Decolorization of olive oil mill wastewaters by Phanerochaete flavido-alba
Abstract
Olive oil mill wastewater (OMW), a major waste product of olive oil extraction, contains many recalcitrant compounds, mainly present in the colored fraction. In this study we attempted to identify optimum culture conditions for the decolorization of OMW by Phanerochaete flavido-alba for subsequent use in bioremediation assays. Of several media tested, nitrogen-limited P. flavido-alba cultures containing 40 mg/L Mn(II) were the most efficient at decolorizing OMW. Decolorization was accompanied by a 90% decrease in the OMW phenolic content. Concentrated extracellular fluids alone (showing manganese peroxidase, but not lignin peroxidase activity) did not decolorize the major OMW pigment, suggesting that mycelium binding forms part of the decolorization process.
INTRODUCTION
Mediterranean countries produce thousands of tonnes of olive oil mill wastewater (OMW) as part of the oil extraction process. Olive oil mill wastewater is black and highly toxic because of a heterogeneous mixture of polymeric molecules (related to lignin) and humic acids [1-3] and aromatic compounds [4-6].
Fungal ligninolytic machinery oxidizes a wide variety of substrates and can play a role in xenobiotic mineralization [7]. As a result, ligninolytic fungi (particularly white rot fungi such as Phanerochaete chrysosporium) and their enzymes have been used in the bioremediation of environmental pollutants [8-13].
In batch cultures, or when immobilized on polyurethane [14], P. chrysosporium is able to degrade OMW macromolecular chromophores [2] and decrease the amount of low molecular weight phenolic compounds [15, 16]. Pleurotus ostreatus and Lentinus edodes also decrease the total phenolic content and reduce the color of OMW-containing cultures [17, 18].
Phanerochaete flavido-alba is as efficient as P. chrysosporium in mineralizing synthetic radiactive lignin [19] and decolorizing paper mill effluents [20] and is effective in removing soluble phenolics present inOMW[21]. Both fungi accumulate similar levels of lignin peroxidase (LiP) and manganese peroxidase (MnP) activity, which are regulated by levels of nitrogen, carbon, and Mn(II) [22; O. Ben Hamman, unpublished results]. More laccase seems to be accumulated in P. flavidoalba than in P. chrysosporium cultures [19, 23].
The objectives of this study were to characterize optimal conditions for the decolorization of OMW-containing cultures by P. flavido-alba and to determine whether extracellular enzymes alone were sufficient for decolorization.
MATERIALS AND METHODS
OMW and OMW major pigment purification
According to the supplier (Instituto de la Grasa y sus Derivados, Seville, Spain), the main characteristics of the OMW used in this study were pH 4.7; conductivity 0.35 × 10−2 Ω−1/cm; chemical oxygen demand 35.5 g/L; biological oxygen demand 25.5 g/L; total phenolic content 2.8 g/L; ammonia 25 mg/L. After filtration, the residue was kept at −20°C.
Olive oil mill wastewater major pigment (OMWMP) was obtained as previously described [2, 3]. Briefly, the OMW (2.5 L) was vacuum concentrated fivefold and precipitated by the addition of 2 L of cold (4°C) acetone. After 24 h, the precipitate was recovered by decantation, solubilized in 250 ml of distilled water, and reprecipitated as before. The precipitate was again dissolved in 275 ml of distilled water, and reprecipitated by adding methanol (2 L). After 3 d at 4°C, pigmented material was recovered by filtration, vacuum dried, and lyophilized to give 24.5 g of OMWMP, which was stored in the dark at room temperature. Centrifuged OMWMP was oxidized with CuO, extracted, derivatized, and analyzed by gas chromatography-mass spectrometry (GC-MS) [24].
Microorganism and culture conditions
Phanerochaete flavido-alba FPL 106507 was kindly provided by Forest Products Laboratory (U.S. Department of Agriculture, Madison, WI). This strain was maintained on yeast extract-malt extract-peptone-glucose slants [25].
Roux flasks containing 70 ml of the basal medium (nitrogen-limited) GLULN (glucose [10 g/L], nitrogen [2.2 mM ammonium tartrate]) [26], were inoculated with mycelia on yeast extract-malt extract-peptone-glucose from slant tubes, and incubated at 30°C for 15 d. Mycelial mats were broken in a blender and the homogenate was used as inocula (0.5 ml per flask).
Four culture media (selected because of their influence on P. flavido-alba LiP, MnP, and laccase production [19, 22]) were evaluated for their ability to support OMWMP decolorization. Different culture media were prepared by modifying the carbon source and nitrogen content of the basal medium. The medium GLYHN 11 Mn(II) consisted of glycerol (10 g/L) and highnitrogen (22 mM ammonium tartrate) medium with 11 mg/L Mn(II) added as sulfate (LiP- and MnP-producing culture media) [22]. The medium GLYHN no Mn was the same as the GLYHN 11 Mn(II) medium, but with no addition of Mn(II) (low LiP and very low MnP activities were detected in cultures). The medium GLULN 40 Mn(II) consisted of glucose (10 g/L) and ammonium tartrate (2.2 mM) supplemented with 40 mg/L Mn(II) (MnP was produced preferentially over LiP). The medium GLUHN no Mn was the same as GLULN 40 Mn(II) but with 22 mM ammonium tartrate and no added Mn (laccase was preferentially produced and minor quantities of LiP were detected) [19].
Cultures were grown for 10 d in 250-ml Erlenmeyer flasks containing 16 ml of culture medium plus veratryl alcohol (0.43 g/L) and Tween 20 (0.5 g/L). Inocula for media not Mn supplemented were obtained by growing the strain three times (at weekly intervals) in the basal media without addition of Mn(II). After 48 h of incubation at 30°C, static cultures were flushed daily with pure O2 (3 L/min for 1 min). In order to induce laccase in GLUHN no Mn media cultures, these were not O2 flushed [19].
Enzymatic activity assays
The MnP activity was measured spectrophotometrically at 30°C with vanillylacetone as a substrate [27]. The LiP activity was measured spectrophotometrically at 30°C with veratryl alcohol as a substrate [28]. Laccase activity was measured by monitoring the oxidation of 2,2-azino-bis (3-ethylbenzothiazoline-6-sulfonic acid) [29]. Enzymatic activity was expressed in nmol/min substrate oxidized per milliliter.
Color measurement
Flasks were harvested in triplicate. Media was adjusted to pH 7.6 and centrifuged (4,000 rpm for 10 min). The color of these solutions (color-soluble [CS]) was determined by absorbance at 465 nm (A465) in the supernatant and expressed as color units on platinum-cobalt scale [30]. Chromophoric material coprecipitated and/or adsorbed on mycelia at pH 7.6 was recovered from the centrifugation pellets by washing in 10 ml of 2 N NaCl in 2 N NaOH. Insoluble material was removed from the alkaline solution by recentrifuging and the supernatant was adjusted to pH 7.6. The color of this solution (colorinsoluble [CI] components) was estimated as previously.
Decolorization of OMWMP and OMW by P. flavido-alba
For OMWMP decolorization assays, an aqueous solution of lyophilized pigment (0.1 g/ml; pH 4.8) was added to the culture media (pH 4.5). After centrifugation (4,000 rpm for 20 min), the remaining soluble material was autoclaved (115°C for 30 min). This solution was added to noninoculated culture media (as a control) and to cultures (1.5 ml per flask). Olive oil mill wastewater major pigment was included in the culture medium (addition at day 0), or added to 5-d-old cultures.
For OMW decolorization assays, crude vacuum-concentrated OMW was added to cultures at a concentration equivalent to the OMWMP used in OMWMP decolorization experiments (8.3 g/L). Blackish concentrated solutions were adjusted to pH 4.5, deprived of insoluble materials (after centrifugation; 4,000 rpm for 20 min), autoclaved, and added to the cultures. Color of cultures was measured for 6 d (day 5 to day 10 inclusive) as described above.
Sephadex G-200, G-100, and G-25 chromatography
Columns (1 × 30 cm) (Sephadex, Pharmacia Biotech, Uppsala, Sweden), calibrated with syringic acid (198 Mr) and Blue Dextran (200,000 Mr) were equilibrated in pH 7.0 NaCl 1 N. Samples were filtered through 0.22-μm pore filters. The flow rate was adjusted to 0.15 ml/min and 1-ml fractions were collected and measured spectrophotometrically at 280 nm.
Decolorization of OMWMP with MnP
To obtain extracellular fluids, 1-L Erlenmeyer flasks (70 ml GLULN 40 Mn(II) medium each) were incubated until extracellular MnP activity peaked. Extracellular fluids, concentrated 100-fold by ultrafiltration (Centricon, Amicon, Beverly, MA, USA; Millipore®, Milli-Q, Bedford, MA, USA, 10-kDa cutoff membrane), were dialyzed against 10 mM sodium acetate, pH 5.0. Further 100-fold concentration of the fluid, as described previously, gave crude MnP preparation (17,204 nmol/ml·min), which was devoid of detectable LiP or laccase activity. The OMWMP was dissolved (2%, w/v) in sodium tartrate buffer (0.5 M, pH 5.0) and filtered through 0.22-m pore filters.
Sodium tartrate buffer (0.92 ml, 2.17 M, pH 5.0), 2.08 ml of OMWMP solution, 2 ml of crude MnP preparation, and 0.125 ml of 2 M sodium lactate (pH 5.0) were incubated at 30°C for 2 h. At the beginning of the reaction (time 0) and at 30-min intervals, 0.025 ml of H2O2 (10 mM) and 0.1 ml of MnSO4.H2O (5 mM) were added. The OMWMP solutions without crude MnP addition were used as controls. Color estimations (performed in solutions obtained by diluting aliquots in potassium phosphate buffer, 0.2 M, pH 7.6) were expressed as percentage color recovered in crude MnP-added solutions versus control solutions.
Determination of phenolic content
Total phenolics were quantified according to Ribéreau-Gayon[31] with p-hydroxybenzoic acid as standard. Growth was estimated after filtration of mycelial mats and drying for 24 h at 105°C.
RESULTS AND DISCUSSION
OMWMP partial purification and analysis
Other substances present in OMW (such as low molecular weight aromatics) may adversely affect the decolorization process by, for example, enzyme deactivation. The OMWMP was therefore purified to establish efficient conditions for decolorization, without interference from other compounds. Autoclaving of the OMWMP solution did not affect its composition (as analyzed by CuO oxidation-GC-MS) (not shown).
Three classes of compounds were detected in OMWMP: short-chain carboxylic acids (2-hydroxypropanoic, 2-hydroxyacetic, butanedioic, methylbutanedioic, 2,3-dihydroxipropanoic, 2-butenedioic), aromatics (2,5-cyclohexadiene-1,4-dione, 2,6-bis(1,1-dimethylethyl), 2,6-bis(1,1-dimethylethyl)-4-methylphenol, 1,2-benzenedioic acid, 3,4-dimethoxybenzoic acid, 3-methoxy-4-hydroxybenzoic acid), and fatty acids (dodecanoic, tetradecanoic, hexadecanoic, octadecanoic acids).
Selection of culture conditions for OMWMP decolorization by P. flavido-alba
Mycelia were able to decolorize CS fractions when grown in three out of four culture media tested (Table 1), with GLULN 40 Mn(II) media (OMWMP added at 5 d) being the most effective. No evaluation of CI color has been performed in P. chrysosporium cultures [14-16, 32]. No significant decolorization of CI fractions by P. flavido-alba mycelia was observed in any of the culture media tested. Indeed, an increase in the color of CI components, notably in GLYHN no Mn media, was observed. No changes were seen in CS or CI fractions in uninoculated controls (not shown).
| A | B | |||
|---|---|---|---|---|
| Culture medium | CI ± SD | CS ± SD | CI ± SD | CS ± SD |
| Samples from 10-d-old cultures | ||||
| GLYHN 11 Mn(II) | 91.0 ± 11.0 | 17.2 ± 2.1 | 116.0 ± 17.0 | 36.0 ± 5.4 |
| GLYHN no Mn | 143.0 + 19.5 | 20.4 ± 2.3 | 137.0 ± 16.0 | 21.0 ± 2.6 |
| GLULN 40 Mn(II) | 129.0 ± 17.0 | 18.6 ± 2.6 | 83.5 ± 14.0 | 15.7 ± 1.5 |
| GLUHN no Mn | 135.0 ± 10.0 | 102.0 ± 4.0 | 132.0 ± 10.5 | 94.0 ± 12.0 |
| Samples from 15-d-old cultures | ||||
| GLYHN 11 Mn(II) | 245.0 ± 30.0 | 10.6 ± 0.5 | NA | NA |
| GLYHN no Mn | 315.0 ± 9.4 | 4.1 ± 0.2 | NA | NA |
| GLULN 40 Mn(II) | NA | NA | 165.0 ± 18.0 | 11.6 ± 0.6 |
- a Color was estimated at 465 nm in triplicated samples. Control solutions in uninoculated media show 350,179 color units in color soluble (CS) and 24,545 color units in color recovered from mycelia (color-insoluble [CI]) fractions. NA = not assayed.
The effect of culture conditions on extracellular enzyme activity
These results are described in detail in previous publications [19, 22]. In GLYHN 11 Mn(II) media, MnP and LiP were produced [22]. In GLYHN no Mn, low levels of LiP and very low levels of MnP were detected (not shown). In GLULN 40 Mn(II) media, high MnP and low levels of LiP were found (Fig. 1a). In GLUHN no Mn media, very low MnP and low LiP activities were detected [19]. Laccase production has been detected in GLUHN no Mn media without O2 flushing [19].
OMWMP chromophore destruction
The ratio of A280 to A465 has been shown to be useful in the estimation of chromophore destruction in bleach plant effluent [33]. In decolorized GLULN 40 Mn(II) cultures the A280/A465 ratios in CI (6.4) and CS (15.0) components increased with respect to those fractions (4.8 and 5.0, respectively) in uninoculated control solutions (Table 2). In decolorized GLYHN no Mn cultures the ratio of A280 to A465 in CS components (11.0) was about twofold increased (Table 2) as compared to control OMWMP solutions. However, that ratio of CI components (5.3) from GLYHN no Mn cultures showed a smaller increase than in CI fractions from GLULN 40 Mn(II) cultures, suggesting a higher adsorption of visible chromophores to mycelia in GLYHN no Mn cultures. These results demonstrate that chromophoric materials were degraded in P. flavido-alba cultures where the production of ligninolytic enzymes, particularly MnP, took place (Fig. 1a).
The role of extracellular enzymes in OMWMP decolorization
Because no decolorization was observed in cultures producing more laccase than LiP or MnP (GLUHN no Mn media) [19], laccase is unlikely to be an important component of the decolorization machinery of P. flavido-alba, at least without the involvement of ligninolytic peroxidases. Overall decolorization efficiency was not augmented by increasing incubation time (up to 15 d). The increased decolorization in CS fractions correlated with a noteworthy increase in the color of CI fractions (Table 1). As in 10-d-old cultures, the color of CI components was less pronounced in GLULN 40 Mn(II) cultures.
These results suggest a saturation of the ability to decolorize OMWMP. After incubation for 10 d, the increased decolorization observed in the cultures seems to have been due to mycelial adsorption.
OMWMP and crude OMW decolorization kinetics in GLULN 40 Mn(II) cultures
Olive oil mill wastewater major pigment decolorization kinetics were studied in P. flavido-alba cultures with maximum CS decolorization capacity (GLULN 40 Mn(II) media, with OMWMP added after 5 d of incubation). Heat-killed mycelia decolorized CS fractions (Fig. 1b). No change in color of CS or CI fractions was observed either in OMWMP- or crude OMW-containing uninoculated media (not shown).
After CI components were recovered, color adsorbed to mycelia was visible, but could not be directly quantified. The CI color changes seen during incubation of cultures did not justify the decrease in the color recovered in CS fractions; about 80% CS decolorization was obtained at the end of the experiment for OMWMP (Fig. 1c).
OMW decolorization
In order to determine the influence of components accompanying OMWMP (in crude OMW) on decolorization, crude OMW decolorization was assayed. About 70 % CS decolorization of crude OMW was observed (Fig. 1d). A stronger color in crude OMW-supplemented cultures than in those containing OMWMP was observed. This can be explained by the loss of methanol-soluble components during purification of the OMWMP. Total phenolics in decolorized OMW decreased by about 90% (Fig. 1d). The OMW decolorization kinetics in P. flavido-alba cultures on GLULN 40 Mn(II) media were similar to those observed in OMW-supplemented P. chrysosporium cultures on GLYHN and GLULN media containing 3.8 mg/L Mn(II) [32].
The role of extracellular enzymes in OMW decolorization
In white rot fungi, the role of ligninolytic peroxidases in organopollutant degradation is still controversial [10, 34]. In spite of the involvement of LiP (and to a lesser extent MnP) in OMW decolorization by P. chrysosporium [16], P. flavidoalba cultures with no detectable MnP activity (GLYHN no Mn media) yielded similar overall decolorization as those showing LiP and MnP activities (GLYHN 11 Mn(II) [22] and GLULN 40 Mn(II); Fig. 1c). However, the fact that more color was recovered in CI fractions in GLYHN no Mn media (which shows no MnP activity) suggested that ligninolytic peroxidases (at least MnP) can mediate the P. flavido-alba decolorization.
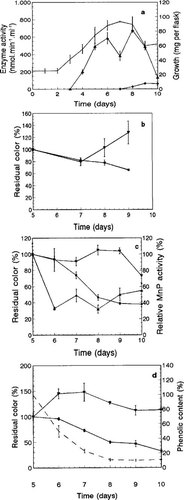
Phanerochaete flavido-alba cultures in GLULN 40 Mn(II) medium (a). Residual color in olive oil mill wastewater major pigment (OMWMP)-containing heat-killed mycelia (b), OMWMP-added cultures (c), and crude olive oil mill wastewater-added cultures (d). Mycelial growth (——), manganese peroxidase (MnP) activity (▴), lignin peroxidase (LiP) activity (♦). ▪, color units of color-soluble components; ▾, color units of color-insoluble components; —, total phenolics. Colored material was added after growth for 5 d. Values are means of three replicates ± SD.
Lignin peroxidase was the main enzyme implicated in P. chrysosporium OMW decolorization [16], whereas the major extracellular ligninolytic enzyme detected in P. flavido-alba OMWMP-added cultures (Fig. 1c) was MnP (LiP was not detected). Thus, MnP is more likely to participate in the decolorization process than LiP. However, because ligninolytic peroxidases are inactivated by aromatic [35] and phenolic substances, LiP activity estimations can be affected [36]. The absence of P. flavido-alba LiP activity cannot definitively exclude this enzyme from the OMWMP decolorization process.
| A280/A465 | ||
|---|---|---|
| Culture medium | CS | CI |
| Control uninoculated solutions | 0.5/0.0986 | 0.356/0.074 |
| GLYHN no Mn | 0.382/0.0344 | 0.5/0.094 |
| GLULN 40 Mn(II) | 0.466/0.031 | 0.457/0.071 |
- a CS = color soluble; CI = color insoluble, color recovered from mycelia.
Crude extracellular MnP did not decolorize OMWMP solutions in the presence of MnSO4 with continuous addition of H2O2 in lactate buffer (not shown). This suggest that the mechanism of OMWMP decolorization is a mycelial-binding-dependent process, as was demonstrated for P. chrysosporium synthetic lignin degradation [37]. Envelope-bound LiP and MnP have been described in certain fungi [38, 39].
Chromatography of decolorized samples
Sephadex gel filtration was used to orientatively determine the molecular weight heterogeneity of OMW chromophoric material [3]. A decrease in the content of low (about 198 Mr) and high molecular weight components was observed in decolorized P. chrysosporium cultures [32], suggesting a correlation between OMW decolorization and depolymerization [16].
The CS and CI components from 10-d-old P. flavido-alba cultures and control solutions were compared by Sephadex G-200 and G-25 gel filtration. An asymmetric major peak, suggesting a heterogeneous composition in OMWMP, was obtained in control CS fractions on Sephadex G-200 (Fig. 2a). In CS samples from P. flavido-alba OMWMP decolorized cultures, a more symmetric peak was obtained, suggesting modifications in molecular mass distribution of pigment components associated with decolorization. Compounds eluting in the fractionation upper limit (200 kDa) were not detected in control fractions on Sephadex G-200 (Fig. 2a). In CS samples from P. flavido-alba, OMWMP decolorized cultures, even when column loading was increased leading to increased absorbance in the major fraction (Fig. 2a). Gel chromatography profiles of CS and CI samples from OMWMP cultures showed no significative difference on Sephadex G-100, with no detection of the high molecular weight fraction in CI components (not shown). This suggested that components detected in CI fractions are identical to those present in CS fractions. The disappearance of a high molecular weight fraction in Trametes versicolor-decolorized bleach plant effluents was interpreted as depolymerization of aromatic lignin compounds [40].
Similar profiles were obtained from Sephadex G-25 column chromatography of decolorized CS samples from OMWMP and crude OMW. In control CS samples from crude OMW, the main absorbance peak eluted at the upper fractionation limit (5,000 Mr). In the CS components from P. flavido-alba-decolorized cultures, a significant decrease in the lower molecular components was observed (Fig. 2b). A slight increase in higher and intermediary molecular mass components was also noted in crude OMW CS decolorized samples.
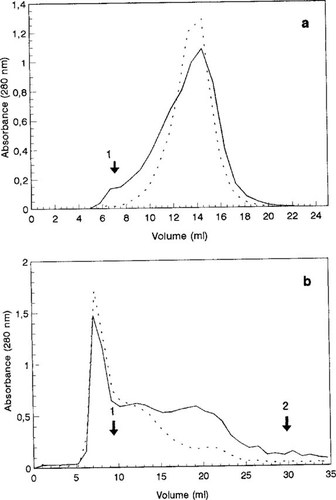
Sephadex G-200 column chromatography of color-soluble (CS) components (– – –) from Phanerochaete flavido-alba 10-d-old cultures on GLULN 40 Mn(II) medium, supplemented with olive oil mill wastewater major pigment after growth for 5 d (a). Sephadex G-25 column chromatography of CS components (– – –) from crude olive oil mill wastewater-added cultures (1 = Blue Dextran; 2 = syringic acid) (b). —, control solutions.
Taken together, analysis of these data on molecular mass distribution in OMWMP and crude OMW decolorized cultures indicates that the fungus (under ligninolytic conditions that favored MnP production, i.e., GLULN 40 Mn(II)) is responsible for a decrease in the proportion of the higher and lower molecular mass components and an increase in the proportion of pigment components of intermediary molecular mass, lower than 5,000 and higher than 198. These results are compatible with decolorization associated with a partial depolymerization of the higher molecular mass components. The observed decrease in lower molecular mass pigment components accompanying decolorization can be associated with degradation or with a repolymerization in components of a molecular mass lower than 5,000. On the other hand, because crude concentrated P. flavido-alba MnP cannot decolorize OMWMP, mycelium adsorption seems to be a part of the decolorization process.
We conclude that OMW decolorization and removal of phenolic compounds by P. flavido-alba make it a valuable organism for bioremediation of these pollutant wastes. Extracellular enzymes alone do not seem to be effective for the observed decolorization and our group is currently studying the mechanisms involved.
Acknowledgements
We are very grateful to the Spanish Comisíon Interministerial de Ciencia y Tecnología for financial support (project Bio 96/0393). The assistance of Juan Carlos Ruiz Ruiz is acknowledged.




