Influence of sludge amendment on transport and sorption ideality of s-triazines in soil columns
Abstract
The aim of this work was to examine the effect of organic soil amendments (municipal sewage sludge and composted municipal sewage sludge) on transport ideality, sorption equilibrium, and s-triazine (atrazine, simazine, ametryn, and terbuthylazine) retardation compared with nonamended soil. Organic-matter amendment resulted in increased nonequilibrium sorption effects, including early breakthrough, increased breakthrough, and elution front-tailing. Organic amendments likewise caused greater solute retardation compared with transport in nonamended soil columns. As the organic matter content increased, retardation increased and the desorption rate constant decreased. The fraction of fast sorption sites also decreased, resulting in greater sorption non-equilibrium. In a given column, the fraction of fast sorption sites was essentially equal for the different s-triazine compounds. The desorption rate constant decreased as the organic carbon partition coefficient (Koc) increased in the order atrazine > simazine ≥ ametryn > terbuthylazine. Soil amendment with sludge and compost resulted in some nonideal physical transport, which was negligible compared with sorption nonideality effects. The linear sorption isotherm nonequilibrium (LNE) model adequately simulated the measured breakthrough curves. The breakthrough curve for ametryn in an amended, nonrinsed column was identical to that in an amended, rinsed column, indicating that sludge-/soil-derived dissolved organic carbon did not affect ametryn sorption and transport in this system.
INTRODUCTION
The use of treated municipal sewage sludge for soil amendment in agriculture may be more environmentally sound than incineration or ocean and surface water dumping, and, simultaneously, may conserve resources such as organic matter and plant nutrients [1-4]. The amendment of soil with sludge causes changes in soil structure and transport characteristics, including increased porosity, decreased bulk density, increased water retention, and changes in pore-size distribution [5-8]. Soil amendment with sludge can also affect pesticide binding [9-13], which may alter pesticide transport and ultimate distribution in the soil profile. Guo et al. [14, 15] studied the sorption of atrazine (ATR) and alachlor on soils freshly amended with carbon-rich waste materials and found that the sorption coefficient (Kd) for these compounds increased on amended versus unamended soils. They also found that amended soil columns retained more pesticide than unamended soil columns. These results suggest that pesticide sorption to sludge-amended soils will be predictably enhanced by freshly added organic matter (sludge) [16]. In contrast, O'Connor et al. [9] found that sorption of dichlorophenoxyacetic acid (2,4-D) was unaffected by freshly applied sludge while sorption to aged soil-sludge increased. Jin and O'Connor [11] found that toluene adsorption was influenced not only by the increase in organic matter content resulting from sludge amendment, but also by amendment type and soil clay content.
The amendment of soil with sewage sludge can add a significant amount of dissolved and colloidal organic macromolecules, which may have an impact on subsequent pesticide binding and transport [e.g., 17–20]. It has been shown that the presence of dissolved or colloidal organic matter, including matter derived from treated sewage effluent, can lead to enhanced movement of pesticides through soil columns and in the field, possibly through complexation with the mobile organic matter [17, 19, 20]. Pesticide transport and binding in soil can be studied directly by displacing an aqueous solution of pesticide through a soil column and measuring the resulting breakthrough curve (BTC) [21]. BTCs can then be analyzed using simulation models that evaluate transport and sorption nonequilibrium. The aim of this work was to examine the effect of organic amendments (municipal sewage sludge and composted municipal sewage sludge) on transport ideality, sorption equilibrium, and retardation of several s-triazines through the use of simulation models based on the well-validated, two-site bicontinuum approach. Triazine herbicides were assessed because they represent a class of compounds in wide agricultural use and because a number of triazine compounds (e.g., atrazine, simazine [SIM], and ametryn [AME]) have been detected in ground water in many parts of the world.
Theoretical considerations
Nonequilibrium transport or sorption processes can influence the behavior and distribution of organic contaminants in the soil. Nonequilibrium processes resulting in early breakthrough and increased elution front-tailing have been demonstrated in column experiments, and the data have been applied to simulation models designed to describe such nonideal processes [22-29].
Physical transport ideality. Transport nonideality in porous media is thought to result from mass transfer between mobile water regions (controlled by advective flow) and immobile water regions (governed by diffusion), and by rate-limited diffusion within immobile water regions [22]. Physical transport ideality can be most effectively evaluated using a nonsorbing tracer so nonequilibrium effects due to sorption nonideality are avoided. In a system that probes only physical nonequilibrium behavior, a parameter <‡> can be defined as the fraction of water in the mobile region. When physical transport is ideal (physical equilibrium, PE), <‡> is unity. As transport becomes nonideal (φ> 1), physical transport nonequilibrium (PNE) effects become apparent [22, 29].
| Common name | IUPACa chemical name | MWb | log K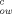 |
Sd (mg/L) | log K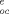 |
|---|---|---|---|---|---|
| Atrazine | 2-chloro-4-ethylamino-6-isopropylamino-1,3,5-triazine | 215.7 | 2.47 | 35.5 | 1.71 (0.02) |
| Simazine | 2-chloro-4,6-ethylamino-1,3,5-triazine | 201.7 | 2.04 | 5 | 1.80 (0.05) |
| Ametryn | -2-ethylamino-4-isopropylamino-6-methylthio-1,3,5-triazine | 227.3 | 2.58 | 185 | 2.04 (0.04) |
| Terbuthylazine | 2-tert-butylamino-4-chloro-6-ethylamino-1,3,5-triazine | 229.7 | 3.04 | 8.5 | 2.35 (0.04) |
- a International Union of Pure and Applied Chemistry.
- b Molecular weight.
- c log Kow (octanol-water distribution coefficient) is from [39].
- d S (aqueous solubility) is from [39].
- e Each log Koc value (soil organic carbon-water distribution coefficient) is the mean of four experimentally determined values; the value in parentheses is the standard deviation. Data from [34].
Sorption ideality. Local equilibrium assumption (LEA) [30] is represented as a single domain sorption process that is instantaneous relative to other processes affecting solute concentration. Models accounting for sorption nonideality may include terms for isotherm nonlinearity and/or multidomain sorption with multiple sorption rate constants. In the current study, sorption nonequilibrium is considered using models based the two-domain, bicontinuum approach where sorption in one domain is instantaneous (fast sites) and sorption in the other domain is rate-limited (slow sites) [22, 26, 28, 29, 31]. Like transport nonequilibrium, sorption nonequilibrium is expressed by asymmetric BTCs displaying early breakthrough and increased time to reach complete breakthrough and desorption. In the linear isotherm nonequilibrium (LNE) model, sorption is modeled as a linear process in both the instantaneous (fast) and rate-limited (slow) domains. The PNE and LNE models are mathematically identical, but the parameters have different physical interpretations. In the nonlinear isotherm, nonequilibrium (NLNE) model, the nonlinear Freundlich exponent (n) is considered the same for both fast and slow sites.
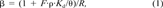 (1)
(1) (2)
(2)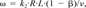
MATERIALS AND METHODS
Materials
The soil studied is a virgin sandy loam (Ap: fine-loamy, mixed, thermic, calcic haploxeralf) from the Gilat Experimental Station in the Northern Negev, Israel (72% sand, 11% silt, 17% clay, 0.4% organic carbon [OC]). These soils are generally structureless and thus are good candidates for amendment by organic matter to improve structure and water retention capacity. The soil was gently crushed and passed through a 1-mm sieve. An acid-washed medium quartz sand from a mine in Maktesh Ramon, Israel, was mixed in a 1:1 w/w ratio with fine loam to prevent overcompaction of the soil during the experiment.
Treated sewage sludge was obtained from the Kishon Sewage Treatment Plant that uses activated sludge followed by anaerobic digestion in holding tanks. The sludge samples were taken from drying beds and further air-dried. Composted Haifa sludge was prepared in aerated windrows at a 2:1 sludge: wood chips ratio and was maintained above 50°C for at least 2 weeks [32].
Atrazine (97.8%), terbuthylazine (TBA) (99%), SIM(99%), and AME (99.4%) were supplied by Agan Chemical Company, Ashdod, Israel. Analytical-grade pentafluorobenzoic acid (PFBA) (99%) obtained from Aldrich Chemical Company, Milwaukee, WI, USA, was used as a nonsorbing tracer. Relevant characteristics of the herbicides studied are listed in Table 1.
Four columns were prepared: (1) quartz sand (quartz), (2) a mixture of loam and quartz sand (1:1, w/w) (loam), (3) Haifa sludge in loam: quartz sand mixture (1:1, w/w) (sludge), and (4) Haifa compost in loam: quartz mixture (1:1, w/w) (compost). Sludge and compost were thoroughly mixed into the soil at a rate of 0.5% w/w prior to mixing with quartz, which is equivalent to a loading rate of 10 tons/ha with a plowing depth of 15 cm. This soil application rate is consistent with tentative guidelines for sludge use in Israeli agriculture distributed by the Israeli Ministry of Environmental Quality in 1994.
The five compounds were prepared in 0.05 M CaCl2 at the following concentrations: ATR, 20 mg/L; TBA, 5.3 mg/L; AME, 25 mg/L; SIM, 2.7 mg/L; and PFBA, 100 mg/L. NaN3 was added to the solutions to inhibit bacterial activity at a rate of 0.005 M for atrazine and PFBA, and 0.002 M for the other compounds. NaN3 had no effect on sorption in preliminary batch sorption studies.
Experimental procedure
A miscible displacement technique [21, 27, 28] was used to study solute sorption and transport behavior in sludge- and compost-amended soil and nonamended soil. A borosilicate glass preparative chromatography column (Kontes, Aachen, Germany) (i.d. 2.54 cm × 15 cm), fitted with Teflon® bed supports lined with a porous Teflon filter, was uniformly packed with soil in 1-cm increments. Each increment was tapped firmly to achieve homogeneous packing and uniform bulk density. The surface of the packed material was slightly disturbed before each new addition to ensure continuity. The column was connected to a dual piston high-pressure minipump (Milton Roy, Ivyland, PA, USA); a three-way switching valve was used to switch between the background electrolyte solution and the test compound-electrolyte solution. The effluent from the column was passed through a flow-through, variable wavelength ultraviolet (UV) absorbance detector (Applied Biosystems, Foster City, CA, USA) connected to a stripchart recorder. The apparatus was designed to minimize void volume between the column and the detector. The solutions came into contact with glass and Teflon only. The soil column was slowly wetted from the bottom to achieve saturation and was rinsed for 3 to 5 d with the electrolyte solution until a stable output from the UV detector was obtained. The detector was then zeroed. The electrolyte solution was replaced with a pesticide solution, which was displaced through the column until no change in UV absorbance was observed. The pesticide was then desorbed by the electrolyte solution under identical flow conditions. The flow rate for all experiments was fixed at 8.5 ±0.1 cm/hr (mean ± standard deviation), except for quartz-PFBA, quartz-ATR, and quartz-TBA, where the flow rate was 7.2 ±0.1 cm/hr. Breakthrough curves were evaluated at wavelengths of 240 nm for PFBA, 260 nm for ATR, 240 nm for AME, and 220 nm for SIM and TBA.
A second identical loam: quartz column amended with Haifa sludge (0.5%) was prepared, and an AME solution (25 mg/L in 0.05 M CaCl2 + 0.002 M NaN3) was displaced through the column after slowly wetting the column from the bottom with 1 pore volume of electrolyte solution. The column was not rinsed with the electrolyte solution prior to displacement of the pesticide solution. The column effluent was collected in an automated fraction collector (ISCO) at intervals of approximately 5 min. Fractions were immediately analyzed for AME concentration by high-performance liquid chromatography (HPLC) using a Diode Array Detector (235C) and a Series 410 pump (Perkin Elmer, Norwalk, CT, USA) and the sampling interval was adjusted accordingly. This experiment was designed to evaluate the effect of column rinsing and removal of dissolved and colloidal organic matter on the breakthrough of AME. The HPLC chromatographic conditions were wavelength, 240 nm; C-8 column, 25 cm long; flow rate, 1.2 ml/min; eluent, 50 mM ammonium acetate: acetonitrile (30: 70 v/v).
Data analysis
Retardation (R) was determined as the first moment of the BTCs. The Peclet number (P), a measure of hydrodynamic dispersion during flow, was optimized with data for the nonsorbing solute PFBA using a curve-fitting program, CFITIM3, based on linear least-squares optimization techniques [33]. Transport and sorption parameters for the sorbing solutes were then optimized using CFITIM3 and incorporating the previously determined R and P values. Relevant data and optimized parameters are listed in Table 2. Measured BTCs for the sorbing solutes were evaluated using the LEA, LNE, and NLNE models. Each of these models assumes physical transport ideality. Breakthrough and elution curves displayed singularity in all cases.
RESULTS AND DISCUSSION
The nonsorbing tracer (PFBA) data for the four columns were used to evaluate physical transport ideality in amended and nonamended soil columns. The four BTCs for PFBA are presented in Figure 1A, with relative concentration (C/C0) on the ordinate and pore volume relative to retardation ([V/V0]/R) (relative pore volume) on the abscissa (C0 = initial concentration, C = concentration, V0 = porosity, V = volume of solution passed through the column). Transport in the quartz and loam columns was ideal, with C/C0 = 0.5 at 1 relative pore volume (Fig. 1A). Slight physical transport nonideality was apparent in both the compost- and sludge-amended columns (Fig. 1A). Figure 1B compares the PE and PNE model descriptions for PFBA in the compost-quartz-loam column (φ = 0.979 ± 0.004). Both models adequately describe the nonsorbing tracer PFBA data (Fig. 1B). Physical nonideality was therefore considered negligible, and BTCs for the sorbing solutes (Table 1) were evaluated without regard for physical nonequilibrium transport. The fraction of water in the mobile domain (φ) was greater than 98% in all cases (Table 2).
To examine the impact of sorption isotherm nonlinearity on transport, BTCs were evaluated using both the LNE and NLNE models. In all cases, the Freundlich exponent n returned by the simulation was >0.94, and in most instances, values for the sum of the squares (SSQ) of the deviations were identical. There were no discernible differences in the fits provided by the two models. Experimentally determined Freundlich exponents for systems exhibiting n ≤ 0.9 [34] were also compared using the NLNE model. In these instances, the experimentally determined n in the NLNE model always returned a worse fit than the LNE model fit. An example of LEA-, LNE-, NLNE-model n, and NLNE-batch n curves for the TBA compost system is found in Figure 2. The LEA model did not provide an adequate fit of the data (Fig. 2); the measured BTC exhibited early breakthrough and increased tailing compared with predictions based on the equilibrium model. In contrast, LNE- and NLNE-model-determined n provided equally good descriptions of the data (Fig. 2). The similar descriptions provided by the two models is a function of the near-unity Freundlich exponent (n = 0.97) returned by the model. The NLNE model using the experimentally determined Freundlich exponent (n = 0.9 ± 0.02) provided a somewhat poorer description of the data (SSQ nearly 4 times greater than for the other two models), particularly in the approach to equilibrium (Fig. 2). It is clear that for the systems investigated, the LNE model adequately describes BTC data. The good correlation found between the data and the LNE and NLNE models, contrasted with the poor description of the data provided by the LEA model, emphasizes the importance of nonequilibrium sorption processes [29]. The LEA model did not adequately describe BTC results for sorbing compounds in any of the three soil columns in this study.
| Sorbent | Sorbate | ρ | θ | P | R | φ(σ) | β(σ) | σ (σ) | F | k2 |
|---|---|---|---|---|---|---|---|---|---|---|
| Quartz | Pentafluorobenzoic acid | 1.88 | 0.37 | 420 | 1.05 | 0.988 (0.002) | NA | NA | NA | NA |
| Simazine | 1.07 | 0.981 (0.002) | 0.08 (0.02) | NA | NA | |||||
| Atrazine | 1.13 | 0.978 (0.003) | 0.07 (0.03) | NA | NA | |||||
| Ametryn | 1.22 | 0.967 (0.003) | 0.17 (0.05) | NA | NA | |||||
| Terbuthylazine | 1.41 | 0.954 (0.006) | 0.7 (0.2) | NA | NA | |||||
| Quartz-loam | Pentafluorobenzoic acid | 1.84 | 0.38 | 197 | 1.06 | 0.995 (0.001) | NA | NA | NA | NA |
| Simazine | 1.40 | 0.915 (0.003) | 0.23 (0.02) | 0.70 | 1.11 | |||||
| Atrazine | 1.37 | 0.938 (0.004) | 0.29 (0.05) | 0.77 | 1.98 | |||||
| Ametryn | 1.66 | 0.882 (0.004) | 0.42 (0.04) | 0.70 | 1.26 | |||||
| Terbuthylazine | 2.42 | 0.839 (0.004) | 0.69 (0.04) | 0.73 | 1.04 | |||||
| Quartz-loam-compost | Pentafluorobenzoic acid | 1.97 | 0.33 | 132 | 0.99 | 0.979 (0.004) | NA | NA | NA | NA |
| Simazine | 1.69 | 0.84 (0.01) | 0.37 (0.08) | 0.65 | 0.77 | |||||
| Atrazine | 1.63 | 0.841 (0.003) | 0.52 (0.03) | 0.59 | 1.18 | |||||
| Ametryn | 2.26 | 0.768 (0.005) | 0.63 (0.04) | 0.59 | 0.72 | |||||
| Terbuthylazine | 3.43 | 0.704 (0.006) | 0.93 (0.05) | 0.58 | 0.54 | |||||
| Quartz-loam-sludge | Pentafluorobenzoic acid | 1.99 | 0.32 | 101 | 1.03 | 0.981 (0.003) | NA | NA | NA | NA |
| Simazine | 1.74 | 0.806 (0.008) | 0.38 (0.05) | 0.57 | 0.65 | |||||
| Atrazine | 1.78 | 0.812 (0.006) | 0.80 (0.06) | 0.57 | 1.41 | |||||
| Ametryn | 2.54 | 0.713 (0.009) | 0.79 (0.08) | 0.53 | 0.62 | |||||
| Terbuthylazine | 4.10 | 0.667 (0.008) | 1.14 (0.07) | 0.56 | 0.49 |
- ρ = column bulk density; θ = fractional water content; P = Peclet number; R = retardation; φ = fraction of water in mobile region; σ standard deviation; β = fraction of retardation in instantaneous sorption sites; ω = parameter describing sorption process in slow sites; F fraction of instantaneous sorption sites; K2 = kinetic rate constant for desorption from slow sites; NA = not applicable.
Breakthrough curves for the sludge-amended column and model predictions for PFBA (PE), and SIM, ATR, AME, and TBA (LNE), are found in Figure 3A. Movement of the sorbing s-triazine compounds was retarded compared with PFBA in all instances, with SIM and ATR displaying the smallest retardations and TBA displaying the greatest (Table 2). Breakthrough curves for SIM and ATR were nearly identical in all four columns (data not shown). Kd and Koc values determined in batch equilibrium studies for ATR were always somewhat lower than for SIM [34] (Table 1), despite the fact that the reported octanol/water partition coefficient (Kow) for ATR is considerably greater than that of SIM (Table 1). Figure 3B displays BTCs for ATR for the four columns. Retardation increased as organic carbon content of the soil columns increased (quartz 0%, loam 0.20%, compost 0.27%, sludge 0.29%). This trend was identical for the other three s-triazine compounds.
After normalizing pore volume to retardation (relative pore volume), as shown in Figure 4A, it is clear that in the compost-amended column, s-triazine compounds experience early breakthrough and increased breakthrough front-tailing as compared with the nonsorbing tracer, denoting sorption nonequilibrium. Sorption nonequilibrium for the s-triazine compounds was observed in all three soil columns (Table 2). Nonequilibrium effects generally increase as log Kow of the compounds increases (Table 1), with the greatest nonequilibrium observed for TBA and the least for SIM (Table 2). A similar effect was noted by Bouchard et al. [27]. The increase in sorption non-equilibrium with increasing Kow can be explained on the basis of hydrophobic interactions of the sorbing compounds with organic matter, with more strongly sorbed molecules, such as TBA, experiencing slower intraorganic matter diffusion than less strongly sorbed molecules [29, 31]. Sorption nonequilibrium increases with increasing organic matter content, with greater nonequilibrium effects in amended columns than nonamended columns (Fig. 4B; Table 2). This is thought to reflect diffusion rate-limited sorption processes, which increase as organic matter content increases [27, 29, 31] and, particularly, where relatively large aggregates of sludge or composted organic matter are added. The effect of increasing organic matter content on AME sorption nonequilibrium is clearly depicted in Figure 4B, where organic carbon increases from 0% (quartz) to 0.29% (sludge).
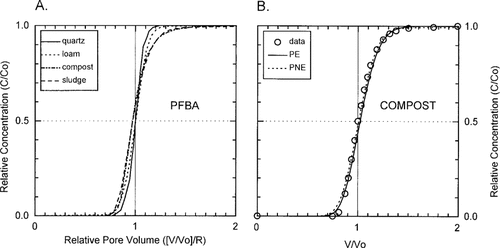
(A) Relative concentration (C/C0) versus relative pore volume ([V/V0]/R) for the nonsorbing tracer PFBA in the four columns. C/C0 of 0.5 at ([V/V0]/R) of unity represents physical transport ideality. (B) Comparison of the physical equilibrium transport (PE) and physical non-equilibrium transport (PNE) models for the compost-quartz-loam column.
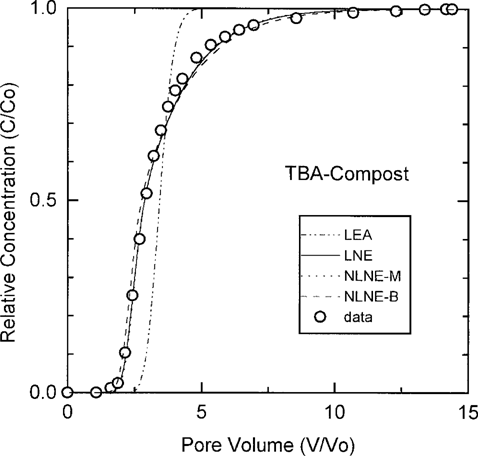
Results of local equilibrium assumption (LEA), linear non-equilibrium (LNE), and nonlinear nonequilibrium model predictions (model-determined n NLNE-M; batch-determined n NLNE-B) for breakthrough of terbuthylazine in the compost-loam-quartz column.
The fraction of fast sorption sites (F) for each column was estimated using Equation 1 with Kd values computed from the data and Equation 2 (Table 2). The fraction of fast sorption sites decreased as the organic carbon content of the soil column increased. Assuming that “instantaneous” sorption sites are those at the organic matter surface, the addition of relatively large organic matter aggregates (sludge or compost) leads to a smaller relative fraction of instantaneous surface sorption sites. In a given column, the estimated fraction of fast sites was essentially equal for the different s-triazine herbicides, supporting the interpretation of physical, rather than chemical, sorption at instantaneous sites. The rate constants for desorption from slow sites (k2), calculated using Equation 3 and data from Table 2, decreased for each compound as the estimated fraction of slow sorption sites (1 — F) and organic carbon content increased. This effect, which has been previously observed [35-38], is attributed to diffusion-limited processes in the organic matter aggregates. Within any given column, the desorption rate constants were most nearly identical for SIM and AME, while for TBA the desorption rate constant was 10 to 40% lower. The desorption rate constants computed for ATR were two to three times those of TBA and significantly higher in all cases than those of SIM and AME (Table 2). In general, the desorption rate constant for hydrophobic organic compounds has been found to decrease as Kd or Kow increase [29, 33, 37, 38]. On the basis of strict hydrophobic theory, the desorption rate constant would be expected to decrease in the order which was observed: ATR > SIM ≥ AME > TBA, such that more hydrophobic compounds, those with higher Koc, experience slower interorganic matter diffusion than do less hydrophobic compounds.
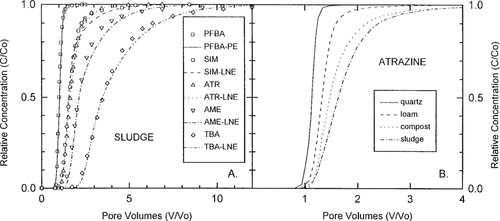
(A) Breakthrough curves and PE or LNE model predictions for pentafluorobenzoic acid, atrazine, simazine, ametryn, and terbuthylazine in the sludge-loam-quartz column. (B) Breakthrough curves for atrazine in the four columns.
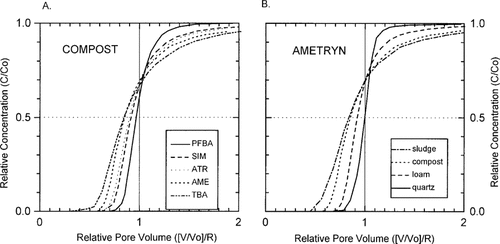
(A) Breakthrough curves normalized to relative pore volumes for the compost-loam-quartz column. (B) Ametryn breakthrough curves normalized to retardation in the four columns.
Ametryn breakthrough from the rinsed sludge-loam-quartz column was compared with breakthrough in an identical non-rinsed column to evaluate the effect of dissolved organic matter on AME breakthrough (Fig. 5). No difference between the two BTCs was observed, indicating that column rinsing did not bias sorption results. The similarity in breakthrough for the rinsed and nonrinsed columns is interpreted to indicate that any potential complexation between AME and sludge or soilderived, dissolved organic matter had a minimal impact on AME transport. In such a system, where the amount of added solid organic matter is substantially greater than the amount of added water-soluble organic matter, transport of sorbing organic solutes is apparently controlled by sorption to the solid organic matter phase rather than by complexation with the dissolved organic matter phase. This may be contrasted with transport of ATR under irrigation with effluent, where downward transport was enhanced, presumably by complexation with dissolved or colloidal effluent-borne organic matter [20].
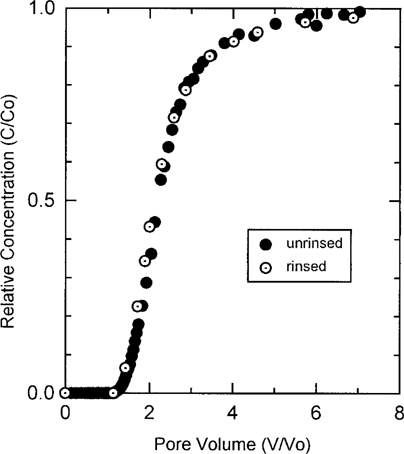
Ametryn breakthrough in rinsed and nonrinsed sludge-loam-quartz columns.
SUMMARY AND CONCLUSION
In this work we examined the means by which soil amendment with sludge and compost can influence transport ideality, sorption equilibrium, and retardation of s-trazines using simulation models based on the bicontinuum approach. Organic matter amendment resulted in increasing sorption nonequilibrium effects such as early breakthrough, increased breakthrough, and elution front-tailing. Soil organic matter amendment likewise resulted in enhanced sorption and increased retardation. Soil amendment with sludge and compost resulted in some nonideal physical transport, although in the particular systems studied, this effect was negligible. The sorption nonideality associated with amendment had a far greater impact on overall transport. Ametryn transport was not affected by sludge-/soil-derived dissolved organic matter. This can be contrasted with studies that have shown enhanced transport of organic compounds in the presence of dissolved organic matter alone [19, 20]. The LNE bicontinuum model with ideal physical transport [22] adequately described the BTC results. The fraction of fast sorption sites was the same for each of the four ä-triazine compounds studied, while the desorption rate constant decreased as log Koc increased, in accordance with hydrophobic theory.
Acknowledgements
This study was supported in part by grants from the European Community (AVICENNE Initiative) and the German Ministry of Agriculture and Forestry: German-Israeli Program for Ecologically Acceptable Agriculture.




