Quantitative structure-activity relationships for weak acid respiratory uncouplers to Vibrio fisheri
Abstract
Acute toxicity values (5- and 30-min Vibrio fisheri 50% luminescence inhibition) of 16 organic compounds thought to elicit their response via the weak acid respiratory uncoupling mechanism of toxic action were secured from the literature. Regression analysis of toxicities revealed that a measured 5-min V. fisheri potency value can be used as a surrogate for the 30-min value. Regression analysis of toxicity (30-min for potency [log pT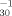 ]) versus hydrophobicity, measured as the 1-octanol/water partition coefficient (log Kow), was used to formulate a quantitative structure-activity relationship (QSAR). The equation log pT
]) versus hydrophobicity, measured as the 1-octanol/water partition coefficient (log Kow), was used to formulate a quantitative structure-activity relationship (QSAR). The equation log pT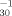 = 0.489(log Kow) + 0.126 was found to be a highly predictive model (r2 adj. = 0.848). This V. fisheri QSAR is statistically similar to QSARs generated from weak acid uncoupler potency data for Pimephales promelas survivability and Tetrahymena pyriformis population growth impairment. This work, therefore, suggests that the weak acid respiratory uncoupling mechanism of toxic action is present in V. fisheri, and as such is not restricted to mitochondria-containing organisms.
= 0.489(log Kow) + 0.126 was found to be a highly predictive model (r2 adj. = 0.848). This V. fisheri QSAR is statistically similar to QSARs generated from weak acid uncoupler potency data for Pimephales promelas survivability and Tetrahymena pyriformis population growth impairment. This work, therefore, suggests that the weak acid respiratory uncoupling mechanism of toxic action is present in V. fisheri, and as such is not restricted to mitochondria-containing organisms.
INTRODUCTION
In their recent review of quantitative structure-activity relationships (QSARs) in aquatic toxicity, Cronin and Dearden [1] noted that three large experimental toxicity databases exist. These data sets are the 4-d Pimephales promelas survivability, the 2-d Tetrahymena pyriformis population growth inhibition, and the 5- to 30-min Vibrio fisheri (formally Photobacterium phosphoreum) luminescence inhibition. The latter is better known as the acute Microtox™ (AZUR Environmental, Carlsbad, CA, USA) assay. In the two eukaryotic systems, P. promelas and T. pyriformis, 1-octanol/water partition coefficient (log Kow)-dependent QSARs have been developed for phenols acting as weak acid uncouplers of oxidative phosphorylation [2]. Using data on the same two toxicity assessment systems, Cajina-Quezada and Schultz [3] expanded QSAR studies on uncouplers to include selected anilines.
As noted in the review by Terada [4], weak acid respiratory uncouplers (WARUs) possess a weak acid assemblage (i.e., an amino or hydroxyl group), a bulky, hydrophobic aromatic moiety, and multiple electronegative groups (i.e., nitro and/or halogen substituents). The WARUs are thought to elicit their toxic response via protonophoric action [5]. McKim et al. [6], using 2,4-dinitrophenol and pentachlorophenol as model compounds, described the fish acute toxicity syndrome (FATS) for WARUs. Additionally, Schultz et al. [7] conducted a volume fraction analysis for WARUs.
Considering evidence from the 12-h Escherichia coli population growth inhibition assay [8], Jaworska and Schultz [9] stated the weak acid respiratory uncoupling mechanism of action did not exist in prokaryotes. In an effort to examine whether this mechanism could be indirectly demonstrated in the V. fisheri light emission impairment assay, the following structure-toxicity evaluations were undertaken. Moreover, to determine if toxic potencies for WARUs can be extrapolated among the three data sets (i.e., V. fisheri, P. promelas, and T. pyriformis), log Kow-dependent models were compared and correlations between endpoints were examined.
MATERIALS AND METHODS
Toxicity data
Current knowledge suggests that the structural features relevant for WARUs include a moderately weak acid moiety (i.e., an amino or hydroxyl group) on a bulky, hydrophobic aromatic ring system with additional electron-withdrawing substituents (i.e., two nitro groups, more than three halogen groups, or a single nitro group and more than one halogen group) [3]. Searching the monograph of Kaiser and Palabrica [10] revealed toxic potency (pT−1) to V. fisheri (5-min and 30-min log pT−1; log pT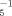 and log pT
and log pT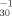 , respectively) for eight phenols and eight anilines meeting these strict structural criteria. When multiple entries were noted, the recommended toxicity value was used. Moreover, toxicity data for the same compounds measured for P. promelas (4-d log median lethal concentration−1 [LC50−1]) and T. pyriformis (2-d log median impairment growth concentration [IGC50−1]) were attained from the five-volume set entitled Acute Toxicities of Organic Pollutants to Fathead Minnows (Pimephales promelas) [11–15-19] and TE-TRATOX (T.W. Schultz, unpublished) databases, respectively. Although more toxicity data for P. promelas and/or T. pyriformis are available [3], only those for the above 16 compounds were used in an effort to make the comparisons of QSARs and toxic potencies appropriate. All potency data are listed as the negative logarithm of the millimolar concentration required to elicit a 50% reduction in biological activity in the stated time.
, respectively) for eight phenols and eight anilines meeting these strict structural criteria. When multiple entries were noted, the recommended toxicity value was used. Moreover, toxicity data for the same compounds measured for P. promelas (4-d log median lethal concentration−1 [LC50−1]) and T. pyriformis (2-d log median impairment growth concentration [IGC50−1]) were attained from the five-volume set entitled Acute Toxicities of Organic Pollutants to Fathead Minnows (Pimephales promelas) [11–15-19] and TE-TRATOX (T.W. Schultz, unpublished) databases, respectively. Although more toxicity data for P. promelas and/or T. pyriformis are available [3], only those for the above 16 compounds were used in an effort to make the comparisons of QSARs and toxic potencies appropriate. All potency data are listed as the negative logarithm of the millimolar concentration required to elicit a 50% reduction in biological activity in the stated time.
Hydrophobicity and QSAR development
Log Kow values were computer estimated or retrieved as measured values from MEDCHEM (CLOGP version 3.55 [BioByte Corp., Claremont, CA, USA]) software. Structure-toxicity model development was performed by least-squares linear regression using the MINITAB (ver. 9.1 [MINITAB, State College, PA, USA]) software.
| Chemical | CAS number | Log Kow | 1/Log pT5 | 1/Log pT30 | 1/Log LC50 | 1/Log IGC50 |
|---|---|---|---|---|---|---|
| 2,4-Dinitrophenol | 51-28-5 | 1.67 | 1.26 | 1.24 | 1.23 | 1.10 |
| 2,4-Dinitroaniline | 97-02-4 | 1.72 | 0.49 | 0.58 | 1.09 | 0.53 |
| 3,5-Dinitroaniline | 618-87-1 | 1.89 | 0.71 | 0.98 | — | 0.94 |
| 4,6-Dinitro-2-methylphenol | 534-52-1 | 2.13 | 1.44 | 1.51 | 2.06 | 1.73 |
| 2,6-Dinitro-4-methylphenol | 609-93-8 | 2.29 | 1.33 | 1.34 | — | 1.23 |
| 4-Chloro-2,6-dinitroaniline | 5388-62-5 | 2.46 | 1.54 | 1.76 | — | — |
| 6-Chloro-2,4-dinitroaniline | 3531-19-9 | 2.46 | 0.98 | 1.03 | — | — |
| 4,5-Dichloro-2-nitroaniline | 6641-64-1 | 3.21 | 1.40 | 1.37 | — | 1.66 |
| 2,4-Dichloro-6-nitroaniline | 2683-43-4 | 3.33 | 1.42 | 1.39 | — | 1.26 |
| 2,3,5,6-Tetrachlorophenol | 935-95-5 | 3.88 | 1.92 | 2.02 | — | 2.22 |
| 4-Phenylazophenol | 1689-82-3 | 3.96 | 2.41 | 2.33 | 2.23 | 1.65 |
| 2,3,4,6-Tetrachlorophenol | 58-90-2 | 4.45 | 2.09 | 2.26 | 2.35 | — |
| 2,3,5,6-Tetrachloroaniline | 3481-20-7 | 4.46 | 2.20 | 2.16 | 2.93 | 1.79 |
| 2,3,4,5-Tetrachloroaniline | 634-66-2 | 4.57 | 2.31 | 2.37 | — | 1.96 |
| Pentachlorophenol | 87-86-5 | 5.12 | 2.46 | 2.71 | 3.04 | 2.07 |
| Pentabromophenol | 608-71-9 | 5.74 | 2.74 | 3.03 | 3.72 | 2.66 |
- alog Kow = 1-octanol/water partition coefficient; pT5 = 5-min toxic potency; pT30 = 30-min toxic potency; LC50 = median lethal concn.; IGC50 = median inhibitory concn.
RESULTS
Table 1 presents a summary of the hydrophobicity and toxic potency data used to develop the QSARs. Hydrophobicity varied over five orders of magnitude.
Comparison of V. fisheri potency with length of exposure
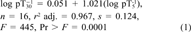 (1)
(1)A slope of near unity and an intercept of near zero for Equation 1 is evidence that toxic potency for WARUs measured in the acute V. fisheri assay differ only slightly with length of exposure. The 30-min data were chosen for the subsequent analyses.
Comparison of hydrophobicity-dependent potency with test system
The log toxic response versus log Kow models are summarized in Table 2. The QSARs expressed by Equations 2 and 3 for V. fisheri and P. promelas, respectively, have high coefficients of determination and exhibit neither statistical nor visual outliers. However, the relationship for T. pyriformis quantitated by Equation 4 has a single chemical, 4,6-dinitro-o-cresol, that was a visual outlier, being more potent than predicted. Removal of this compound prior to reanalyses resulted in Equation 5. The statistics for Equation 5 are very comparable to the statistics of Equations 2 and 3.
| Slope | Intercept | n | r2 adj. | s | F | Pr>F | Equation | |
|---|---|---|---|---|---|---|---|---|
Log pT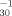 |
0.489 | 0.126 | 16 | 0.848 | 0.267 | 85 | 0.0001 | 2 |
| Log LC50−1 | 0.526 | 0.408 | 8 | 0.858 | 0.337 | 43 | 0.0001 | 3 |
| Log IGC50−1 | 0.363 | 0.371 | 13 | 0.717 | 0.307 | 32 | 0.0001 | 4 |
| Log IGC50−1 | 0.401 | 0.189 | 12 | 0.824 | 0.253 | 52 | 0.0001 | 5 |
- a pT30 = 30-min toxic potency; LC50 = median lethal concn.; IGC50 = median inhibitory concn. Pr = Probability.
Additional support of the V. fisheri WARU model
As an effort to verify Equation 2, two chemicals not included in the previous analyses, but having molecular structural characteristics of WARUs and measured 30-min V. fisheri toxicity data, were evaluated. A summary of this exercise is presented in Table 3.
Comparisons of toxic potencies between V. fisheri, P. promelas, and T. pyriformis
The comparative toxicity models are summarized in Table 4. Except for the slopes, the statistics for Equations 6, 7, and 8 are remarkably similar.
DISCUSSION
The fact that toxic potency values of WARUs deviate little between the 5-min and 30-min exposure schemes (see Eqn. 1) bolsters the premise that these data can be used interchangeably.
Because of the unfaltering statistical similarities observed between Equations 2, 3, and 5, we offer Equation 2 as the QSAR for the WARU mechanism of toxic action for the acute V. fisheri luminescence inhibition endpoint.
The impressive statistics observed for regressions of potency measured for the V. fisheri, P. promelas, and T. pyriformis endpoints (see Eqns. 5, 6, and 7) allows, in the case of WARUs, a measured potency in one endpoint to be used to estimate the potency in either of the other endpoints. Similar results were noted by Schultz et al. [16] for a limited number of nonpolar narcotics representing the baseline mechanism of toxic action.
| Chemical | CAS number | Log Kow | Observed 1/log pT30 | Predicted 1/log pT30 |
|---|---|---|---|---|
| 2,6-Dinitro-4-methylaniline | 6393-42-6 | 2.22 | 1.18 | 1.20 |
| 2,6-Dichloro-4-nitroaniline | 99-30-9 | 2.79 | 1.75 | 1.49 |
- a pT30 = 30-min toxic potency.
Considering the 12-h E. coli population growth inhibition data of Nendza and Seydel [8], it was suggested that the WARU mechanism of action did not exist in bacteria but was limited to mitochondria-containing organisms [9]. Results of the research here provide convincing evidence to the contrary.
To avoid resource-demanding testing, QSARs have been developed as a means of modelling toxic potency from molecular descriptor data and predicting the toxicity of untested chemicals. Such QSARs have been established as valid methods of hazard assessment [17, 18].
For aquatic toxicity, the most thorough QSARs are ones advanced on the basis of a common mechanism of toxic action [1, 19]. Many such QSARs use hydrophobicity, quantitated by log Kow, as a single universal descriptor. Although the approach of using log Kow as a single predictor works for various mechanisms of action, this approach does not work for all toxicants [24-26].
The accuracy in predicting potency when only using log Kow as the descriptor hinges on selecting the correct mechanism of action. Because the activity of chemicals towards biological systems can depend upon a multitude of physical and/or chemical interactions between the toxicant and an often ill-defined site of action, mechanism selection is not an easy task. Despite the general lack of knowledge about the molecular basis for toxicity, several methods have been developed to group toxicants by mechanism of action. These methods include strict concentration addition in joint toxic action models [23], similar whole-animal behavioral and cardiovascular-biochemical response sets [6], and similar values from volume fraction analyses [24].
Over the past decade, different investigations have pointed to the fact that specific aromatic polar compounds, including phenols and anilines, were significantly more potent than predicted by baseline nonpolar narcosis models [2, 3, 25]. Based on their degree of electronegativity, such compounds were deemed to be either polar narcotics or WARUs [26, 27].
Log pT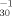 vs. Log LC50−1 vs. Log LC50−1 |
Log pT vs. Log IGC50−1 vs. Log IGC50−1 |
Log IGC50−1 vs. Log LC50−1 | |
|---|---|---|---|
| Slope | 1.030 | 0.728 | 1.352 |
| Intercept | 0.295 | 0.310 | 0.101 |
| n | 8 | 13 | 7 |
| r2 adj. | 0.844 | 0.810 | 0.884 |
| s | 0.354 | 0.251 | 0.330 |
| F | 39 | 52 | 47 |
| Pr > F | 0.001 | 0.0001 | 0.0001 |
| Equation | 6 | 7 | 8 |
- a pT30 = 30-min toxic potency; LC50 = median lethal concn.; IGC50 = median inhibitory concn. Pr = Probability.
Weak acid uncoupling of oxidative phosphorylation is the result of the inhibition of coupling between electron transport and phosphorylation reactions [4]. As such, ATP synthesis is inhibited with no effect on the respiratory chain or ATP synthase. Although several classes of chemicals have been demonstrated to be respiratory uncouplers [4], weak acidic ones are the most thoroughly studied. As noted previously, structural requirements for WARUs include an acid-dissociable group, a bulky hydrophobic moiety, and strong electron-withdrawing groups.
Structure-toxicity studies [3, 26-30] have indicated that potency of WARUs is typically depicted as a linear function of hydrophobicity measured as log Kow and electron-withdrawing capability is depicted by the acid dissociation constant, pKa. Substitution of the acid-dissociable moiety of a WARU with a nonacid-dissociable substituent results in the loss of decoupling action [31]. Although high hydrophobicity and strong electron-withdrawing capability are the principal physiochemical properties for uncoupling, the proportion of contribution of these attributes to decoupling is compound dependent. The fact that electron-withdrawing capacity was not considered in the QSARs represented by Equations 2, 3, and 5 probably explains the fact these log Kow-dependent models only explain about 85% of the variability in the observed toxic potency.
In a recent investigation using T. pyriformis, toxicity data for a large series of phenols [32] demonstrated that the molecular orbital quantum chemical term ELUMO (energy of the lowest unoccupied molecular orbital), when used in conjunction with log Kow, provided a high-quality QSAR for predicting toxic potency. In another analysis with T. pyriformis data, Schultz et al. [27] modelled the toxicity of selected phenols using log Kow and pKa as molecular descriptors. They noted that the threshold for electron-withdrawing capacity for phenols acting as WARUs was a pKa value of 6.3. All eight of the phenols included in this investigation had pKa values < 6.3. These results, although limited, suggest this threshold is applicable to V. fisheri.
As summarized by Terada [4], the following molecular mechanism has been hypothesised. In the most simple terms, at the membrane-water interface, an anionic uncoupler traps a hydrogen ion and becomes neutral. The neutral assemblage crosses the membrane to the inner surface where it releases the hydrogen ion. Subsequently, the anionic uncoupler returns to the original surface, and the process is repeated. By this cycle, hydrogen ions are transported to the inside of the mitochondria through the ion-impermeable membrane, thereby disintegrating the hydrogen ion gradient.
Whole-animal experimental evidence to support this mechanism of toxic action was gleaned from the assessment of physiological response sets from spinally transected rainbow trout exposed to model chemicals. In this FATS approach [6], in vivo biochemical and respiratory responses were measured during lethal aqueous exposures. The responses and their interdependence formed a complex data matrix. The best response variables for the specific FATS were determined through multivariate statistical techniques.
The FATS for WARUs were described using 2,4-dinitrophenol and pentachlorophenol as model toxicants [6]. The rapid and continuous increase in ventilation volume and oxygen consumption were the most impressive diagnostic responses for WARUs. These responses, which corresponded to a continuously rising metabolic rate, were not a result of increased activity because the trout were immobile. Moreover, the increase in ventilation volume and oxygen consumption did not include a change in either oxygen uptake efficiency or ventilation frequency. Therefore, the trout were increasing the water flow across their gills by increasing the ventilatory stroke volume, while at the same time maintaining a constant oxygen uptake. The elevated oxygen consumption was reflected by an initial increase in total arterial oxygen content. This elevated arterial oxygen level lasted for almost half of the survival period and then fell slowly, presumably as the tissues used more oxygen in an effort to generate ATP.
Using classical nonpolar and polar narcotic chemicals, Jaworska and Schultz [24] demonstrated that volume fraction analyses can be used to distinguish mechanisms of toxic action. They used potency data from both the P. promelas survivability and T. pyriformis population growth assay. Analyses of target hydrophobicity indicated that the hydrophobicity of the molecular site of action was constant for chemicals eliciting the same mechanism of action but distinct for a given test system. Moreover, their mean volume fraction analyses indicated that the volume fraction in the target phase was constant for each mechanism of action regardless of test system or protocol [24]. Schultz et al. [7] extended these volume fraction analyses to include WARUs. Again, using P. promelas and T. pyriformis toxicity data, they determined the mean volume fraction for WARUs to be ∼ 7 ± 2 × 10−5.
In conclusion, the QSAR, log pT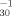 = 0.489(log Kow) = 0.126, was found to be a highly predictive model for the 30-min V. fisheri 50% luminescence inhibition endpoint for organic compounds eliciting their toxicity via the WARU mechanism of action.
= 0.489(log Kow) = 0.126, was found to be a highly predictive model for the 30-min V. fisheri 50% luminescence inhibition endpoint for organic compounds eliciting their toxicity via the WARU mechanism of action.
Regression analysis further revealed that for these uncouplers, a measured 5-min V. fisheri potency value can be used as a surrogate for the 30-min value. The V. fisheri QSAR was similar to hydrophobicity-dependent QSARs generated for WARUs from P. promelas survivability and T. pyriformis population growth impairment data. These findings suggest that the WARU mechanism of toxic action is present in V. fisheri, and, therefore, not restricted to mitochondria-containing organisms.




