Accelerated increase in mercury contamination in north Atlantic mesopelagic food chains as indicated by time series of seabird feathers
Abstract
Concentrations of mercury in the environment have increased manyfold since preindustrial times as a result of anthropogenic emissions of gaseous mercury to the atmosphere. However, most records of historical change are affected by regional inputs and evidence of global impact of human activities at pristine oceanic sites is scanty. Seabird feathers contain a valuable record showing historical trends in methylmercury contamination. Some seabirds are top predators in epipelagic and others in mesopelagic food chains and thus reflect methylmercury contamination of these ecosystems. Here, we report the first measurements of mercury concentrations in a time series of animals from the subtropical northeast Atlantic over the last 100 years. These data show increases in mercury levels by 1.1 to 1.9%/year in epipelagic foodchains and by 3.5 to 4.8%/year in mesopelagic food chains. While the rate of increase in the epipelagic ecosystem is in close agreement with model predictions, the higher rate in the mesopelagic ecosystem has not previously been detected. However, the latter concurs with methylmercury production below the thermocline and conveys new insights into the understanding of the anthropogenic impact in the marine cycle of mercury.
INTRODUCTION
The global cycle of mercury is dominated by anthropogenic and natural emissions of gaseous mercury (Hg°) to the atmosphere that are subjected to long-range atmospheric transport [1, 2]. Anthropogenic emissions started in the late 15th century with precious metal mining [3], and current human activities, notably fossil fuel combustion and waste incineration, are responsible for 50 to 75% of the total current atmospheric emissions [4]. Air-sea exchange processes, such as atmospheric deposition of divalent mercury—Hg2+—and oceanic production with subsequent evasion of Hg°, are a crucial part of the global biogeochemical cycle of mercury [1, 5]. Recycling of mercury between the surface oceans and the atmosphere prolongs the global anthropogenic influence and a major proportion of contemporary oceanic effluxes are a re-emission of deposited anthropogenic mercury [2, 6], yet there is considerable uncertainty about the relative importance of mixing into the ocean interior as a sink for anthropogenically derived mercury [6].
Historical records of mercury deposition in aquatic ecosystems, as shown by lake sediment and peat bog cores or top predator tissues, indicate two- to fivefold increases in contamination levels since preindustrial times in midlatitudes of the Northern Hemisphere [7-13]. Slight increases in the sub-Antarctic were also indicated by seabird feathers [14]. Ice cores from remote regions have not provided reliable data because of sampling and analytical limitations [15, 16]. Nonetheless, existing records of historical change may be affected by regional inputs and evidence of global impact of anthropogenic emissions is still scanty [17, 18]. The need to assess trends in deposition at pristine oceanic sites has been identified as a high priority [1, 17]. Because assimilation of methylmercury by animals is extremely efficient while assimilation of inorganic mercury is very poor, it is also crucial to discriminate between marine vertical compartments, given the potential transport of anthropogenic-derived mercury into deep waters [6] where methylation and bioaccumulation rates are highest [19-21] and photodegradation will not occur [22].
We measured mercury concentrations in feather time series (1886–1994) from seabirds breeding in Azores, Madeira and Salvages (30–40°N, 8–32°W). This subtropical sector of the northeast Atlantic is remote from continental anthropogenic emissions of mercury and, thus, suitable to investigate globalscale historical trends of mercury contamination in marine food chains due to deposition of anthropogenic mercury at long distance from emission sources.
MATERIALS AND METHODS
Monitor species and monitoring units
The monitor species used here (Table 1) were selected on the basis of a priori information on the ecology of the local seabird assemblage. Although they all feed predominantly on fish, ecological segregation leads to dietary specialization on epipelagic or mesopelagic resources [23, 24] ensuring vertical integration of mercury contamination. Species and sites sampled were: epipelagic monitors—Cory's shearwater Calonectris diomedea borealis (Azores, Madeira, Salvages), little shearwater Puffinus assimilis baroli (Azores, Salvages), common tern Sterna hirundo (Azores); mesopelagic monitors—Bulwer's petrel Bulweria bulwerii (Madeira, Salvages), Madeiran storm petrel Oceanodroma castro (Madeira, Salvages). The main taxa in the diet of each seabird species are [24]: for Cory's shearwater, trumpetfish Macroramphosus scolopax, Atlantic saury Scomberesox saurus, boarfish Capros aper; for common tern, trumpetfish; for Bulwer's petrel and Madeiran storm petrel, lanternfish, especially Myctophum punctatum and Electrona rissoi.
| Species | Feeding nichea | Pre-1931b | Post-1990 | Increasec(%) | Increased (%/year) |
|---|---|---|---|---|---|
| Cory's shearwater | Epipelagic | 1921 | 1993 | ||
| Calonectris diomedea | 2.7 ± 0.1 (0.6–4.6), 2.7 | 5.4 ± 0.1 (1.9–10.6), 5.3 | 100 | 1.4 | |
| (48) | (219) | ||||
| Little shearwater | Epipelagic | 1895 | 1993 | ||
| Puffiniis assimdis | 1.7 ± 0.2 (0.8–3.2), 1.6 | 2.8 ± 0.2 (1.8–6.9), 2.4 | 65 | 0.7 | |
| (15) | (34) | ||||
| Common tern | Epipelagic | 1927 | 1992 | ||
| Sterna hirundo | 1.1 ± 0.2 (0.5–2.1), 1.1 | 2.0 ± 0.1 (1.2–3.5), 2.1 | 82 | 1.3 | |
| (15) | (22) | ||||
| Bulwer's petrel | Mesopelagic | 1903 | 1993 | ||
| Bulweria bulwerii | 6.0 ± 0.7 (0.9–15.1), 5.1 | 21.6 ± 0.7 (12.2–33.8), 21.9 | 260 | 2.9 | |
| (30) | (55) | ||||
| Madeiran storm petrel | Mesopelagic | 1896 | 1993 | ||
| Oceanodroma castro | 3.0 ± 0.1 (1.3–6.8), 2.8 | 14.9 ± 0.5 (9.3–24.9), 14.3 | 397 | 4.1 | |
| (16) | (47) |
- a Categorization based on predominant (>2/3) origin of prey species and their respective frequency of occurrence [26].
- b Values for each time period are: above, median year; middle, mercury concentrations x ± SE (range), median; below, sample size.
- c Absolute percent increase based on mean mercury concentrations.
- d Average annual rate of increase equal to absolute percent change divided by range of median years in the two periods.
Adult ventral feathers were used as monitoring units. Contemporary samples (post-1990) were obtained from live birds at breeding colonies. Historical samples (pre-1970) were obtained from dated preserved study skins of birds collected at the same colonies and held in museum collections as indicated in the Acknowledgement. Contour feathers are the most representative for estimating whole-bird mercury content [25], and up to 12 ventral feathers were collected from each individual by plucking from live birds or cutting at the basis with scissors from study skins and placed in polyethylene bags prior to analysis (numbers of birds sampled by species and time period are given in Table 1). An assessment of the effect of two washing regimes on feather mercury concentrations showed identical concentrations in unwashed and washed samples [26] and only four historical feather samples with major dust-surface contamination, likely to alter feather weight, were subjected to a chloroform/acetone washing regime [27].
Determinations of total and organic mercury
Contemporary feather samples were analyzed for total mercury. Subsamples of 0.02 to 0.1 g were subjected to a wet mineralization digestion in sulfuric acid and potassium permanganate as described in detail elsewhere [28], prior to total mercury determination by cold vapor atomic absorption spectrophotometry (CVAAS) with a Bacharach mercury analyzer system Coleman 50B. The limit of detection of the method, taken as twice the standard deviation of triplicate analysis at blank concentrations [29], was 10 ng, equivalent to 0.01 μg/g for a 1-g sample. Within- and between-laboratory quality control procedures were employed throughout the study period. Accuracy (expressed as relative error) was within 10% and monitored with standards of inorganic mercury and reference materials (NRCC dogfish muscle DORM 1: measured = 0.829 ± 0.013 SE [n = 9]; certified = 0.798 [±95% confidence limits = 0.074]) and by participation in the intercomparison program for mercury in human hair undertaken by Health and Welfare Canada. Precision (or reproducibility; expressed as coefficient of variation) within and between batch was within the usual 10% for total mercury determinations in biological samples [29], and it was independent of the mercury concentration in the feather sample over a wide range of levels (0.1–30 μg/g; product-moment correlation, r = 0.14, p = 0.14, n = 110). Interference on sensitivity due to matrix and pretreatment were assessed by the method of standard additions before the wet mineralization digestion, and the mean recovery of added inorganic mercury was 99.8% (SE = 5.6, n = 8).
Historical samples were analyzed for organic mercury to overcome potential postmortem contamination of study skins with inorganic mercury used as preservative [27, 30]. Mercury in seabird feathers is entirely organic [27] and this allows a direct comparison with total mercury concentrations determined in uncontaminated (with preservative) contemporary feather samples. That was confirmed for the monitor species by replicate determinations of organic and total mercury concentrations (range: 0.9–22.8 μg/g) in contemporary samples, which yielded a mean organic fraction of 101.0% (SE = 4.1, n = 10). Subsamples of 0.04 to 2 g were subjected to an extraction of methylmercury into toluene, as methylmercury bromide, followed by stripping of methylmercury from toluene into thiosulfate as described in detail elsewhere [26, 27]. Methylmercury in the extracted samples was subjected to the wet mineralization digestion used for contemporary samples (except for a reduction to 1 h digestion in sulfuric acid) prior to determination as total mercury by CVAAS. The efficiency of extraction was 0.910, and concentrations were corrected accordingly. Accuracy and precision of organic mercury determinations were within 10% and monitored throughout the study with standard reference materials and standards of methylmercury chloride. The mean organic mercury in NRCC dogfish muscle DORM 1 was 0.770 ± 0.040 μg/g (SE, n = 3) compared with the certified value of 0.731 (±95% confidence interval = 0.060); the mean organic mercury in IAEA tuna fish RM 350 was 3.90 ±0.14 μg/g (SE, n = 3, CV = 6.2%) compared with a value of 3.96 ± 0.12 μg/g (SE, n = 5) given by Horvat [31]. The mean coefficient of variation for replicate standard solution samples with 0.5 to 1 μg of mercury as methylmercury chloride was 4.1 ± 1.0% (SE, n = 9).
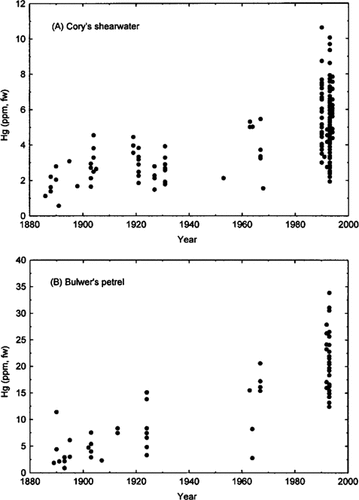
Historical variation of feather mercury concentrations in an epipelagic monitor (A) and a mesopelagic monitor (B).
Because consistent weights of dried oven feather samples were difficult to obtain due to rapid reabsorption of moisture, feather weights were taken at ambient laboratory temperature, and all feather mercury concentrations are expressed in micrograms per gram on a fresh weight basis (μg/g fw).
Data analysis
Data were tested for goodness of fit to a normal distribution using the Kolmogorov-Smirnov one-sample test and the requirements of homogeneity of variances using the Levene test, prior to statistical analysis. Parametric and nonparametric statistics were employed as appropriate, and analysis followed standard statistical procedures [32].
RESULTS
The numbers of preserved historical specimens available preclude an assessment of historical changes by location. However, mercury concentrations in contemporary adult feather samples of each species did not differ significantly among locations [26], and combined mercury concentration data were used in subsequent analysis for each species.
The comparison of mercury concentrations in birds sampled pre-1931 and post-1990 shows significant increases in all monitor species (Table 1; Mann-Whitney tests, p < 0.0005), which rise well above the baseline noise quantified in an a priori study of the intrapopulation contemporary variability of mercury in each species [26]. Assuming linearity, overall estimates of the increase rate differ significantly between epipelagic monitors (1.1%/year, SE = 0.2) and mesopelagic monitors (3.5%/year, SE = 0.6; t3 = 4.48, p < 0.05).
Fine-time resolution of historical trends was possible for two species (Fig. 1 and Table 2) and shows highly significant increases (one-way ANOVAs on logarithmic transformed data: Cory's shearwater, F3,272 = 78.84, p < 0.0001; Bulwer's petrel, F3,91 = 69.47, p < 0.0001). Increases occurred as early as 1900–1931 relative to 1885–1900 and at a higher rate than in subsequent time periods. Whereas the value of this indication of short-term patterns is weakened by some small sample sizes, it is strengthened by the consistency of indications from epipelagic (Cory's shearwater) and mesopelagic (Bulwer's petrel) monitors. This analysis produces higher long-term increase rates (epipelagic: 1.9%/year; mesopelagic: 4.8%/year) because contemporary levels were compared with historically older background values.
DISCUSSION
Validity and assumptions
The rationale for using seabird feathers as a monitor for mercury is well established [33, 34]. Briefly, seabirds excrete dietary methylmercury in a linear dose-dependent fashion into the plumage by endogenous incorporation during feather growth [26, 35], and mercury is stably bound to the feather keratin [36, 37] and thus can be measured in feathers from museum study skins [8, 30, 37].
Though the trends observed here are clear and well supported, their representativeness relies on the validity of the assumption that the diet of monitor species has remained constant over the study period. This is reasonable because the species selected have narrow and relatively inflexible diets [26] and ecological processes underlying segregation and partitioning of epipelagic and mesopelagic resources should remain unchanged [23, 26]. Furthermore, increase rates are consistent between species feeding on different prey within the same vertical compartment (Table 1). Field and experimental evidence indicate that mercury intake during the breeding period is largely responsible for concentrations in contour feathers of these monitor species [26], and at the very least, trends may be taken as reflecting contamination over the wide north-south Atlantic midlatitude sector where the migratory monitors spend the annual cycle [24].
| Species | 1885–1900a | 1900–1931 | 1950–1970 | 1992–1994 | Long-term increase (%/year) |
|---|---|---|---|---|---|
| Cory's shearwater | 1890 | 1921 | 1967 | 1993 | |
| 1.8 ± 0.2 (10) | 2.9 ± 0.1 (38) | 3.9 ± 0.5 (9) | 5.4 ± 0.1 (219) | 1.9 | |
| — | 2.0 | 0.7 | 1.5 | ||
| Bulwer's petrel | 1892 | 1919 | 1967 | 1993 | |
| 3.7 ± 1.0 (10) | 7.1 ± 1.0 (20) | 14.3 ± 1.6 (10) | 21.6 ± 0.7 (55) | 4.8 | |
| — | 3.4 | 2.1 | 2.0 |
- a Values for each time period are: above, median year; middle, mercury concentration x̄ ± SE (n); below, short-term annual rate of increase relative to previous period, estimated as in Table 1.
Trends
The long-term increase of mercury levels near the apex of the epipelagic food web observed here (1.1–1.9%/year) is consistent with a predicted threefold increase of global mercury concentrations in atmosphere and surface oceans since pre-industrial times (i.e., 1.3%/year over 150 years) due to anthropogenic inputs [2] and increase rates of 1.2 to 1.5%/year between 1977 and 1990 in the global background of atmospheric mercury measured over the Atlantic Ocean [18]. The equality of increases in anthropogenic atmospheric inputs of mercury and levels in epipelagic organisms is presumably due to the rapid equilibrium of mercury between the atmosphere and the surface ocean [5, 38]. The trends observed here for the epipelagic oceanic environment are consistent with peat bogs [7, 9], lake sediments [1-12], and seabird feathers [8], all recording two- to fivefold increases since the beginning of the 19th century in midlatitudes of the Northern Hemisphere. Slight increases in the subAntarctic region between pre-1950 and post-1950 were also indicated by seabird feathers [14].
The long-term increase of mercury contamination near the apex of the mesopelagic food web observed here (3.5–4.8%/year) represents a threefold magnification of the anthropogenic-derived pulse of mercury comparative to analogous rates for the epipelagic food web, atmosphere, and surface ocean. Explanations for this difference must be sought in the particular biogeochemistry of mercury in low-oxygen subthermocline seawater, which results in net production of highly bioaccumulative methylmercury through microbial-mediated methylation of reactive mercury supplied by scavenging of particulate mercury from the mixed layer [21]. Assuming linearity in the food chain transfer of methylmercury, the mesopelagic trend indicates that methylmercury production and bioaccumulation grew faster at depth than at the surface, where photodegradation [22] and loss due to gas exchange result in no detectable accumulation of methylmercury [21]. Concurrently, given that methylation is substrate limited [21], the faster historical increase of mercury in depth may reflect long-term increase in fluxes of particulate mercury from the mixed-layer to subthermocline waters. Indeed, upwelling intensification due to climate change enhanced primary productivity [39] and may have increased both oceanic evasion of elemental mercury and scavenging of particulate matter to subthermocline waters for subsequent methylation. There is clearly considerable scope for more research in this topic regarding conflicting views on the role of mixing into the ocean interior as a sink of anthropogenic-derived mercury (see Mason et al. [2] versus Hundson et al. [6].
Historical increases do not appear to have been linear in the epipelagic and mesopelagic food chains due to enhanced increases at the turn of the century (cf. Table 2). This pattern is remarkably consistent with variations in rates of atmospheric mercury deposition in remote midcontinental North America in the same period [7]. Such coincidence may be accounted for by the peak use and loss of mercury to the atmosphere from gold and silver mines in 1850 to 1900 [3] and some slowness of mixing causing a slight time lag. Apparent declines in atmospheric mercury deposition post-1960 recorded in midcontinental North American peat bog presumably reflect regional-scale changes in emissions [7] and were not recorded here in seabird feathers.
Implications
The trends presented above must be perceived under the extreme role of atmospheric processes on mercury environmental cycling. The flux of mercury from the atmosphere at any location on the Earth's surface is a sum of contributions from the global cycle, and regional and local sources [1]. If the only significant mode of deposition of atmospheric mercury is associated with the global cycle, then mercury distribution should be relatively uniform on a regional basis [13]. This is true for the contemporary levels of mercury contamination in the epipelagic and mesopelagic environments of the study area [26]. Because the main regional-scale mercury-related process (fluxes from volcanism and occurrence of Mediterranean water below 1,000 m) were steady over the study period, the historical increases in mercury concentrations in seabirds feeding near the apex of food chains reported here should reflect the impact of the perturbed global cycle of mercury. This provides an empirical linkage between increasing accumulation of methylmercury in aquatic organisms and the anthropogenic influence in the global mercury cycle. Large increases, especially in mesopelagic organisms, are of concern because of the current public-health problem resulting from widespread incidence of elevated levels of methylmercury in fish [40] and the increasing importance of deep-sea resources as a source of protein for humans. Besides, potential interactions of environmental change, as global warming and increases in oxidants and particles in the atmosphere, on the global mercury cycle should not be neglected [6]. The uncertainties concerning the outcome of anthropogenic-derived global processes in current levels of mercury contamination beg for an appraisal of long-term trends at remote locations. In this respect, and given that mercury cycling in marine ecosystems includes many biologically mediated processes [19-21], seabird study skins present good prospects for a comprehensive appraisal of the ecological hazards of global pollution by mercury into epipelagic and mesopelagic food webs.
Acknowledgements
This research was supported by Junta Nacional de Investigação Científica through grant BD/2289/92-IG to L.R. Monteiro and research contract STRDB/C/MAR/228/92. We thank Mário Laranjo, Encarnacion Sola, José Pedro Granadeiro, Paulo Oliveira, Luís Pires, Ramon Ferris, Dr. Keith Hamer, Valentina Costa, and Cláudia Pereira for assistance with field work, collection of feather samples, and/or mercury determinations. For permission to collect feather samples of study skins in their collections, we thank the British Museum of Natural History, American Museum of Natural History, Muséum National d'Histoire Naturelle, Museu Municipal do Funchal, Royal Scottish Museum, Museu Carlos Machado, and Museu de História Natural.




