Dechlorination of chloromethanes on iron and palladium-iron bimetallic surface in aqueous systems
Abstract
Iron granules (< 10 μ) (Fe°-H2O) and palladium-treated iron granules (Pd/Fe°-H2O) in contact with water have been tested as a potential means to dechlorinate chloromethanes (CCl4, CHCl3, and CH2Cl2) that are commonly generated in teaching chemistry laboratories. Palladium treatment enhanced the rate of dechlorination of CCl4 by a factor of about seven compared to the untreated Fe°-H2O, but the potential toxic effect of palladium remains a concern. Oxygen in the aqueous solution reduced the dechlorination rates of CCl4 with both Fe°-H2O and Pd/Fe°-H2O by at least a factor of three. Nevertheless, from the consideration of remediating solvent wastes, both systems appear suitable for treating CCl4 and possibly CHCl3 wastes, even in the presence of oxygen. The reactivities of the three chloromethanes toward the Fe°-H2O were vastly different. The dechlorination rate of CCl4 was by far the highest, followed by that of CHCl3, and CH2Cl2 was virtually unreactive toward the Fe°-H2O system. Dechlorination reactions of CCl4 and CHCl3 were systematically examined on both Fe°-H2O and Pd/Fe°-H2O. On the basis of the data, it appears that CCl4 is successively dechlorinated to form CH4 via the formation of partially dechlorinated intermediates. Proton transfer to various intermediates produces the chloromethanes: CHCl3, CH2Cl2, and CH3Cl. Furthermore, CH4 formation, independent of the proton-transferring reactions, appears to be selectively stimulated by palladium (Pd/Fe°-H2O).
INTRODUCTION
A wide range of chemical wastes are generated by teaching chemistry laboratories. As a part of continuing efforts to reduce the wastes, comprehensive programs are being developed for the in situ remediation of the potentially toxic wastes. Chloromethane solvents are among the chemicals that are generated in substantial quantity and are under consideration for treatment. These wastes are relatively difficult to remediate under ambient conditions partly because they are typically concentrated and frequently contain metals and other substances that might be toxic to microorganisms. Therefore, the use of microbes of dechlorinating competency does not appear to be suitable.
In the early 1970s, Sweeney [1] and Gillham [2] reported the use of zero-valent metals, transition metals in particular, as a practical means to decontaminate organohalides. This approach has attracted a great deal of interest because of its realistic and cost-effective means to degrade organohalides in groundwater. The underlying chemistry of reductive dehalogenation of chlorinated aliphatic hydrocarbons in aqueous solution has since been studied under different reaction conditions [3-8]. For example, Matheson and Tratnyek [3], while studying the kinetics and mechanisms of dechlorination of carbon tetrachloride (CCl4) for the metal iron–water systems (Fe°-H2O), found that CCl4 was dechlorinated to form chloroform (CHCl3) as a dominant product, which in turn is partly transformed to methylene chloride (CH2Cl2), although at a much slower rate. However, the fate of CH2Cl2 remained unclear.
Although the Fe°-H2O approach appears to be promising for dechlorinating CCl4 and possibly CHCl3 to CH2Cl2 in such environmental matrices as groundwaters and sediments, where the oxygen levels are relatively low, the Fe°-H2O-mediated treatment of the wastes that come in contact with air for periods of days might be too inefficient, for the half-reaction involving O2 as electron acceptor, 2 Fe° + O2 + 2 H2O ⇌ 2 Fe2+ + 4 OH−, can compete with the dechlorination reaction of CCl4, which can be described by the reaction Fe° + RCl + H+ → Fe2+ + RH + Cl−. Recent studies have indicated that the dechlorination rates by the iron–water system can be improved at least in two ways. Wang et al. [9] showed that the dechlorination rates of trichloroethene could be vastly increased by increasing the surface area of iron granules from 10 μm (with surface area of 0.9 m2/g) to 1 to 100 nm (with surface area of 33.5 m2/g). In addition, palladium-treated iron (Pd/Fe°) has been shown to be highly effective in the dechlorination of polychlorinated biphenyls [10] and chloroethenes [11]. A study on the palladium–iron surface indicated that prolonged exposure of the Pd/Fe surface to a saturated solution of aqueous trichloroethene resulted in the accumulation of the hydroxylated iron oxide but could be readily removed by washing with a dilute mineral acid without desorbing palladium [12].
The present study was undertaken with three purposes. First, we wanted to determine the relative effects of both Fe°-H2O and Pd/Fe°-H2O on the dechlorination rate of CCl4. Second, we considered the effect of oxygen on the reductive dechlorination, as the matrices of the solvent wastes are normally in equilibrium with air. Finally, we wanted to further explore the degradation products of chloromethanes for the iron–water system by the use of palladium as a catalyst, an aspect clearly important to the remediation of chloromethanes.
MATERIALS AND METHODS
Iron particles (<10 μ) and potassium hexachloropallidate (K2PdCl6; fresh weight 397.32) were obtained from Aldrich Chemical (Milwaukee, WI, USA). Surface oxides of the iron were removed with 2 M HCl. Residual acid was removed by rinsing the acid-treated particles with a bicarbonate solution (0.05 M). The washed iron particles were used in the dechlorination experiments or were palladized by suspension in an aqueous solution of K2PdCl6 (7 × 10−3 mole of Pd per mole of Fe). The mixture was gently shaken on a shaker-incubator at ambient temperature. The coating process is complete in about 8 min, as indicated by the appearance of a bright yellow solution. The palladized iron was thoroughly washed with 20 to 30 volumes of water under aspiration or until the pH of the rinses rose to about 7.
Dechlorination reaction
Stock solutions of carbon tetrachloride (CCl4) of high purity (HPLC grade, Fisher Scientific, Fairlawn, NJ, USA) were prepared in distilled water. Degradation experiments were performed in 15-ml borosilicate vials with a Teflon®-lined rubber septum with an aluminum crimp cap. The aqueous CCl4 solution, 10 ml in most cases, was placed in the vial containing premeasured amounts of Fe or Pd/Fe. Vials were sealed and laid on their side on the flat platform of a linear shaker such that the vials would gently roll sideways when shaking. Reactions were carried out at ambient temperature (25 ± 2°C), and headspace samples were withdrawn with a calibrated syringe through the septum. Vials containing CCl4 (or CHCl3) solution without Fe° or Pd/Fe° were used as blank controls. The controls were analyzed at the beginning and end of the experiments. The overall loss of the parent compound for the experimental period that lasted for as long as 37 h was less than 3%.
Instrumentation
Gas chromatography and gas chromatography/mass spectrometry were used throughout this study in the dechlorination of CCl4 and the formation of CHCl3, CH2Cl2, CH3Cl, and CH4 in the headspace samples. Gas chromatography was performed on a Hewlett-Packard Model 5885A Gas Chromatograph (Palo Alto, CA, USA) equipped with a flame ionization detector (FID) and fitted with a Hewlett-Packard Ultra 2 fused silica capillary column (25 m × 0.32 mm × 0.52 μm film thickness; cross-linked 5% PhMe silicone). The FID was operated at 250°C, whereas the injection port was held at a constant 100°C. The column was operated isothermally at 55°C. Detector gas flows to the FID were 200 ml/min of air (Ultra zero-air, App-Tek, Brendale, Australia) and 20 ml/min of hydrogen (99.9%). Helium was used as the carrier gas at a flow rate of 4 ml/min. The headspace samples were injected manually using a gastight syringe with an injection volume of 25 μL, unless stated otherwise. The peaks were identified by comparison with the retention times of reference compounds. A Finnigan 4000 GC/MS (Finnigan, Bremen, Germany) operated under similar conditions was used to confirm identities of the peaks.
Quantitation
A calibration curve for each compound was established in the following way. To evaluate the detector response, independent mixtures of CCl4, CHCl3, CH2Cl2, CH3Cl, and CH4 were prepared by injecting variable amounts of the individual components into tightly sealed vials containing 10 ml of distilled water. The solutions were allowed to equilibrate (about 40 min) under the conditions of analysis, followed by manual injection of headspace samples with an injection volume of 25 μL into the gas chromatograph. The detection limits for CCl4, CHCl3, and CH2Cl2 were 100 to 500 ppb and about 8.0 and 3.0 nmol for CH3Cl and CH4, respectively. The FID responses were linear up to three orders of magnitude from their detection limit for all compounds.
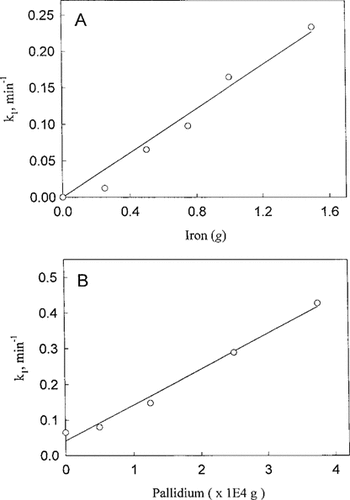
(A) Effects of Fe on pseudo-first-order rate constant (k′) for dechlorination of CCl4. Each reaction vial contained 253 μM CCl4 and varying amounts of Fe, as indicated. (B) Effects of Pd on pseudofirst-order rate constants (k′) for dechlorination of CCl4. 0.5 g of Fe particles were treated with Pd at the following levels: 50, 125, 250, and 375 μg The untreated Fe served as the control.
RESULTS
To assess the catalytic role of palladium in the dechlorination of CCl4, control experiments were initially performed with iron alone (Fe°-H2O). Aqueous solutions of CCl4 (263 μmol or 40 ppm) were reacted with the incremental amounts of iron particles at room temperature. The reaction was monitored until the CCl4 reached its detection limit. Figure 1A shows the apparent increasing rate constant (k′) for the disappearance of CCl4, which increases as a function of iron mass. The rate constants were derived from the plots of natural log concentration of CCl4 versus time that were fitted to the firstorder reaction model. The coefficient of determination (r2) on the data gives 0.88 for n = 10. Under the experimental conditions, dechlorination of CCl4 assumed pseudo-first-order kinetics with respect to the mass of iron granules. Parallel experiments were next carried out to investigate the effect of palladium on the dechlorination of CCl4. For comparative purposes, the iron weights were maintained at 0.5 g, and the amount of palladium used to palladize the iron ranged from 50 to 375 μg (5.2 × 10−5−3.9 × 10−4 moles per mole of Fe). Figure 1B shows that the rate constant for the disappearance of CCl4 increases linearly with the amount of palladium (Pd/Fe°-H2O) used in the study. Linear regression on the data for the disappearance of CCl4 yields r2 = 0.95 for n = 6.
| Aerobic | Anaerobic | |||
|---|---|---|---|---|
| k1, min−1a | t1/2, min | k, min−1 | t1/2, min | |
| Fe°-H2O | 0.0041 ± 0.0012 | 120 | 0.0160 ± 0.0011 | 39 |
| Pd/Fe°-H2O | 0.0220 ± 0.0010 | 33 | 0.1360 ± 0.0420 | 5 |
- aMean (±SE) for n = 10 − 15.
The estimated relative half-reduction potentials of oxygen and CCl4 in water at pH 7.4 are +0.40 [13] and +0.67 [14], respectively. Thus, Fe° is expected to react with CCl4 more favorably than with oxygen. Nevertheless, the rate of reductive dechlorination by the Fe°-H2O might be substantially reduced in the presence of oxygen. The aqueous solution of CCl4 was deoxygenated as follows. Immediately before the start of the experiment, 10 ml of distilled, deionized water was placed in the vial containing 0.5 g iron or 0.5 g iron treated with 250 μg Pd. The headspace was quickly flushed with nitrogen before crimping the vial. The air in the reaction medium was purged by passing a stream of nitrogen with a slight positive pressure through the septum by means of a microliter-size syringe that was coupled to a compressed nitrogen tank. A second syringe placed on the septum allowed the gas to be evacuated. Microliter aliquots of the aqueous CCl4 were then injected into the reaction medium. As shown in Table 1, an inhibitory effect of oxygen is clearly evident. It took nearly three to four times as long to dechlorinate the same quantity of CCl4 in the presence of oxygen. The oxygen effect was even more pronounced when the experiments were performed with the Pd/Fe-H2O.
To investigate the potential dechlorination of CCl4 to CH4, we performed several experiments. For purposes of comparison, dechlorination reactions of CCl4 and the partially chlorinated products, CHCl3 and CH2Cl2, by the Fe°-H2O were individually measured in separate experiments. All the reactions were performed under aerobic conditions unless stated otherwise. The chloromethane concentrations were measured over the experimental period of 23 d. As shown in Figure 2, the disappearance of CCl4 was by far the fastest; it took only a few hours for the compound to fall below the detection limit. On the other hand, it took nearly 23 d for the CHCl3 to disappear. It is also evident that the dechlorination rates of CH2Cl2 were barely measurable over the entire experimental period of 23 d. In the next series of experiments, the transformation of CCl4 was analyzed on both Fe°-H2O and Pd/Fe°-H2 O. For the Fe°-H2O system, the dechlorination of CCl4 was accompanied by the formation of CHCl3 (Fig. 3). In terms of mass balance, CHCl3 accounts for about 65% of the starting CCl4. In addition, formation of CH4 was detected, and its appearance was nearly concurrent with the formation of CHCl3. The early formation of CH4 was somewhat surprising in view of the observations that the reactivity of CH2Cl2 toward the Fe°-H2O was extremely low (Fig. 2). Parallel experiments performed with Pd/Fe° yielded additional information (Fig. 4). The dechlorination of CCl4 on the bimetallic surface proceeded with much greater rate; within 2 h, the dechlorination of CCl4 was essentially complete, with the level of CHCl3 already declining from its peak. By the end of the 10-h experimental period, virtually all CHCl3 was dechlorinated, with the attendant appearance of CH4 as the major dechlorination product. In addition, the formation of CH3Cl, although relatively minor, was also evident.
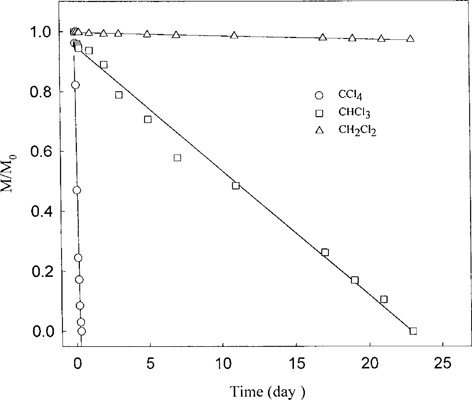
Kinetics of dechlorination of CCl4, CHCl3, and CH2Cl2. The reactions were independently carried out in the presence of each of the chloromethanes in the presence of 0.5 g Fe under aerobic conditions. Effect of Pd on pseudo-first-order rate constants (k′) for dechlorination of CCl4.
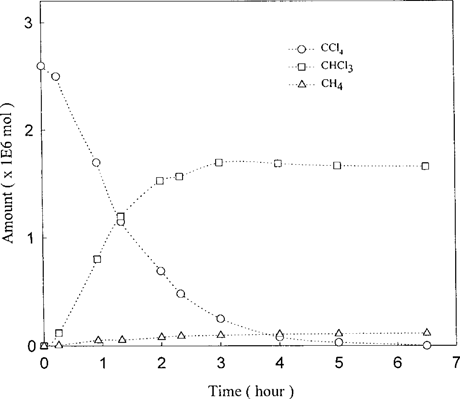
Kinetics of CCl4 dechlorination on Fe°-H2O. The reactions were performed in the presence of 0.5 g Fe under aerobic conditions.
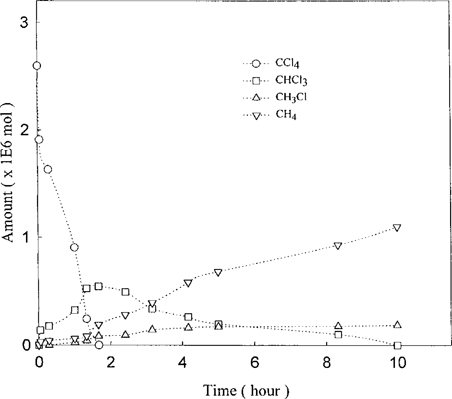
Kinetics of CCl4 reduction on Pd/Fe°-H2O. The reaction was performed in the presence of 0.5 g Fe treated with 250 μg Pd.
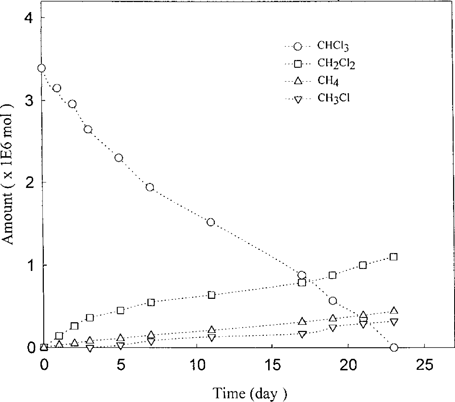
Kinetics of dechlorination of CHCl3 on Fe°-H2O. The reaction was performed in the presence of 0.5 g Fe. The reaction conditions were those of Figure 3.
When CHCl3 was reacted with the Fe°-H2O as starting substrate, it took nearly 23 d for the CHCl3 to fall below the detection limit, and its dechlorination was accompanied by the appearance of the putative intermediates: CH2Cl2 and CH3Cl plus CH4 (Fig. 5). The aggregate amounts of these products account for less than 50% of the starting CHCl3; however, it should be noted that the formation of all these products continued to rise when the experiments were terminated. When the parallel experiments were carried out with the Pd/Fe°-H2O, the dechlorination rates of CHCl3 dramatically increased: its dechlorination took only about 1 h (Fig. 6). Furthermore, the concomitant appearance of CH4 as the single dominant product was even more evident. Both CH2Cl2 and CH3Cl also appear as minor products, but their formation was clearly delayed.
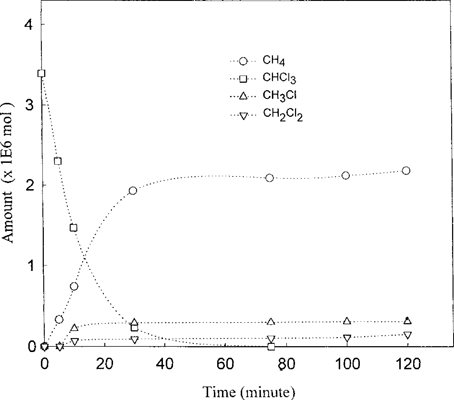
Kinetics of CHCl3 on Pd/Fe°-H2O. The reaction conditions were those of Figure 4.
DISCUSSION
The present data demonstrate that micron-scale iron granules suspended in water (Fe°-H2O) are highly effective in dechlorinating CCl4. The kinetics of CCl4 dehalogenation was related to the amount of iron, suggesting that the rate of dechlorination is set by the availability of iron surface area, as described in the proposed mechanisms of dehalogenation of halogenated hydrocarbons by metals in aqueous systems [3]. A recent study by Wang et al. [9] on the dechlorination of trichloroethene by nanoscale iron powder further illustrates how the reaction rates could be improved by increasing the metal surface area. The present study demonstrates that dechlorination of CCl4 can also be increased by treating iron granules with palladium (Pd/Fe°-H2O), verifying the high catalytic effects previously demonstrated in connection with polychlorinated biphenyls [10] and trichloroethenes [11]. However, the catalytic role of palladium for the dehalogenation of organohalides for the Fe°-H2O system is speculative. Although palladized iron has a clear advantage in the promotion of dechlorination of organohalides, a possibility of desorption of palladium from the Pd/Fe surface as a result of repeated exposure to aqueous organohalides remains a concern because of its potential toxicity. On the other hand, the short-term analysis of the Pd/Fe surface with X-ray photoelectron spectroscopy shows that the Pd/Fe surface is fairly stable [12].
We have examined the effects of oxygen on the reductive dechlorination rates to better estimate treatment of chloromethane wastes that are normally in a state of equilibrium with atmospheric oxygen. In aqueous systems, zero-valent iron is readily oxidized by the presence of H+ and H2O as electron acceptors (corrosion). As anticipated from the reduction potentials of oxygen and organohalides, dissolved oxygen strongly competes with the reaction of CCl4 with Fe°, as evidenced by the reduced rates of halomethane disappearance. Nevertheless, the application of the Fe° or Pd/Fe° system is practical in the treatment of the concentrated chloromethane wastes under aerobic conditions.
The reaction of CCl4 by the Fe°-H2O under the conditions of this study produced CHCl3 as the dominant product (Fig. 3), which is estimated to be 65 to 70% of CCl4 consumed. These results are in general agreement with the earlier observations of Matheson and Tratnyek [3], although our experiments were conducted under aerobic conditions. In addition, the degradation of CCl4 by the Fe°-H2O system was also accompanied by the formation of CH4 as a significant minor product, raising doubts on any mechanism in which partially chlorinated chloromethanes are formed as obligatory intermediates in the formation of CH4. The dechlorination of CCl4 by the Pd/Fe° system performed under similar conditions also produced CH4, but its formation as the dominant product accounted for about 50% of the CCl4 that disappeared.
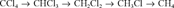
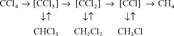
It could be that the [CCl3] is a radical that is formed via the one-electron reduction of CCl4, as, for example, Criddle et al. [7] described for the electrolytic model system for reductive dehalogenation in aqueous environments. These investigators are supportive of the mechanism that involves oneelectron reduction of CCl4 to give a trichloromethyl radical. The radical might then be transformed to various products, including CO2, HCOOH, and CHCl3, via different routes. In the present system (Fe° or Pd/Fe°), a trichloromethyl radical might be formed as the first step on the iron surface, which is then either hydrogenated to form CHCl3 or further dechlorinated to form the less chlorinated radical intermediates. Matheson and Tratnyek [3] showed that the kinetics of CCl4 dehalogenation on the Fe°-H2O is highly pH dependent. However, whether the H+ participates in the protonation of the radical intermediates remains to be investigated.
According to the previously described scheme, for CHCl3 to degrade to form CH4, it must first undergo deprotonation to form the trichloromethyl intermediate followed by successive dechlorination while remaining on the iron surface. Likewise, CH2Cl2 as well as CH3Cl could be similarly dechlorinated to form CH4; however, on the basis of the extremely low rate of degradation of CH2Cl2 by the Fe°-H2O, its degradation to CH4 should be negligible.
The increased formation of CH4 by the Pd/Fe°-H2O (Figs. 4 and 6) also suggests that the palladium accelerates the successive dechlorination reactions with the formation of the intermediates without directly affecting the proton transfer reactions. For example, in the presence of palladium, the formation of CH4 from CCl4 increased from 6 to 44% and the formation of CH4 from 12 to 63% when CHCl3 was used as the parent compound. That the formation of CH4 is nearly concurrent with the dechlorination of CHCl3 indicates that the hydrogenation of [CCl3] is faster than that for the less chlorinated intermediates, [CCl2] and [CCl], to form CH2Cl2 and CH3Cl, respectively (as shown by their delayed appearance). It should be noted that the experiments here were shortened to 2 h (vs 23 d without palladium), so that the formation of these two products was not complete when the experiments were terminated.
In terms of the carbon mass balance between the products (CH4, CH2Cl2, and CH3Cl) and the starting substrate (CHCl3), the combined mass of the products account for about 70% of the parent compound, implying that perhaps the CCl4 or the other chlorinated compounds could be partially degraded to form other carbon products, such as CO2 and formate, when the reactions are performed in the presence of oxygen. For example, the reaction of CCl4 with pyrite (FeS2) under aerobic conditions produced CO2, whereas in an anaerobic pyrite system, about one-half of the CCl4 was transformed to CHCl3 [4]. Other experimental conditions that include other metals could also affect the manner in which CCl4 is decomposed. Boronina and Klabunde [15] reported that decomposition of CCl4 by the Zn-H2O produced CH4 via the partially chlorinated methanes (CHCl3, CH2Cl2, and CH3Cl), whereas CO2 and CHCl3 were the major products in the case of a Sn-H2O system.
CONCLUSIONS
Of the three chloromethanes examined, iron granules (Fe°-H2O) were most effective in dechlorinating CCl4. The Fe°-H2O is far less effective in the dechlorination of CHCl3 and is virtually unreactive toward the CH2Cl2. Palladized iron (Pd/Fe°-H2O) dramatically increases the dechlorination reactions. The presence of oxygen significantly reduces the rate of dechlorination of CCl4 by both the Fe°-H2O and the Pd/Fe-H2O. From the mass balance and the time-course appearance of the chloromethanes, it seems that the partially chlorinated products, CHCl3, CH2Cl2, and CH3Cl, are not obligatory intermediates in the formation of CH4 from CCl4. It is proposed that the dechlorination of CCl4 initially forms a reactive trichloromethyl intermediate, possibly a free radical, that is then successively dechlorinated to produce additional reactive intermediates, hydrogenation of which then results in the formation of the corresponding chloromethanes. Palladium (Pd/Fe°-H2O) accelerates the decomposition of either CCl4 or CHCl3 to form CH4 as a major product.
Acknowledgements
We thank John Masnovi for providing many useful comments in the preparation of this manuscript.




