1 × 101 | 2.3 × 103 | 2.1 × 103 | 2.8 × 104 | 6.9 × 104 | |||
| B[ghi]F | 2.0 × 10−2 | 1.0 × 10−2 | 7.0 × 10−2 | 8.2 | 9.5 × 10−1 | 1.4 × 101 | 2.5 × 101 | 1.3 × 101 | 8.0 × 101 | 6.1 × 101 | 3.7 × 103 | 3.3 × 103 | 3.4 × 104 | 6.2 × 104 |
| B[a]A | 5.0 × 10−2 | 3.0 × 10−2 | 4.1 × 101 | 4.1 | 9.0 × 101 | 1.9 × 101 | 2.5 × 101 | 3.3 × 102 | 1.3 × 102 | 3.9 × 104 | 3.4 × 104 | 2.2 × 105 | 4.3 × 105 | |
| Chr | 2.0 × 10−1 | 9.0 × 10−2 | 5.6 × 10−1 | 1.5 × 102 | 1.7 × 101 | 3.8 × 102 | 2.0 × 102 | 1.9 × 102 | 9.2 × 102 | 3.5 × 102 | 1.4 × 105 | 1.3 × 105 | 5.6 × 105 | 1.1 × 106 |
| B[k]F | 2.3 × 10−1 | 1.5 × 10−1 | 1.6 × 101 | 1.3 | 1.0 × 102 | 2.0 × 101 | 1.7 × 101 | 1.8 × 102 | 5.3 × 101 | 1.9 × 104 | 1.6 × 104 | 1.3 × 105 | 2.4 × 105 | |
| B[e]P | 1.7 × 10−1 | 1.8 × 101 | 1.2 | 1.3 × 102 | 1.8 × 101 | 1.6 × 101 | 2.8 × 102 | 6.4 × 101 | 2.1 × 104 | 1.8 × 104 | 1.8 × 105 | 3.7 × 105 | ||
| B[a]P | 1.0 × 10−1 | 7.8 | 2.6 × 10−1 | 5.7 × 101 | 9.3 × 10−1 | 1.9 | 1.4 × 102 | 2.2 × 101 | 9.4 × 103 | 7.6 × 103 | 1.1 × 105 | 2.3 × 105 | ||
| Per | 2.4 | 7.0 × 10−2 | 1.5 × 101 | 8.3 × 10−1 | 1.7 | 2.6 × 101 | 4.5 | 1.5 × 103 | 1.9 × 103 | 1.9 × 104 | 4.1 × 104 | |||
| Ind[l,2,3-cd]P | 9.1 | 7.3 × 101 | 4.4 × 10−1 | 1.0 | 7.9 × 101 | 1.3 × 101 | 5.6 × 103 | 4.8 × 103 | 4.0 × 104 | 9.6 × 104 | ||||
| B[ghi]P | 8.0 | 6.1 × 101 | 3.7 × 10−1 | 9.0 × 10−1 | 1.0 × 102 | 3.5 × 101 | 5.2 × 103 | 4.2 × 103 | 3.8 × 104 | 8.8 × 104 | ||||
| Cor | 1.5 × 101 | 1.0 × 101 | 8.8 × 10−1 | 3.6 × 102 | 5.7 × 101 | 1.7 × 103 | ||||||||
| Total | 3.8 | 2.9 | 3.2 | 2.5 × 103 | 3.0 × 102 | 1.4 × 103 | 1.8 × 103 | 9.6 × 102 | 4.5 × 103 | 2.1 × 103 | 8.9 × 105 | 8.3 × 105 | 3.0 × 106 | 6.1 × 106 |
- aPhe = phenanthrene; Ant = anthracene; 3-mePhe = 3-methylphenanthrene; 1-mePhe = 1-methylenephenanthrene; Flu = fluoranthene; Pyr = pyrene; 2-mePyr = 2-methylpyrene; 1-mePyr = 1-methylpyrene; BghiF = benzo[ghi]fluoranthene; BaA = benzo[a]anthracene; Chr = chrysene/triphenylene; BkF = benzo[k]fluoranthene; BeP = benzo[e]pyrene; BaP = benzo[a]pyrene; Per = perylene; Ind1,2,3-cdP = indeno[1,2,3-cd]pyrene; BghiP = benzo[ghi]perylene; Cor = coronene.
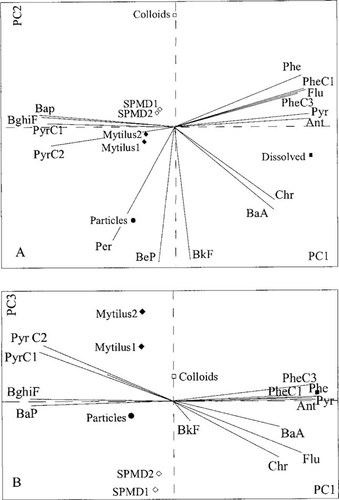
Standardized priciple components analysis for comparing the profiles of different sampling matrices. (A) Principal component 1 and 2. (B) Principal component 1 and 3. Indeno[1,2,3-cd]pyrene, benzo[ghi]perylene, and coronene were excluded from the analyses because of concentrations below detection limit.
The RDAs based on Monte Carlo permutations showed significant trends in the data set. Applying the procedure of forward selection in the RDA, the dissolved fraction as a group accounted for as much as 89% of the variance and was the only variable significant at p < 0.01. This implies that the profile in the four other matrix groups (mussels [attributable to 12% of the variance], particles [11%], SPMDs [4%], and colloids [3%]) could not be significantly (p > 0.05) differentiated internally. It must be pointed out that the data set is limited, although the same pattern that emerged in the data set from the smelter site was also generally evident at the reference site (results not shown). Because of the number of MWh compounds that were below the detection limit in the dissolved and colloidal fractions, the analysis of the reference site had to be restricted to fewer individual PAHs. The results showed that SPMDs did not mimic the relative PAH distribution in the dissolved phase as often assumed [5, 8, 19]. However, the PAH profiles in the SPMDs and mussels were similar, falling between the profiles seen for particles and for colloids.
| Sampling rate (L/d) | ||
|---|---|---|
| Compounda | 1 ng/L | 100 ng/L |
| Phe | 2.2b | 2.4 |
| Ant | 1.84 | 1.8 |
| B[a]A | 2.7 | 3 |
| Chr | 1.4 | 1.9 |
| B[k]F | 1.9 | 1.5 |
| B[a]P | 1.9 | 2 |
| Ind | 1.8 | 1.6 |
| B[ghi]P | 1.2* | 1.3 |
- aPhe = phenanthracene; Ant = anthracene; BaA = benzo[a]anthracene; Chr = chrysene/triphenylene; BkF = benzo[k]fluoranthene; BaP = benza[a]pyrene; Ind = indeno[1,2,3-cd]pyrene; BghiP = benzo[ghi]perylene.
- bDetermined for 10 ng/L.
Prediction of ambient concentrations
SPMDs. The dissolved phase was sampled in two different ways: by PUF adsorbent after GF/F filters, and by SPMDs. The PUF adsorbents provide “snapshots,” whereas the SPMDs show integration over time. Using laboratory-calculated rates for initial linear uptake (Table 2), the concentration of individual PAHs in the dissolved phase can be back-calculated on the basis of the PAH content in the SPMDs at the end of the sampling period. The concentrations measured by the two different methods represent average concentrations in similar volumes of water, that is, 30 to 40 L of water were passed through the PUF adsorbents, and the SPMDs sampled water for 26 and 34 d, respectively, for reference and smelter waters. Multiplying 26 and 34 d times general sampling rates of 1 to 3 L/d (Table 2) equals 30 to 100 L. Unfortunately, only few SPMD sampling rates are available in the reference literature for the PAHs included in this study, especially for the MWh PAHs. This, in turn, limits the applicability of the following discussion on these compounds.
Dissolved concentrations of individual PAHs at the smelter and reference sites were calculated using the sampling rates for 100 ng/L and 1 ng/L, and comparing them with those measured by the PUF absorbent (Fig. 3). Interestingly, although the estimated concentrations are on the same order of magnitude, the results indicate a systematic difference between the two methods. The ratio between the dissolved concentrations determined by SPMD (CSPMD) and by PUF adsorbent (Cdis) for individual PAHs (R) is plotted against log Kow in Figure 4. A significant positive correlation was found between log R and log Kow for the smelter water. However, this correlation was not significant (p = 0.08) at the reference site, probably because of the limited number of observations. Although the slopes were similar for the two sites, the intercepts were different. The R values of the smelter site for some of the MWl PAHs were less than 1, whereas for the reference site, all R values were >1 (Fig. 4). In the smelter site water, the PUF adsorbent tended to overtrap (R < 1) MWl PAHs and undertrap (R > 1) MWh PAHs, relative to SPMDs. One explanation for R < 1 for MWl PAHs may be that they are rapidly desorbed from the organic colloids and subsequently trapped by the PUF adsorbent [12]. A complimentary explanation is that the linear uptake phase of SPMDs for MWl has been exceeded, resulting in a back flux of these PAHs from the SPMDs during the latter part of the sampling period. This is not applicable for MWh PAHs because their elimination rates are substantially lower.
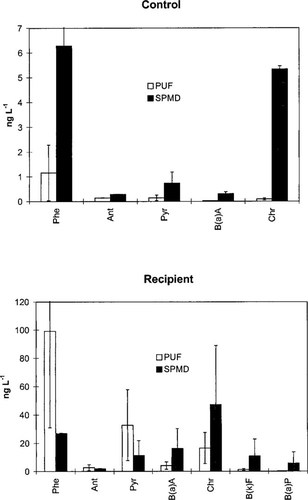
A comparison of the estimates of the dissolved concentrations of PAHs as determined by polyurethane foam (PUF) and semipermeable membrane device (SPMD) at the two sampling locations: control and recipient. The bars indicate a standard error of n = 2 or 3, depending on the number of sampling occasions on which the compound was detected.
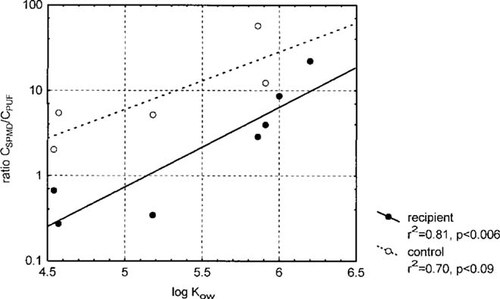
The ratio (R) between the estimates of the dissolved concentrations, using the semipermeable membrane device (SPMD) (CSPMD) and polyurethane foam (PUF) (Cdis) of individual PAHs plotted against log Kow.
Explaining the undertrapping of MWh PAHs by the PUF adsorbent in relation to SPMD (R > 1) is more difficult. The PUF adsorbent has been thoroughly evaluated and found to be efficient in trapping dissolved hydrophobic compounds from seawater, although the possible effect of colloids as a sorbing matrix has not been evaluated [22]. The volumes sampled in the present study were far too small for any filtration breakthrough of dissolved PAHs to occur [22]. Consequently, the problem seems to be related to the SPMD sampling methodology. A systematic increase in overtrapping seems to exist with increasing PAH hydrophobicity.
The SPMD methodology requires that algae and other fouling organisms be removed from the surface of the membranes before extraction. This was done by means of rinsing in tap water and mechanical removal using a facial tissue. The total amount of BaP extracted from the two SPMDs in the smelter was 4.3 and 3.7 μg. In the smelter water, the carbon-normalized concentration of BaP in suspended particles ranged from 170 to 370 μg/g C. A residue of 10 mg C on the SPMD surface after cleaning could therefore account for an amount of BaP corresponding to the amount found in the SPMD. Accordingly, further attention should be devoted to the cleaning method. The explanation that residual organic film or soot particles can affect total amounts is supported by the finding that the CSPMD of chrysene, the most hydrophobic PAH detected by both PUF and liquid-extraction on all three occasions at the reference site, was actually eight to nine times higher than the sum of Cdis and Ccol.
Mussels. Several studies have reported that accumulation of contaminants in aquatic organisms can be described by the BCF, which is an equilibrium partitioning between the organism and the dissolved phase in the surrounding water [23, 24]. The driving force for this partitioning is the hydrophobicity of the contaminant, often expressed as the partition coefficient in an octanol-water system (Kow). Pruell et al. [25] reported that steady state was achieved within 20 to 40 d, in which interval our sampling times fall. Mussels in the field may accumulate PAHs both directly from the dissolved phase as a result of the equilibrium partitioning process, and from ingested food particles. If the equilibrium partitioning process is fast relative to the food ingestion rate the elimination process will counteract an additional uptake via food.
By using an allometric relationship to estimate approximate filtration rates for the individual mussels [26], estimation of the total amount of PAHs the mussels were exposed to in the different phases during the deployment period was possible F = 3.9000.60 where F is the filtration rate (L/h) and W the weight (g tissue dry weight). Using the measured average dry weight of the analyzed mussels the filtration rates were estimated to 2.6 to 3.0 L/h and 1.8 to 2.1 L/h for the mussels at the reference and smelter sites, respectively. At the end of the deployment period the mussels were significantly larger at the reference site (0.51–0.63 g dry weight per individual) than at the smelter site (0.27–0.36 g dry weight per individual).
The relationship between log BCF and log Kow at the reference and smelter site is presented in Figure 5 on a wet weight basis. Good linear correlation was found between log BCF and Kow. The slopes of the regression lines were near unity (1.08 and 1.10) for the two sites. The results reported by Geyer et al. [23] and Pruell et al. [25] are also included in Figure 5. Compared with the results presented in these papers and also by Murray et al. [27], the BCFs at the reference site are almost an order of magnitude higher. The BCFs at the smelter site were another order of magnitude higher.
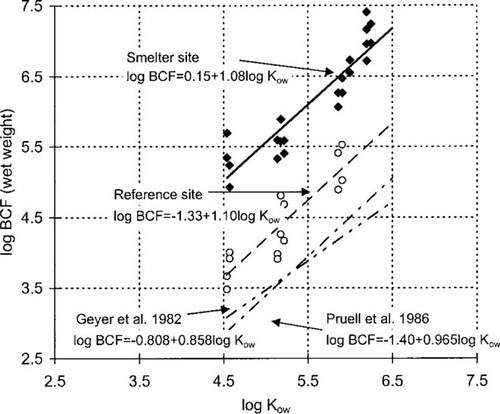
Explaining the observed high in situ BCFs at the smelter site in terms of equilibrium partitioning is difficult. One explanation for the extremely high field BCFs could thus be that the dissolved phase was not in equilibrium at the times when the PUF filter measurements were performed. Because of the highly contaminated discharge water, the PAH levels in the receiving water were significantly elevated compared to background concentrations. As the discharge water is diluted into the receiving water the concentration in the dissolved phase drops significantly whereas for the particulate phase it may take some time before the desorption and subsequent equilibration with the surrounding water takes place. The sampling point was located a few hundred meters away from the discharge point and the dilution factor of the effluent water was there only ∼34 [16]. A significant desorption from the discharged particles possibly takes longer than the average time needed for these particles to travel from the discharge to the sampling point. However, this cannot be quantitatively evaluated with the available data. A slow desorption may also explain the very high particle partition coefficients observed at this site in relation to other sites [16]. Considering the total amount of the individual PAHs in the dissolved phase passing through the mussel bodies up to 1 year of deployment would have been needed to account for the concentrations of the MWh PAHs in the mussels, even if the uptake efficiency from the dissolved phase was 100%. Therefore, the results indicate additional uptake of either colloidal PAHs or particulate PAHs for which sootlike carbon probably was an important sorbing matrix. The analyses of the PAH phase distribution in the water column indicated that the sampled colloidal PAHs accounted for a substantial fraction of the MWh PAHs at the smelter site, and were the dominating pool for MWl PAHs.
Also, the in situ BCFs at the reference site are high relative to what would be expected from previous studies [23, 25]. Ideally, log BCFlipid is near log Kow at equilibrium. However, deviations from this are observed often and may be attributed to the fact that, as a sorbing matrix, octanol solubility does not represent lipids completely. In addition, other components of the tissue than lipids may serve as a sorbing matrix, which may result in higher BCFlipid. The determination of the dissolved phase is also complex from an analytical viewpoint. For example, experimentally determined BCFs may be underestimates if the presumed dissolved phase also includes the fraction sorbed to organic colloids.
At the both the smelter and the reference sites log BCFlipid is considerably higher than log Kow. At the reference site the organic carbon-normalized PAH concentrations in the particles were an order of magnitude higher than the lipid-normalized concentrations in the mussels. The mussels at the reference site did grow considerably because their dry weight was on average 80% higher than the dry weight of the smelter site mussels at the end of the deployment. This means that they have ingested suspended particles and possibly thereto sorbed PAHs. Because of the high PAH concentrations on the suspended particles at the reference site, only a small amount of particles ingested may have affected the mussel tissue concentration significantly, thus driving the tissue concentrations to above equilibrium concentrations. The exposure of dissolved-phase PAHs to the mussels was significant. Only 1 to 10 d of exposure accounts for the amounts of PAHs observed in the mussels. The higher values refer to MWh PAHs.
These findings at the smelter site illustrate the difficulties of using the organisms as indicators of ambient contaminant loading in discharge situations where significant dilution occurs. Therefore, normal relationships between the uptake from the dissolved phase and via food may not be valid in such situations. In addition, the BCFs observed at the reference site alone indicate that in situ-determined, site-specific values are required to accurately predict ambient concentrations from mussel tissue concentrations, an important consideration when using the blue mussel for spatial distribution studies.
Acknowledgements
We wish to thank the following organizations for the financial resources that have made this project possible: Elkem's aluminium and manganese alloy smelters, the Norsk Hydro aluminium smelters, Tinfos Jernverk A/S, the Norwegian Pollution Control Authority, the Research Council of Norway, and the Norwegian Institute for Water Research. We are also indebted to Linda Sivesind for language corrections.




