Multispecies toxicity assessment of compost produced in bioremediation of an explosives-contaminated sediment†
Publication 4674, Environmental Sciences Division, Oak Ridge National Laboratory.
Abstract
A multispecies terrestrial test system was used to assess the environmental effectiveness of composting for bioremediation of explosives-contaminated soils. The assessment involved comparing biological responses, from the individual to the community level, in remediated and reference composts. A 6-month greenhouse study incorporated two soil invertebrate species, three plant species and an associated symbiont, and the naturally occurring complement of soil microorganisms. Measured parameters included growth and reproduction of earthworms and isopods; soil mite diversity; soil lipid class composition as an indicator of soil microbial community structure; plant growth, photosynthesis, and reproduction; and root nodulation and symbiotic N2 fixation. Additional short-term toxicity tests of seed germination and earthworm survival were performed to supplement the mesocosm data. Compost prepared from the explosives-contaminated soil inhibited several aspects of plant growth and physiology, but few adverse effects on soil invertebrates were detected. An initial lag in earthworm and isopod reproduction occurred in the reference compost, reflecting some inherent compost differences not associated with contamination, and highlighting the importance and the difficulty of finding appropriate reference soils for assessing hazardous waste sites or remediation technologies. Nonetheless, the results from this study suggested some nonlethal effects from the contaminated-soil compost, primarily to plants. The mesocosm methodology used in this study can bridge the gap between traditional short-term toxicity testing and longer term field assessments, and provide information on ecological effects by explicitly including measurements of multiple species across several levels of ecological organization.
INTRODUCTION
Composting has been investigated by the U.S. Army as an alternative to incineration for the purpose of treating explosives-contaminated soils and sediments at several sites in the United States [references in 1]. In the composting process, contaminated sediments are mixed with organic materials and formed into long narrow mounds (windrows) that are turned periodically by machine. In such a system, a 40-d composting period reduced the concentrations of explosives in composts by >98% and in aqueous leachates from the composts by >97% [1]. These studies have also shown that as composting proceeds, toxicity of aqueous leachates and mutagenicity of organic solvent extracts tend to decline [1]. However, because the metabolites observed during composting did not fully account for the decline in concentration of 2,4,6-trinitrotoluene (TNT), and because TNT may accumulate in soils in a chemically bound fraction [1, 2], the long-term suitability of this type of compost for land application could not be predicted.
Toxicity tests currently available for terrestrial systems tend to focus on short-term assessments of either acute toxicity or effects on sensitive life stages, for example, the 14-d earthworm toxicity test, or the test of germination and early growth of plant seedlings [3]. These tests are economical, but because they are limited in terms of exposure time and taxonomic breadth, may not be able to detect more subtle effects that develop only at the population or community levels, occur to different degrees in different species, or disrupt interactions between species.
In this study we used soil mesocosm systems containing a range of terrestrial taxa to assess the suitability of two composts to sustain biological activity over the longer term. The two composts assessed were generated as part of a field-scale demonstration of composting technology for explosives bioremediation [1 and references therein]. One compost was produced using an explosives-contaminated sediment; the reference was prepared from uncontaminated soil. The responses of the organisms, including plants, invertebrates, and existing soil microbial communities (assessed via lipid class analysis), were followed in the mesocosms over a 6-month period to establish a chain of evidence linking acute toxicologic activity with ecologically relevant chronic effects at higher levels of organization, including populations and communities of soil organisms, and plant-microbe symbiotic relationships. Results were compared with those from more traditional laboratoryscale testing conducted in growth chambers.
MATERIALS AND METHODS
Composts
The composts tested in this study were prepared at the Umatilla Army Depot Activity (UMDA) at Hermiston, Oregon, USA, by Roy F. Weston (West Chester, PA, USA) as part of a larger field-scale composting experiment comparing techniques of windrow composting [1]. The contaminated compost (CWR-8) was prepared by mixing 30% (v/v) contaminated soil and sediment from a dry explosives washout lagoon, with 70% (v/v) organic amendment (cow and chicken manure, sawdust, alfalfa, and potato waste) to promote microbial growth and metabolic activity during composting [1]. At the beginning of the composting period (day 1), concentrations of TNT, hexahydro-1,3,5-trinitro-1,3,5-triazine (RDX), and octahydro-1,3,5,7-tetranitro-1,3,5,7-tetrazocine (HMX) in the contaminated compost were 2,326 mg/kg, 884 mg/kg, and 266 mg/kg, respectively (dry weight basis) [1].
To provide a comparable reference material for the effects testing, a reference compost (UWR-5) was prepared along with the contaminated-sediment compost, using the same organic amendments, but using 30% (v/v) uncontaminated soil from an adjoining area and of the same soil type as that underlying the lagoon [1]. Both composts were characterized in terms of the concentration of explosives and explosives metabolites over the 40-d composting period [1]. Data on the mutagenicity and aquatic toxicity (to Ceriodaphnia dubia) of compost leachates and extracts were also reported [1]. At the end of the composting period, measurable (extractable) concentrations of TNT, RDX, and HMX in the contaminated compost had declined to 2.84, <2.9, and 3.91 mg/kg dry weight of compost, respectively. No explosives or explosives metabolites were detected in the reference compost [1]. After the composting study ended, the material was stored in Oregon for approximately 6 months before being shipped to testing facilities at Oak Ridge National Laboratory, Oak Ridge, Tennessee, USA. All data presented here refer to the final composts, as received.
Chemical analyses
Three replicate subsamples of each of the two composts were tested for differences in pH, total Kjeldahl N, total organic matter (by combustion), soil-available plant nutrients (in a saturated media extract test), cation exchange capacity, and total metal concentrations (by inductively coupled plasma spectrometry and atomic absorption for As, Se, and Hg). Organic carbon concentrations used to calculate C to N ratios were approximated by dividing total organic matter concentrations by two [4]. Five replicate samples were tested for water-holding capacity, and on day 82 of the experiment, five replicate samples of mesocosm leachate were analyzed for pH.
Mesocosm description and experimental design
The main study used 30 mesocosms, each consisting of a galvanized steel container with a vinyl liner and central drain, containing about 100 L of compost. The mesocosms were maintained in a greenhouse under natural light, supplemented with 400-W sodium vapor lamps to assure a standard 15-h light period. Mesocosms were watered to drip point as needed (every 2–3 d) with dechlorinated water. The leachates were collected and returned to the mesocosms at the next watering to avoid potential losses of contaminants by leaching. Half of the mesocosm pots contained contaminated compost (i.e., compost prepared from contaminated sediments, CWR-8), and half contained the reference compost (UWR-5).
Each mesocosm was planted with three species of plants chosen to represent a range of taxa and carbon allocation patterns. The species used were lettuce (Lactuca sativa, cv. Buttercrunch), radish (Raphanus sativus, cv. Cherry Belle), and soybean (Glycine max cv. Essex). Lettuce is commonly used in short-term toxicity tests [3] and allocates most of its carbon to the leaf. Radish allocates more carbon to the root, in direct contact with the soil; soybean is a species for which seed production is an important carbon sink and biological endpoint. Soybean also has an extensive fine root system capable of exploiting the full soil volume of the mesocosm. The soybean seeds were inoculated with Bradyrhizobium japonicum bacteria (Urbana Laboratories, St. Joseph, MO, USA) prior to planting to allow testing of the sensitivity of symbiotic nitrogen-fixing associations in root nodules. We also added 25 earthworms (Eisenia foetida Savigny), obtained from cultures maintained in the laboratory, and 25 isopods (pillbugs, Armedillidium vulgare, Carolina Biological Supply, Burlington, NC, USA) to each mesocosm at the time of planting. Survival, growth, and reproduction were endpoints of interest for all species.
Three harvests of 10 mesocosms each (five replicates per treatment) were conducted 1 month (H-1), 2 months (H-2), and 6 months (H-3) after planting, except for lettuce and radish seedlings; for these two species H-3 was set at 3 months, when the plants had reached maturity. Because these two species were planted at the perimeter of the mesocosms, the rest of the system remained intact.
Plant methods
Mesocosm. Germination rates for soybean and radish in the mesocosms were obtained by planting four seeds at each of four locations in each mesocosm and counting the number that had germinated by day 7. The plants were then thinned to a single seedling at each location. Lettuce germination in the mesocosms was not quantified, but seedlings were thinned as for radish and soybean. Plant growth and carbon allocation in the mesocosms were assessed for each species by determining aboveground and below-ground dry mass of each plant part at each harvest. Photosynthesis of soybean leaves was measured between harvests, at 6 weeks and at 4 months, with a portable closed-loop photosynthesis system (model LI-6200, LI-COR, Lincoln, NE, USA). Two leaf measurements were taken from each mesocosm at 6 weeks, when 20 mesocosms remained, and four measurements each were taken at 4 months when only 10 mesocosms remained unharvested. Photosynthetic pigment concentrations (chlorophylls a and b, and total carotenoids) were measured in ethanol extracts [5] of the same leaves (6 weeks only).
Nitrogenase activity (nitrogen fixation potential) was assayed in detached soybean root systems at H-2 and H-3, using standard acetylene reduction methods [6]. At H-2, all four soy root systems from a mesocosm were assayed together in a single incubation flask, but at H-3, plants were much larger and only one root system per mesocosm was assayed. Dry mass of the nodules assayed was determined by separating nodules from roots after the assay.
Laboratory. Supplemental 5-d tests of seed germination and early growth of lettuce, and germination tests of three other species, were conducted to compare with results in the mesocosm study, and to provide more detailed information on sensitive life stages. Tests were conducted in three replicate petri dishes containing compost, sand, or a 50:50 (w/w) mixture of compost and sand, maintained in growth chambers, with 40 seeds per replicate. Test procedures were modified from the United States Environmental Protection Agency (U.S. EPA) protocols for short-term toxicity screening of hazardous waste sites [3], and included assessment of early root and shoot growth in lettuce seedlings growing directly in the compost and/or sand. Moisture content in all laboratory screening tests was adjusted according to U.S. EPA protocols [3]. The germination portion of the test was repeated with seeds of cabbage (Brassica oleracea, cv. Earliana), clover (Trifolium repens), and Arabidopsis thaliana, but only with petri dishes of pure compost and pure sand. Germination of these species was assessed at 7 d (cabbage and clover) or at 10 d (Arabidopsis).
Invertebrate methods
At the start of the test, 25 adult earthworms were added to each mesocosm. Initial fresh masses for each group of 25 worms were not different, as determined by a one-way analysis of variance. The earthworms were not given additional food during the test. Twenty-five adult isopods of similar size (not weighed) were also added to each mesocosm. A shallow clay saucer (10 cm diameter) was placed on the surface of the compost in each mesocosm to provide shelter for the isopods.
At each harvest, after plants had been removed, the soil in each mesocosm was hand sorted to recover earthworms and isopods. The earthworms in each mesocosm were counted, rinsed in distilled water, dried at 100°C for 48 h, and weighed as a group to obtain total dry mass per mesocosm. Isopods were counted but not weighed.
Additional 56-d tests of earthworm toxicity, growth, and reproduction were performed in growth chambers with these composts for comparison. Details of those supplemental tests are reported elsewhere [7].
Soil microarthropods (mites and insects) in each mesocosm were evaluated taxonomically for community- and trophiclevel changes as the study progressed. One-liter samples of each compost type were taken for this assessment at time zero, and one-liter samples were taken by coring into the soil of each mesocosm harvested. Soil microarthropods were extracted and counted; abundant taxa were classified by genus (and species where possible), and by trophic level, by the Ohio State University Acarology Laboratory (Columbus, OH, USA).
Extraction and analysis of lipids for microbial community characterization
Lipid analyses were performed on two 25-g samples taken from each compost at time zero, and on five additional samples per compost type at each of the three harvests (one per mesocosm harvested). The lipids were extracted from the compost samples immediately or after storage for a few days at −20°C. Lipids were extracted with chloroform-methanol-water in the proportions 1:2:0.8 (v/v), following the classic method of Bligh and Dyer [8]. An Iatroscan analyzer (Iatron Laboratories, Tokyo, Japan) equipped with a flame-ionization detector (thinlayer chromatography-flame ionization detection [TLC-FID]) was used for the analyses of the lipid classes, following the general procedure described by Ackman [9] and Napolitano and Ackman [10]. Lipid classes were separated by a procedure involving multiple developments with three solvent systems of increasing polarity, followed by partial scans. Several solvent systems were employed: first, hexane-ethyl ether-acetic acid (80:20:0.1, v/v); second, 100% acetone; third, chloroform-methanol-water (50:20:3, v/v). The third development was followed by a full scan of the rods. Lipid classes were also identified by monodimensional thin-layer chromatography; bands were sprayed with rhodamine and visualized under ultraviolet light.
Statistical analyses
In all cases mesocosm data were reduced to a single value per mesocosm (e.g., total earthworm mass in the mesocosm, or mean lettuce biomass in the mesocosm), and analyzed with the mesocosm as the experimental unit, with n = 5 at each harvest. Differences between biological responses to the two compost types were evaluated with t tests at each harvest, comparing whole-mesocosm results. Means and standard deviations are presented for the five samples (mesocosms) of each compost type at each harvest.
RESULTS
Compost chemistry
Both composts were high in organic matter, nitrogen, and other plant nutrients, compared with agricultural soils, and with respect to requirements for plant growth. We found no significant differences between the composts in extractable nutrient concentrations (Table 1). Total Kjeldahl N was higher in the contaminated compost than in the reference (0.48% vs 0.30%), but C to N ratios were similar and high (13 for the contaminated compost and 16 for the reference) (Table 1). Few differences in the concentrations of potentially toxic metals were found (Table 1), although extractable Al was higher in the reference compost (6.3 vs 3 mg/kg in the contaminated compost). Concentrations of total Cr, Cd, Ni, and Mo were higher in the composts than in average soils in the United States [11], but this was true for both composts. Values for Cr, although high, were particularly variable in compost samples, ranging from 46 to 224 mg/kg and 37 to 162 mg/kg in contaminated and reference composts, respectively; Ni concentrations were almost as variable. In addition, the composts had relatively high pH values, and high organic matter contents (Table 1), two characteristics that could potentially reduce availability and phytotoxicity of the metals [11, 12]. The pH values were similar in the two composts, and within the optimum range of pH 5 to 8 for E. foetida [3]. The contaminated compost had a higher water-holding capacity (Table 1) and appeared to have a lower sand content. The differences noted in compost chemistry and texture were generally small, and may have resulted from differences between the lagoon sediments and the uncontaminated soil used to prepare the reference compost, or possibly from differences introduced in the composting process. The leachates from the mesocosms were also analyzed for pH after 82 d (after equilibration and repeated recycling of leachate through the mesocosms). Mean leachate pH was 8.5 in both sets of mesocosms.
Seed germination and early growth
Soybean and radish seed germination in all 30 mesocosms was high and did not differ between composts. Mean germination rates were 94% for soybean in both composts, and 95 and 96% in radish for contaminated and uncontaminated composts. Lettuce germination was not assessed in the mesocosms, but in the short-term tests, lettuce germination was 24% lower in contaminated compost than in reference compost. Lettuce germination was reduced even in the uncontaminated reference compost (−25%) relative to germination in sand, and was intermediate in a 50:50 (v/v) mixture of compost and sand. Conversely, lettuce root length at 5 d was greater in all compost and compost-sand mixtures than in sand alone, but roots in the contaminated compost were 24 to 35% shorter than roots in the same concentration of reference compost. Lettuce shoot length was also greater in both composts than in sand, but was similar in the two composts (15 and 17 mm in contaminated and reference composts versus 9 mm in sand alone).
| Parameter | CWR-8 | UWR-5 | p Value |
|---|---|---|---|
| Total nitrogen (%) | 0.48 ± 0.03 | 0.30 ± 0.05 | <0.01 |
| Organic matter (%) (by combustion) | 12.4 ± 2.7 | 9.6 ± 0.1 | 0.22 |
| C to N Ratio | 13.0 ± 2.9 | 16.1 ± 2.3 | 0.22 |
| PH | 7.7 ± 0.1 | 7.5 ± 0.2 | 0.10 |
| N (mg/kg)a | 397 ± 207 | 274 ± 149 | 0.45 |
| P (mg/kg)a | 7.7 ± 1.5 | 7.7 ± 1.5 | 1 |
| K (mg/kg)a | 1,427 ± 125 | 1,094 ± 184 | 0.06 |
| Ca (mg/kg)a | 115 ± 30 | 204 ± 58 | 0.08 |
| Mg (mg/kg)a | 93 ± 24 | 92 ± 28 | 0.94 |
| Fe (mg/kg)a | 4.8 ± 1.6 | 9.1 ± 2.5 | 0.07 |
| Mn (mg/kg)a | 0.21 ± 0.08 | 0.54 ± 0.28 | 0.13 |
| Zn (mg/kg)a | 0.13 ± 0.02 | 0.44 ± 0.43 | 0.34 |
| Cu (mg/kg)a | 0.13 ± 0.02 | 0.25 ± 0.12 | 0.18 |
| B (mg/kg)a | 0.57 ± 0.14 | 0.54 ± 0.04 | 0.79 |
| S (mg/kg)a | 68 ± 29 | 69 ± 17 | 0.96 |
| Na (mg/kg)a | 235 ± 45 | 210 ± 44 | 0.53 |
| Al (mg/kg)a | 3 ± 1 | 6 ± 2 | 0.03 |
| Se (mg/kg) | 0.04 ± 0.03 | 0.02 ± 0.02 | 0.50 |
| As (mg/kg) | 1.4 ± 0 | 1.5 ± 0.1 | 0.37 |
| Ba (mg/kg) | 77.7 ± 2.0 | 87.5 ± 7.8 | 0.10 |
| Cd (mg/kg) | 5.84 ± 0.38 | 6.60 ± 0.38 | 0.07 |
| Cr (mg/kg) | 147.1 ± 91.2 | 119.7 ± 71.5 | 0.70 |
| Pb (mg/kg) | 21 ± 1 | 23 ± 2 | 0.28 |
| Hg (μg/kg) | 27 ± 21 | 17 ± 6 | 0.47 |
| Mo (mg/kg) | 6.41 ± 0.68 | 7.54 ± 0.97 | 0.17 |
| Ni (mg/kg) | 72.8 ± 40.8 | 89.2 ± 43.9 | 0.66 |
| Soluble salts (mmhos/cm)a | 6.61 ± 1.44 | 6.26 ± 1.67 | 0.79 |
| Cation exchange capacity (meq/100 g) | 13.04 ± 2.35 | 9.92 ± 3.14 | 0.23 |
| Water holding capacity (ml/g) | 1.19 ± 0.09 | 0.88 ± 0.07 | <0.01 |
| Leachate pH | 8.5 ± 0.1 | 8.6 ± 0.2 | 0.76 |
- a Saturated media extract.
Germination of the other three species ranged from no effect of compost type (cabbage) to 98% reduction (A. thaliana). Clover germination was 52% lower in the contaminated compost than in the uncontaminated compost. Germination of these three species was not reduced in the reference compost compared to their germination in sand.
Photosynthesis and leaf pigments in soybean
After 6 weeks, leaves of the soybean plants growing in mesocosms of contaminated-soil compost were visibly chlorotic, whereas leaves in the reference compost appeared normal. This was confirmed by measurement of photo synthetic pigments in the leaves: concentrations of chlorophylls a and b and accessory pigments were 58 to 65% lower in leaves of soybean plants growing in the contaminated compost (p < 0.01).
Reductions in photosynthesis accompanied the reduced chlorophyll concentrations at 6 weeks, when photosynthesis was 29% lower in soybeans growing in the contaminated compost (p = 0.03). After 4 months, however, the relative reduction in photosynthesis had decreased (−14%, not significant, p > 0.2).
Plant growth
Plant biomass in the contaminated-compost mesocosms was lower, in many cases, than in the reference mesocosms (Fig. 1), but the reductions were statistically significant only for soybean. At H-1, no significant differences occurred in biomass, but total soybean biomass was reduced by 15%, resulting from a 17% reduction in aboveground growth. By H-2, biomass of aboveground components (stem and leaf) in soybean was reduced by 46 to 56% (p < 0.01), resulting in a 43% reduction in total soybean biomass, although root biomass was not significantly reduced. By H-3, reduction in total soybean biomass in the contaminated compost was only 19%. The reductions in mean biomass of specific soybean components at H-3 ranged from 12 to 30% in the contaminated mesocosms, but were not statistically significant. Reproduction in soybean at H-3 was most strongly affected; the 30% reduction in seed biomass reflected a 38% reduction in mean mass per seed (p = 0.03). The number of seeds per plant was not affected.
Root nodulation and nitrogenase activity
Soybean root nodule formation, activity, and senescence appeared to follow a normal time course in both composts. Few nodules were present at H-1, and number and mass of nodules increased with time. Nitrogenase activity was high at H-2 (in the middle of the plant's life cycle), and decreased substantially at seed set in both groups of plants. However, major differences existed in number, size, and activity of the root nodules in the two composts. Although soybean root biomass was not significantly lower in the contaminated compost, the mass of symbiotic nitrogen-fixing nodules was strongly reduced (84% lower), both at H-2 and H-3 (p = 0.01 and 0.09 respectively, Table 2). At H-3, all four soybean plants lacked nodules in one of the five contaminated-compost mesocosms harvested. Nitrogenase activities (mass-specific rates of acetylene reduction) were also reduced in the contaminated compost, particularly at H-2 (50% lower, p = 0.09), when overall nodule activity was still high. Because nitrogen fixation is a product of nodule mass and specific activity, the effect at the whole-plant level could be even greater. This is shown by the calculated whole-plant acetylene reduction (Table 2), which was reduced in the contaminated compost by 92% and 85% at H-2 and H-3, respectively (p = 0.06 and 0.15).
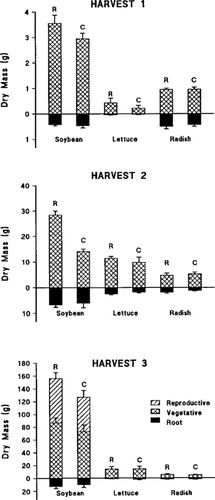
Dry mass of plants (g/plant) at each of three harvests. Error bars are standard error of the mean of the five mesocosms of each compost type at each harvest. R designates plants in the reference compost (UWR-5); C designates plants in the contaminated-soil compost (CWR-8). Mass of roots and aboveground vegetative and reproductive tissues are as indicated in the third panel.
Earthworms and isopods in the mesocosms
The earthworms and isopods appeared to grow and reproduce at a faster rate in the contaminated-soil compost than in the reference compost, at least initially. At H-1 the earthworms had not yet reproduced, and growth was assessed both as total dry mass per mesocosm at harvest (Table 3) and as the increase in mean fresh mass for the individuals recovered. Total dry mass at H-1 was approximately 1.5 times greater in the contaminated compost (Table 3, p < 0.01); the increase in earthworm mean individual fresh mass was 0.355 g in the contaminated compost and 0.046 g in the reference compost (p < 0.01). At H-2, both the number of earthworms and the total dry mass of earthworms per mesocosm were much higher in the contaminated compost (Table 3). By H-3, however, the number of earthworms per mesocosm was similar in both composts, and the dry mass per mesocosm was lower in the contaminated compost (p = 0.05).
Numbers of isopods recovered at H-2 and H-3 followed the same trends as numbers of earthworms (Table 3). Recovery (and presumably survival and reproduction) was approximately four times greater in the contaminated compost than in the reference compost at H-2 (p < 0.01), and did not differ between composts at H-3. Isopods were not counted at H-1, because few adults were recovered, although apparently they had reproduced, as evidenced by the numbers present at H-2 and H-3.
Soil microarthropods
Soil microarthropod community structure changed over time in both compost types. These changes included increases in number of individuals and species richness, and a changing taxonomic composition, but the changes were similar in the two compost types. Very few microarthropods were found in samples of either compost at time zero, but representatives from a total of eight mite genera and two insect orders were identified in samples taken from H-1 through H-3. Variation among replicates (one sample from each mesocosm) was high, and we found no significant differences between compost types at any of the harvests, either in the abundance of any particular taxon, or in total abundance (Table 4). At each harvest, both compost types contained representatives of three to eight taxa, although samples from individual mesocosms contained fewer (zero to five). At H-1 only fungivorous mites were found. Fungivorous, predatory, and plant-feeding mites as well as predatory and scavenging insect larvae were present at H-2 and H-3, increasing in abundance with time.
| Rates of acetylene reduction | ||||
|---|---|---|---|---|
| Harvest | Compost | Nodules per plant (g dry mass) | (μmol/h/g nodule dry mass) | Per plant (μmol/h/plant) |
| H-2 | CWR-8 | 0.07 ± 0.03 | 32 ± 21 | 2.21 ± 1.85 |
| H-2 | UWR-5 | 0.40 ± 0.18 | 64 ± 30 | 28.2 ± 21.9 |
| H-3 | CWR-8 | 0.24 ± 0.51 | 0.62 ± 0.61 | 0.22 ± 0.48 |
| H-3 | UWR-5 | 1.52 ± 1.44 | 0.83 ± 0.83 | 1.46 ± 1.53 |
- a Mean ± SD of five samples per compost type at both harvests (one sample measured per mesocosm harvested).
| Harvest | Compost | No. earthworms (per mesocosm) | Earthworm dry mass (g per mesocosm) | No. isopodsb |
|---|---|---|---|---|
| H-1 | CWR-8 | 20 ± 1 | 1.97 ± 0.36 | |
| H-1 | UWR-5 | 16 ± 2 | 0.80 ± 0.13 | |
| H-2 | CWR-8 | 481 ± 314 | 11.7 ± 9.6 | 87 ± 21 |
| H-2 | UWR-5 | 46 ± 27 | 1.3 ± 1.0 | 21 ± 15 |
| H-3 | CWR-8 | 2,615 ± 1,160 | 16.3 ± 7.0 | 115 ± 85 |
| H-3 | UWR-5 | 2,873 ± 1,276 | 30.7 ± 12.2 | 100 ± 94 |
- a Mean ± SD of five mesocosms per compost type at each harvest.
- b Isopods were not recovered quantitatively at H-1.
Soil lipid class analyses
The contaminated-soil compost contained about 35% more lipids than did the reference compost. The difference in the total lipid content between the two compost types was evident in samples taken at the start of the study, and persisted through H-3. Mean lipid content of the reference compost varied between 268 (SD =115) and 425 (SD = 312) μg/g soil, whereas that of the contaminated compost varied from 312 (SD = 54) to 853 (SD = 182) μg/g. The trends in lipids revealed by gravimetric determination were similar to those identified by TLC-FID. However, the gravimetric method of analysis yielded greater variation among replicates and tended to overestimate the amount of lipids present due to the inclusion of large amounts of nonlipid materials.
Iatroscan analysis permitted the identification of 11 lipid classes in the composts (Table 5). In order of increasing polarity, these lipids were: hydrocarbons, triacylglycerols, free fatty acids, fatty alcohols, sterols, acetone-mobile polar lipids (AMPL, which include monoacylglycerols, glycolipids, and chloropigments), and four major phospholipids (diphosphatidylglycerols [DPG] phosphatidylglycerols [PG], phosphatidylethanolamines, and phosphatidylcholines). In all samples of both compost types, AMPL was the major lipid class. Hydrocarbons, free fatty acids, and triacylglycerols followed as the quantitatively important components. Among phospholipids, DPG and phosphatidylethanolamines were the dominant classes. The proportion of hydrocarbons in the two composts was relatively high, ranging between 8 and 23% of the total lipids in the two composts. Although these hydrocarbons were not characterized further, they may represent contamination by petroleum compounds.
| Harvest | Compost | Total abundancea (number/L) | Species richness (minimum, maximum)b |
|---|---|---|---|
| H-1 | CWR-8 | 5.4 ± 6.4 | 3 (0, 3) |
| H-1 | UWR-5 | 27 ± 47 | 5 (0, 4) |
| H-2 | CWR-8 | 78 ± 112 | 4(1,3) |
| H-2 | UWR-5 | 136 ± 263 | 5 (0, 3) |
| H-3 | CWR-8 | 76 ± 45 | 8 (3, 5) |
| H-3 | UWR-5 | 129 ± 144 | 5 (3, 4) |
- a Mean ± SD.
- b Total number of species per compost type, across all five replicates. Minimum and maximum number of species present in any one replicate are given in parentheses.
Despite the differences in the total lipid content between the contaminated and reference composts, the two composts had very similar lipid-class profiles. Few statistically significant differences (p < 0.05) existed in concentrations of the lipid classes (in relation to compost type) at the start of the study, or at the three harvests. Exceptions included higher concentrations of hydrocarbons and DPG in the contaminated compost samples at some harvests, and lower concentrations of free fatty acids (Table 5).
DISCUSSION
Potentially important environmental effects of soil contaminants can range from short-term individual responses, as measured in traditional toxicity assessments, to responses that may occur only after a prolonged exposure, or after exposure to multiple generations. This idea is summarized in Figure 2, which shows a continuum of possible responses at different time scales and across levels of ecological organization. This conceptual framework also forms a basis for interpreting results from the current study, which included a spectrum of endpoints for multiple species representing a range of terrestrial organisms potentially affected by soil contaminants. The combination of the mesocosm format with short-term toxicity tests provided information on responses of selected species throughout their life cycles, on symbiotic processes, and on effects at the population and community level. Trends emerged within general taxonomic classes, as described below; these trends help link toxicologic activity at lower levels with ecologically relevant effects.
Multiple lines of evidence from this experiment suggest a more negative effect of the contaminated-soil compost on plants than on invertebrates or soil microbes. For example, in the contaminated-soil compost, three of the plant species had reduced germination, and lettuce root growth was inhibited in short-term tests. Early root growth is often more sensitive than germination or early shoot growth [12, 13], but an integrated approach using all sensitive stages (from germination to reproduction, and plant-microbe interactions) can be more reliable for predicting long-term ecological consequences of soil contamination, or assessing the effectiveness of remediation and the capacity of soils to support a plant community [12, 14]. The inclusion of multiple species in the main mesocosm study was important, because although two of the species showed few adverse effects, soybean appeared sensitive to the contaminated-soil compost, based on both aboveground (reductions in growth, photosynthesis, and reproduction) and below-ground (reduced symbiotic nodule formation and nitrogenase activity) observations.
| Time 0 | Harvest 1 | Harvest 2 | Harvest 3 | |||||
|---|---|---|---|---|---|---|---|---|
| Compost type | Compost type | Compost type | Compost type | |||||
| Lipid classesa | UWR-5 | CWR-8 | UWR-5 | CWR-8 | UWR-5 | CWR-8 | UWR-5 | CWR-8 |
| Neutral lipids | ||||||||
| HC | 12.7 ± 1.2 | 15.2 ± 3.7*a | 14.0 ± 3.9 | 21.5 ± 5.6 | 10.4 ± 2.7 | 22.5 ± 5.0* | 8.0 ± 4.8 | 19.4 ± 2.0* |
| TAG | 3.6 ± 0.0 | 7.5 ± 2.4 | 7.0 ± 1.7 | 9.9 ± 5.9 | 7.1 ± 0.7 | 3.5 ± 1.9* | 2.5 ± 1.4 | 1.6 ± 1.2 |
| FFA | 12.5 ± 1.0 | 6.7 ± 2.8* | 6.0 ± 3.3 | 5.9 ± 3.3 | 3.8 ± 2.1 | 0.9 ± 0.4* | 7.2 ± 2.4 | 4.2 ± 2.1 |
| AL | 4.3 ± 0.4 | 2.7 ± 0.2* | 1.0 ± 1.0 | 2.3 ± 2.1 | 1.5 ± 1.2 | 0.8 ± 0.9 | 0.7 ± 0.4 | 0.7 ± 0.3 |
| ST | 4.7 ± 0.2 | 5.0 ± 0.7 | 6.4 ± 2.0 | 3.7 ± 0.8 | 3.6 ± 0.3 | 2.6 ± 0.6 | 2.3 ± 1.3 | 1.6 ± 0.8 |
| AMPL | 47.9 ± 0.5 | 39.8 ± 1.5* | 39.7 ± 7.1 | 33.6 ± 3.5 | 44.5 ± 4.1 | 38.8 ± 5.2 | 57.5 ± 4.9 | 52.7 ± 4.7 |
| Phospholipids | ||||||||
| DPG + PIG | 6.6 ± 1.6 | 6.3 ± 0.1 | 10.1 ± 2.2 | 12.8 ± 2.6 | 8.9 ± 1.6 | 13.8 ± 2.2* | 14.5 ± 1.3 | 17.5 ± 2.1 |
| PG | 2.1 ± 0.9 | 3.3 ± 0.1 | 1.5 ± 1.6 | 1.3 ± 1.2 | 3.9 ± 1.3 | 2.1 ± 2.1 | 1.6 ± 1.3 | -c |
| PE | 2.2 ± 0.5 | 4.3 ± 1.6 | 5.1 ± 1.4 | 5.6 ± 4.0 | 6.0 ± 0.8 | 8.4 ± 2.4 | 3.2 ± 2.2 | 0.9 ± 0.6 |
| PI + PS | 0.7 ± 0.0 | 0.9 ± 0.2 | 0.6 ± 0.4 | 0.6 ± 0.5 | 0.3 ± 0.3 | 0.4 ± 0.8 | 0.8 ± 0.7 | 0.2 ± 0.3 |
| PC | 1.8 ± 0.01 | 3.5 ± 0.1* | 3.3 ± 1.1 | 2.0 ± 1.3 | 4.1 ± 0.3 | 2.8 ± 1.1 | 1.8 ± 0.7 | 1.2 ± 0.3 |
- a HC = hydrocarbons, TAG = triacylglycerols, FFA = free fatty acids, AL = fatty alcohols, ST = sterols, AMPL = acetone-mobile polar lipids, DPG = diphosphatidylglycerols, PIG = pigments, PG = phosphatidylglycerols, PE = phosphatidylethanolamines, PI = phosphatidylinositols, PS = phosphatidylserines, PC = phosphatidylcholines.
- b * = comparisons that differed significantly (p ≤ 0.05, based on a t test).
- c Not separated from DPG + PIG.
Because the plant species we used had different sensitivities to the composts, a single-species test, even if it involved a sensitive life stage, would not provide conclusive evidence for or against phytotoxicity. Taken together, however, the multiple adverse effects in some of the plant species (e.g., early lettuce root growth; germination in three species; growth, reproduction, and symbiotic N2-fixation in soybean) form a chain of evidence, as in Figure 2. This chain demonstrates at least the potential for sublethal effects on plant growth, development, and symbioses, and may be predictive of effects at the higher levels of organization that are not practical to test in plant species.
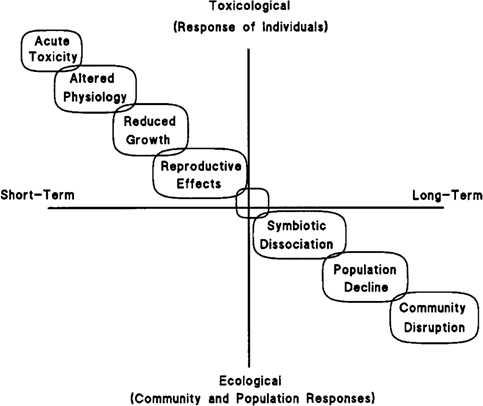
A hypothetical chain of evidence linking potential responses across time scales and levels of organization. Such relationships can be used to establish a continuum associating early effects with longer-term responses, within and across taxonomic groups, to predict potentially important environmental effects. Modified from Gunderson et al. [19].
In contrast, the contaminated compost did not have clear or consistent adverse effects on soil arthropods or soil microbial communities. The rapid early growth and reproduction of earthworms and isopods in the mesocosms of contaminatedsoil compost (H-1 and H-2) paralleled an increase in earthworm reproduction seen in short-term tests with that compost [7]. In the short-term tests, no adult earthworms had died by day 21, and although adults weighed less in the contaminatedsoil compost than in the reference, they had produced more cocoons and more juveniles per cocoon. This outcome resulted in much greater juvenile biomass at 56 d, and 69% greater total biomass, in the contaminated-soil compost [7]. After 6 months in the present mesocosm study, however, the reproductive effects had apparently reversed: total earthworm biomass was then lower in the contaminated-compost mesocosms than in the reference-compost mesocosms, and the numbers of earthworms and isopods per mesocosm were similar in the two composts. Possible explanations for this apparent reversal include delayed effects of residual contaminants, differences in population structure, or indirect effects (e.g., gradual development of nutritional differences between the composts). Within the framework of Figure 2, however, none of the links seen in plants exist to tie short-term or lower level effects to the population effects in soil arthropods or to suggest mechanisms for toxicologic responses.
Microarthropod enumeration and taxonomy did not reveal major differences between microfaunal communities in the two composts either, even though community- and trophic-level analysis of soil microarthropods can be a sensitive measure of the effects of chemical pollutants in laboratory microcosms [2]. The apparent low toxicity of residual explosives to soil microfauna in the present study is in keeping with the results of Parmalee et al., who found no significant negative effects of added TNT (up to 200 μg/g) on numbers of soil nematodes or microarthropods [2]. They also found that very little TNT could be extracted with acetonitrile from their microcosm soils, and attributed the low toxicity of added TNT to low biological availability [2]. Experiments using 14C-labeled TNT have also suggested that TNT may accumulate in composts in a chemically bound fraction that resists organic and aqueous extraction [1]; the biological availability of the labeled compounds was not determined.
Lipid class analysis, like the microarthropod enumeration, suggested more similarities than differences between soil communities in the two compost types. Various studies have shown that analysis of cellular membrane lipids extracted and concentrated from the environment can provide useful information regarding physiologic and environmental processes in the microbial community [15]. The TLC-FID procedures for lipidclass analysis have been extensively used in aquatic ecosystems [16]. Nevertheless, this may be the first attempt to use the TLC-FID method as an assessment of soil microbial communities. Phosphatidylcholine and phosphatidylethanolamine are the major phospholipids in all higher plants and Metazoa, whereas DPG and PG are dominant in gram-negative soil bacteria. Other lipids, such as free fatty acids and alcohols (i.e., phytol) and chloropigments (AMPL), reflect the magnitude of biological degradation, and the presence of fresh plant detritus and soil microalgae, respectively. Contaminated-soil compost and reference compost did not differ substantially in the nature of their basic lipid compositions, but the relatively large amount of lipids in the contaminated-soil compost indicated a larger microbial biomass in that compost type. The difference in the proportions of DPG among compost types noted in H-2 samples was interpreted as an increase in the numbers of gramnegative bacteria. A more detailed analysis of the phospholipid fatty acid composition of the soils by gas chromatography (data not shown) confirmed these results.
The relatively small differences observed between compost types in either microbial or microarthropod communities are in keeping with the minimal effects on earthworm or isopod populations. This again suggests a trend of few toxic effects across the continuum of responses for these taxonomic groups, in contrast with the effects observed for plants.
A number of potentially confounding factors must be considered in assessing the possibility of sublethal effects of soil contaminants. Plants and animals can be affected by many variables unrelated to the contaminants of concern, including differences in soil pH, nutrient availability for plants and animals, or the presence of unsuspected cocontaminants. These factors make selection of an appropriate reference soil essential but difficult. In this study, use of a composted soil was required to provide a comparable reference, and materials used to prepare the two composts were closely matched, within the limits of the technology. Nevertheless, any inherent differences between lagoon sediments and nearby reference soils could be biologically important. Despite these factors, the two composts appeared to be similar, although not identical, in terms of plant and animal nutrient supply and other soil parameters (Table 1). Thus, other than the presence of explosives residuals, it is not clear that soil differences would have caused the observed effects.
Another common problem in evaluating soils from hazardous waste sites is that of pseudoreplication: often only one sample of the contaminated soil is available (e.g., one lagoon, or one sample from a particular point on a transect across a disposal site). In this study, the composts available for evaluation came from only one windrow of each type (contaminated and reference). Thus, data from our replicate mesocosms were useful for distinguishing between the two composts, but may not be adequate demonstration that composting of TNT-contaminated soils will consistently render them suitable for land application. Additional confidence could be gained by including samples from other composted windrows prepared similarly, preferably from sites with a range of soil types and contamination levels.
Despite the potential complications from compost differences and a lag in invertebrate reproduction in the reference compost, the results from this study suggest, through weight of evidence, that the contaminated-soil compost had some effects on plants. Because toxicity was low, however, the level of remediation offered by composting could be deemed environmentally acceptable, and land application of such a compost might be appropriate, depending on the sensitivity of plant and animal species at the site of application. Composting clearly reduced the risks to terrestrial species associated with the pretreatment soils at the lagoon site, where soil berms were completely devoid of vegetation [17], presumably because of the toxicity of the explosives, whose initial concentrations far exceeded those shown elsewhere [17, 18] to be acutely toxic to plants.
This example of using a mesocosm approach to environmental evaluation of soils revealed certain challenges in the selection of reference media, and demonstrated how application of mesocosm methodology may allow a pattern of responses to be traced within and across taxonomic groups. A chain of evidence developed by such an approach may help predict more subtle long-term ecological effects than are apparent in traditional toxicity tests, by explicitly including multiple species in a single test, as well as multiple levels of ecological organization. This approach may also prove useful in selecting environmentally acceptable endpoints for remedial activities.
Acknowledgements
We thank Emily Childs for technical assistance throughout the project, and Clay Runck, Sheri Wampler, and students from the U.S. Department of Energy High School Honors Program for assistance during harvests. Thanks also to Fred Allen for sharing his expertise and soybean seeds. Lynn Kszos and Glenn Suter provided helpful reviews of earlier drafts of the manuscript. G.E. Napolitano was a participant in the Oak Ridge National Laboratory Research Associates Program, administered jointly by Oak Ridge National Laboratory and Oak Ridge Institute for Science and Education. The participation of J.M. Kostuk and M.H. Gibbs was made possible through the U.S. Department of Energy Science and Engineering Research Semester Program. This research was sponsored by the U.S. Army Environmental Center, Interagency Agreement 2134-H018-A1. Oak Ridge National Laboratory is managed by Lockheed Martin Energy Research Corp. for the U.S. Department of Energy under contract number DE-AC05–96OR22464.




