Organic pollutants in the coastal environment off San Diego, California. 1. Source identification and assessment by compositional indices of polycyclic aromatic hydrocarbons
Abstract
Samples collected in January and June 1994 from the Point Loma Wastewater Treatment Plant (PLWTP) effluent, Tijuana River runoff, and microlayer, sediment trap, and surface sediment at several locations adjacent to the PLWTP outfall, mouth of the Tijuana River, and San Diego Bay were analyzed in an attempt to identify and assess the sources of hydrocarbon inputs into the coastal marine environment off San Diego. Several compositional indices of polycyclic aromatic hydrocarbons (PAHs), for example, alkyl homologue distributions, parent compound distributions, and other individual PAH ratios, were used to identify the sources of PAHs. Partially due to the decline of PAH emission from the PLWTP outfall, PAHs found in the sea surface microlayer, sediments, and water column particulates near the PLWTP outfall were predominantly derived from nonpoint sources. The sea microlayer near the mouth of the Tijuana River appeared to accumulate enhanced amounts of PAHs and total organic carbon and total nitrogen, probably discharged from the river, although they were in extremely low abundance in the sediments at the same location. Surprisingly, PAHs detected in the microlayer and sediments in San Diego Bay were mainly derived from combustion sources rather than oil spills, despite the heavy shipping activities in the area.
INTRODUCTION
The main sources of anthropogenic hydrocarbons entering the marine ecosystem off southern California are municipal and industrial waste discharge, surface runoff, inadvertent spills, and aerial deposition. The pollutants in only the former two sources can be easily measured and regulated. In addition, in situ biochemical processes also produce chemicals [1, 2] of marine origin. The mixing of hydrocarbons from various sources yields a complicated assemblage that may not be readily separated. In addition, degradation of organic compounds may occur in the water column before deposition. All of these compromise efforts to evaluate the impact of wastewater discharges on the coastal marine environment.
The compositional patterns of polycyclic aromatic hydrocarbon (PAH) and alkane assemblages have been used to discern the origins of hydrocarbon compounds found in the aquatic environment [3, 4]. The PAHs are sensitive indicators of petrogenic and pyrogenic inputs of hydrocarbons, whereas alkanes are useful in identifying biological- or petroleum-derived products. In general, PAHs from petroleum-related sources have abundant alkylated homologues relative to their parent compounds, whereas combustion of fossil fuels yields PAHs generally devoid of alkylated homologues.
These observations prompted the introduction of two useful indicators, the alkyl homologue distributions (AHDs) and the parent compound distributions (PCDs) [5]. Other individual ratios generally involving two PAH compounds, for example, phenanthrene to anthracene (P/A), methylphenanthrene to phenanthrene (MP/P), fluoranthene to pyrene (FL/PYR), and benz[a]anthracene to chrysene (BZ[a]A/CHR), have also been applied to source recognition. Similarly, several molecular indices derived from alkane components have been shown to be sensitive in differentiating biogenic and petrogenic inputs of hydrocarbons [4].
To gain an insight into the contributions of organic pollutants from various sources to the coastal region off San Diego, we conducted a study to measure PAHs, alkanes, and linear alkylbenzenes in various sample media. The objectives of this study were to identify the possible sources of hydrocarbons and to evaluate the extent of organic contamination exerted by the point and nonpoint inputs. In particular, we evaluated the molecular compositions and indices associated with the PAH and alkane assemblages. We also evaluated the ratios of internal to external isomers [6] of phenyldodecanes. The data were used to further assess the input levels of these sewage-derived organic contaminants and their fate in the coastal marine environment. The results from the study are presented in three separate articles in this journal. This first part in the series reports the results from PAHs.
STUDY SITE
The coast off San Diego begins at the United States—Mexico border and extends northwest approximately to Point La Jolla (Fig. 1). A major municipal wastewater treatment facility, Point Loma Wastewater Treatment Plant (PLWTP), discharged treated sewage (advanced primary treatment) into the ocean in the area through a submarine outfall at a water depth of 60 m. An extension of the sewage outfall to a depth of 100 m commenced operation in November 1993.
The Tijuana River drains a 4,480-km2 area, of which 27% lies in Mexico and 73% lies in the United States [7], and flows into the ocean at the southern San Diego coast. Although discharges from the Tijuana River accounted for only 2% of total gauged runoff to the Southern California Bight in 1987 and 6% in 1988, the runoff contained the highest concentrations of suspended solids, metals (Cd, Cr, Cu, Ni, Pb, and Zn), and total polychlorinated biphenyls (PCBs; measured as Aroclor 1242 + Aroclor 1254) and second highest concentration of total dichlorodiphenyl trichloroethan (DDTs; DDT and metabolites) among the eight largest creeks and rivers in Southern California [8].
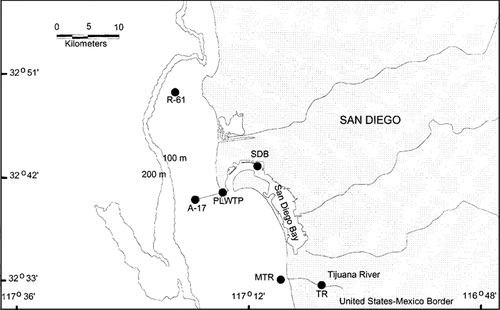
Location of sampling sites in the San Diego, California, area in 1994.
San Diego Bay is a southeast-northwest embayment; its width varies from 1 to 4 km and its length is over 18 km with a maximum water depth of 21 m [9]. Due to the highly elevated level of contamination in San Diego Bay, the National Benthic Surveillance Project under the National Oceanic and Atmospheric Administration National Status and Trends Program established five sampling stations in the vicinity from 1984 to 1993 to monitor the distributions of contaminants in fish and sediments [10].
Four stations were selected in the present study (Fig. 1): A-17 (32°40.28′N, 117°17.03′W), R-61 (32°49.64′N, 117°19.67′W), SDB (32°43.53′N, 117°10.86′W), and MTR (32°32.94′N, 117°08.81′W). In addition, effluent was collected from PLWTP and runoff from the Tijuana River at Dairy Mart Road, about 6 km above the ocean.
MATERIALS AND METHODS
Materials
Glass fiber filters (GF/C 47-mm with 1.2μm pore size) were obtained from Whatman International Ltd. (Maidstone, UK) and baked at 420°C for at least 4 h prior to use. The PAH standard solutions were acquired from AccuStandard (New Haven, CT, USA) and Ultra Scientific (North Kingstown, RI, USA). Internal (nitrobenzene-d5, 2-fluorobiphenyl, and p-terphenyl-d14) and surrogate (naphthalene-d8, acenaphthalene-d10, phenanthrene-d10, chrysene-d12, and perylene-d12) standards were purchased from AccuStandard and Ultra Scientific, respectively. The PAH reference standard (SRM 1491) was acquired from the National Institute of Standards and Technology (Gaitherburg, MD, USA). Ultra-resi-analyzed-grade hexane and methylene chloride were obtained from J.T. Baker (Phillipsburg, NJ, USA) and used as supplied. All the glassware (except for volumetric flasks, which were sonicated with solvent) was soaked with detergent water, rinsed with distilled water, and baked at 420°C for 4 h before use.
Sample collection
Sediment traps were deployed at 1 m and 5 m from the sea floor at both A-17 and R-61 in December 1993 and May 1994; they were retrieved 30 d after deployment. All other samples were collected both in January and June 1994.
The sediment trap consists of two components: a glass centrifuge bottle at the bottom and a glass funnel positioned on the bottle through a Teflon-lined silicone rubber seal [11]. Three moorings were deployed at each location; each mooring carried one trap at 1 m and three traps at 5 m. All the traps deployed in December 1993 were recovered, whereas all three traps deployed at A-17 1-m, six traps at A-17 5-m, and one trap at R-61 5-m in May 1994 were lost. Upon retrieval, the traps were disassembled and water over the particulates was removed slowly. The centrifuge bottles were then capped with Teflon-lined caps.
Sediments were collected using a modified 0.1-m2 van Veen grab [12]. Subsamples of the top 2 cm of sediments were transferred into glass jars. Microlayer samples (upper 50 μm of surface water) were collected into 1-L amber glass bottles using a Teflon-coated, rotation-drum sampler; the procedure for sample collection and handling was similar to that detailed previously [13]. Effluent samples (24-h composite) were obtained from PLWTP and stored in 1-gallon amber bottles. Tijuana River runoff was collected by immersing a 1-gallon glass bottle into the middle of the stream from a bridge. All the samples collected were immediately cooled with ice. Upon returning to the laboratory, the sediment and sediment trap samples were stored at −20°C and microlayer, effluent, and runoff samples were stored at 4°C until further treatment.
Sample extraction
Sediments and sediment trap particulates. A weighed sub-sample of sediments or trap particulates in a 250-ml centrifuge bottle was centrifuged at approximately 50 g for 5 min and the top water layer was decanted. The sample was mixed with ∼30 g of anhydrous sodium sulfate, spiked with surrogate standards, and extracted successively three times (16, 6, and 16 h) with methylene chloride (100 ml each) using a roller table. The combined extract was concentrated and solvent-exchanged to hexane using a rotary evaporator at 30°C and 650 mm Hg vacuum pressure. After sulfur removal with activated copper granules (overnight in the dark), the extract was further concentrated to ∼250 μl under nitrogen. The extract was applied to a 1:2 (v/v) alumina : silica gel glass column for separation of different fractions. The first fraction, containing alkanes, was eluted with 15 ml of dry hexane. The second fraction, containing PAHs and linear alkylbenzenes (LABs), was collected by eluting 5 ml dry hexane and 30 ml of a 30:70 (v/v) mixture of methylene chloride and hexane. Each fraction collected in a pear-shaped flask was concentrated to ∼0.5 ml using the rotary evaporator and transferred to a half-dram vial. The flask was rinsed with additional hexane, which was also added to the half-dram vial. The final volume of the extract was adjusted to 500 μl. The internal standards at 2 μg/ml were added to the final extract prior to instrumental analysis.
Effluent, runoff, and microlayer. Aqueous samples were filtered prior to extraction. The sample bottle was shaken vigorously, and a measured subsample was gradually poured into an all-glass filtration assembly with a preweighed GF/C filter and filtered under vacuum. The filter was replaced when the flow dropped to approximately half of the initial speed. The particle-loaded filters from each sample were cut in pieces and combined in a glass centrifuge bottle. The filters were extracted and fractionated using the same sample procedure as for sediments and sediment trap particulates. The filtrates were extracted three times with methylene chloride using the conventional neutral liquid-liquid extraction method. The combined extract was concentrated and fractionated in the same fashion as for sediments and sediment trap particulates.
Sample analyses
The PAHs were measured using a Hewlett-Packard 5890 II GC with a 5970 mass selective detector equipped with a 60-m × 0.25-mm-i.d. (0.25-μm film thickness) DB-5 column (J&W Scientific, Folsom, CA, USA). The column temperature was programmed from 70°C to 200°C at 6°C/min, followed by a ramp of 10°C/min to 285°C where it was held for 42 min. Other experimental conditions are described elsewhere [14].
The PAH concentrations were determined using the internal calibration method. Twenty-six PAH compounds were quantified in this study (Table 1). ΣC1-Naphthalene consisted of 1-methylnaphthalene and 2-methylnaphthalene. C2-Naphthalenes were quantified using the relative response factor of 2,6-dimethylnaphthalene; C3-naphthalenes using 2,3,6-trimethyl-naphthalene; and C1-phenanthrenes using either 2-methyl-phenanthrene or 1-methylphenanthrene, depending on the closeness of their retention times.
Procedural performance and matrix effects were monitored by the recoveries of the surrogate standards. The mean ± 1 standard deviation in 31 field samples were as follows: naphthalene-d8, 33.8 ± 12.6; acenaphthalene-d10, 69.2 ± 14.5; phenanthrene-d10, 82.0 ± 14.4; chrysene-d12, 84.8 ± 24.4; and perylene-d12, 98.6 ± 31.8. Instrumental performance was checked for each batch of samples (less than 10) by analyzing the SRM 1491 solution. The measured concentrations were within ± 20% of the certified values provided by the National Institute of Standards and Technology for more than 80% of the PAH compounds (or times).
| DL | |||
|---|---|---|---|
| PAH Compound | Molecular ion for quantitation (m/z) | Solid (ng/g) | Aqueous (ng/L) |
| Naphthalene | 128 | 40 | 35 |
| ΣC1-Naphthalene | 142 | 40 | 35 |
| Biphenyl | 154 | 40 | 35 |
| ΣC2-Naphthalene | 156 | 40 | 35 |
| Acenaphthylene | 152 | 40 | 35 |
| Acenaphthene | 154 | 40 | 35 |
| ΣC3-Naphthalene | 170 | 40 | 35 |
| Fluorene | 166 | 40 | 35 |
| Phenanthrene | 178 | 40 | 35 |
| Anthracene | 178 | 40 | 35 |
| ΣC1-Phenanthrene | 192 | 40 | 35 |
| 3,6-Dimethylphenanthrene | 206 | 40 | 35 |
| Fluoranthene | 202 | 40 | 35 |
| Pyrene | 202 | 40 | 35 |
| 2,3-Benzofluorene | 216 | 40 | 35 |
| Benzo[a]anthracene | 228 | 40 | 35 |
| Chrysene | 228 | 40 | 35 |
| Benzo[b]fluoranthene | 252 | 40 | 35 |
| Benzo[k]fluoranthene | 252 | 40 | 35 |
| Benzo[e]pyrene | 252 | 40 | 35 |
| Benzo[a]pyrene | 252 | 40 | 35 |
| Perylene | 252 | 40 | 35 |
| 9,10-Diphenylanthracene | 330 | 200 | 200 |
| Indeno[1,2,3-cd]pyrene | 276 | 200 | 200 |
| Dibenzo[a,h]anthracene | 278 | 200 | 200 |
| Benzo[g,h,i]perylene | 276 | 200 | 200 |
Concentrations of PAH compounds were not corrected for the recoveries of the surrogate standards. The detection limits (Table 1) were set slightly higher than the method detection limits determined using the procedure given previously [15]. These detection limits were based on 1 g dry weight or 1 L volume for a sample. The actual detection limits for samples of different weights or volumes were corrected accordingly. Total suspended solids, moisture contents, total organic carbon (TOC), and total nitrogen (TN) were also determined.
RESULTS
Spatial and temporal distributions of PAHs
PLWTP effluent. A large difference in particulate PAH concentration was observed between the effluent samples collected in January 1994 (5,920 ng/g) and in June 1994 (17,500 ng/g) (Table 2). In the present study, different subsamples were used to measure total suspended solids and organic compounds, due to the concern that some organic compounds might be lost if the same subsample was used for both measurements. Sample heterogeneity may be partially responsible for the large difference in the total suspended solid concentrations, resulting in elevated particle-based PAH concentrations in the effluent collected in June 1994. However, if the aqueous volumes were used, the particulate PAH concentrations were 332 ng/L in the January sample and 481 ng/L in the June sample. The PAH concentrations on a whole-effluent basis were similar in both samples (1,780 ng/L in January vs. 1,550 ng/L in June).
| Sample sizea | Total PAHs (dry wt. based)b | Total PAHs (TOC based)c | % Two-, three-ring PAHsd | % FL + | PYRe | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Sample type | January 1994 | June 1994 | January 1994 | June 1994 | January 1994 | June 1994 | January 1994 | June 1994 | January 1994 | June 1994 |
| Point Loma Wastewater | ||||||||||
| Treatment Plant effluent | ||||||||||
| Filtrates | 2.4 | 10 | 1,450 | 1,070 | —f | — | 100 | 99 | 0 | 1 |
| Particulates | 0.134 | 0.275 | 5,920 (332) | 17,500 (481) | 17,200 | 49,000 | 100 | 90 | 0 | 5 |
| Tijuana River runoff | ||||||||||
| Filtrates | 10 | 30 | 10.8 | 78.5 | — | — | 38 | 92 | 62 | 8 |
| Particulates | 0.888 | 0.450 | 158 (13.9) | 104 (1.6) | 1,550 | 1,280 | 0 | 0 | 100 | 100 |
| Microlayer | ||||||||||
| A-17 filtrates | — | 5 | — | NDg | — | — | — | ND | — | ND |
| A-17 particulates | — | 0.0575 | — | 1,770 (20.4) | — | 13,700 | — | 0 | — | 100 |
| R-61 filtrates | — | 5 | — | ND | — | — | — | ND | — | ND |
| R-61 particulates | — | 0.580 | — | 381 (44.2) | — | 1,840 | — | 33 | — | 67 |
| MTR filtrates | — | 5 | — | ND | — | — | — | ND | — | ND |
| MTR particulates | — | 0.140 | — | 2,830 (79.2) | — | 28,500 | — | 55 | — | 32 |
| SDB filtrates | — | 5 | — | 42.2 | — | — | — | 0 | — | 100 |
| SDB particulates | — | 0.105 | — | 68,600 (1,440) | — | 798,000 | — | 18 | — | 23 |
| Sediments | ||||||||||
| A-17 | 41.1 | 42.0 | 257 | 122 | 47,600 | 23,800 | 8 | 15 | 21 | 21 |
| R-61 | 39.3 | 40.6 | 23.9 | 7.1 | 2,840 | 1,270 | 6 | 0 | 25 | 44 |
| MTR | 41.9 | 44.1 | 16.5 | ND | 4,690 | ND | 13 | ND | 43 | ND |
| SDB | 34.1 | 38.3 | 983 | 898 | 120,000 | 108,000 | 6 | 11 | 11 | 15 |
| Sediment trap particulates | ||||||||||
| A-17 (1 m) | 47.6 | NRh | 325 | NR | 17,200 | NR | 13 | NR | 15 | NR |
| A-17 (5 m) | 46.8 | 4.58 | 270 | 224 | 12,100 | 7,650 | 17 | 13 | 16 | 22 |
| R-61 (1 m) | 5.61 | 6.96 | 145 | 120 | 5,900 | 4,690 | 7 | 6 | 29 | 29 |
| R-61 (5 m) | 9.60 | 6.96 | 163 | 130 | 5,960 | 4,030 | 7 | 12 | 23 | 31 |
- a In g dry wt. for solid and L for aqueous samples.
- b In ng/g dry wt. for solid samples and ng/L for aqueous samples; the numbers in parentheses are concentrations based on the aqueous volumes, in ng/L.
- c In ng/g total organic carbon (TOC) for solid samples; TOC was not measured for aqueous samples.
- d Percent of the concentrations from naphthalene to 3,6-dimethylphenanthrene (see Table 1) relative to total PAHs.
- e Percent of the concentrations of fluoranthene (FL) and pyrene (PYR) relative to total PAHs.
- f —, not analyzed; see text for reasons.
- g ND, not detectable.
- h NR, not recovered. A-17 1-m traps were not recovered in June 1994.
Tijuana River runoff. The runoff samples were collected during low flows. The runoff filtrate of June 1994 had higher PAH concentrations than did the sample from January 1994 (Table 2). This difference likely resulted from the different sample volumes used for analyses, as in the case of the PLWTP effluent. The total PAH concentration based on the aqueous volume was similar in the particulate and aqueous portions of the January sample, but was much higher in the filtrates than in the particulates of the June sample. Again, this discrepancy was partially due to the large differences in total suspended solid concentrations.
Microlayer. In processing the microlayer samples collected in January 1994, black ribbon filter papers were used to hold anhydrous sodium sulfate for removal of water content from extracts. Excessive, mostly unresolved peaks were found in the chromatograms of these samples, as well as in those of procedural blanks (pure solvent going through the extraction processes). Gas chromatography—mass spectrometry (GC-MS) analyses indicated that ion fragments typical of hydrocarbon compounds (e.g., m/z 59, 71, 85, and 99) were dominant. Due to the possible interference with sample quantitation, the analytical results for these samples were abandoned. Baked glass wool replaced the filter papers in the subsequent extraction of the other samples. The same interfering peaks did not appear in the procedural blanks and samples extracted later.
Among the four microlayer samples, only the SDB sample contained detectable PAHs in the filtrates (Table 2). The particulate PAH concentration was highest in the SDB sample, with a decreasing trend of MTR > A-17 > R-61. The MTR particulate sample had more abundant (55%) with two- and three-ring PAHs than all the other samples.
Sediments and sediment trap particulates. The magnitude of sediment PAH contamination followed the order of SDB > A-17 > R-61 > MTR (Table 2). In addition, the PAH concentrations were higher in the samples collected in January than those collected in June. The A-17 sediment trap particulates contained slightly higher PAH concentrations than the R-61 particulates, but the difference was not as substantial as in the case of sediments. Again, the samples collected in January contained slightly higher PAH concentrations than those collected in June.
| Site | Collection date | Level off bottom (m) | Particulates (g/m2/d) | TOC (g/m2/d) | TN (g/m2/d) | PAHs (μg/m2/d) |
|---|---|---|---|---|---|---|
| A-17 | December 21, 1993-January 20, 1994 | 1a | 255.2 | 4.82 | 0.459 | 82.9 |
| 5b | 89.4 | 1.99 | 0.189 | 24.1 | ||
| May 30, 1994-June 29, 1994 | 1c | — | — | — | — | |
| 5a | 22.0 | 0.65 | 0.070 | 4.9 | ||
| R-61 | December 21, 1993-January 20, 1994 | 1a | 87.8 | 2.16 | 0.214 | 12.7 |
| 5b | 55.8 | 1.52 | 0.156 | 9.1 | ||
| June 30, 1994-January 20, 1994 | 1a | 34.7 | 0.89 | 0.090 | 4.2 | |
| 5d | 14.6 | 0.47 | 0.051 | 1.9 |
- a Three traps.
- b Nine traps.
- c Traps lost.
- d Eight traps.
Vertical fluxes of organic materials at A-17 and R-61
The fluxes of particulates, TOC, TN, and total PAHs at A-17 and R-61 varied spatially and temporally (Table 3). The flux of particulates at each depth was the average of multitrap measurements. Fluxes of TOC, TN, and PAHs were calculated directly from the concentrations of TOC, TN, and PAHs and the fluxes of particulates. Due to insufficient sample amounts for the PAH measurements in the May-June 1994 sediment trap particulates, PAH concentrations were possibly underestimated in these samples.
The maximum flux of particulates was 255.2 g/m2/d1 at the A-17 1-m traps deployed between December 21, 1993, and January 20, 1994. The A-17 1-m traps were lost in the second sampling during May-June 1994, hence no temporal comparison can be made at this depth. A substantial decline in fluxes of particulates between January and June was obvious at all other traps. Because TOC, TN, and PAH contents were only slightly higher in the sediment trap particulates collected in June 1994 than those collected in January 1994, the temporal variation in the fluxes of TOC, TN, and PAHs followed the same pattern as did the fluxes of particulates.
DISCUSSION
PAH origins: Compositional indices
The PAH assemblages in the samples can be divided into two distinct groups based on their compositional patterns. The first group includes PLWTP effluent, Tijuana River runoff, and MTR microlayer particulates which were dominated by low molecular weight PAHs, that is, a high proportion of two-, three-ring PAHs (38-100%, Table 2). The PAH compositions in the second group (all the sediments, sediment trap particulates, and microlayer particulates except for MTR microlayer particulates) were characterized by the predominance of four-, five-ring PAH compounds.
Alkylated naphthalenes were more abundant than the parent compound in both the PLWTP effluent filtrate and particulate samples, although the patterns were slightly different (Fig. 2a and 2bb). The relatively high abundance of alkylated naphtha lenes suggested that PAHs (especially those with low molecular weights) received by the PLWTP were mostly petroleum related. The PCDs in these samples could not be determined, because almost all the concentrations of the related parent compounds were not detectable (Fig. 3a and b).
Among the sediment and sediment trap samples from A-17 and R-61, only the A-17 1-m sediment trap sample collected in January 1994 contained a recognizable AHD pattern (Fig. 2c through e; R-61 samples not shown), which was similar to those in the PLWTP effluent samples, suggesting that low molecular weight PAHs were likely derived from petroleum-related sources. On the other hand, PCDs determined in these samples (Fig. 3c through e; R-61 samples not shown) were similar to those found in the sediments from Narragansett Bay (Rhode Island), which is believed to be contaminated by combustion-generated PAHs [5]. Apparently, PAH assemblages in the water column particles and sediments at A-17 and R-61 originated from both combustion and petroleum-related sources. For the microlayer samples collected at A-17 and R-61, only a few mid-range molecular weight PAHs were detected in the particulates. Thus, no AHDs and PCDs could be accurately determined. As also discussed in the following section, nonpoint source inputs, presumably produced by combustion-related processes, may exert a greater impact on the microlayer than the PLWTP outfall.
Both AHD and PCD in the Tijuana River runoff filtrate sample collected in June 1994 (Figs. Fig. 2., Fig. 3.) suggest a petrogenic source for PAHs. As noted previously [8], a large percentage of the Tijuana River discharge has reportedly been raw sewage and industrial and agricultural wastes, the composition of which may be comparable to that of the wastes received by the PLWTP. In addition, only 3% of the drainage land of the Tijuana River on the U.S. side is urban and suburban [7]. Therefore, combustion-derived PAHs that were normally abundant in urban riverine runoff [16-18] should not be the major components in the Tijuana River runoff.
Unlike the other microlayer samples, the MTR microlayer particulates contained high concentrations of low molecular weight PAHs, indicating a predominant petrogenic origin for PAH compounds. This observation was corroborated by the patterns of AHD and PCD in this sample (Figs. Fig. 2., Fig. 3.). By contrast, the MTR sediment displayed a low proportion of 2,3-ring PAHs (Table 2); its AHD and PCD patterns were indicative of predominant pyrogenically produced PAHs, showing little or no effect from Tijuana River runoff.
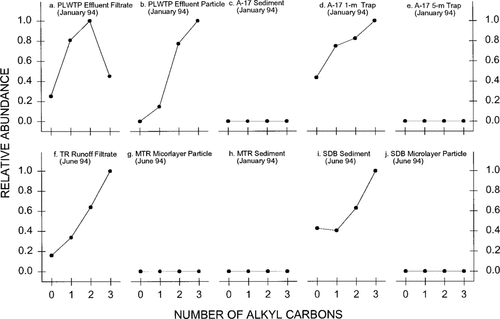
Alkyl homologue distributions of naphthalene in selected samples collected off San Diego, California.
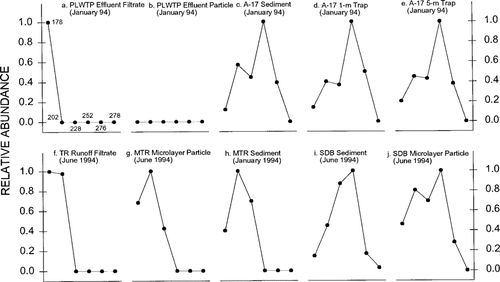
Parent compound distributions in selected samples collected off San Diego, California.
The relative abundance of four- to six-ring PAHs in the SDB sediments ranged from 89 to 94% (Table 2), similar to the result (∼90% were four- to six-ring PAHs) of McCain et al. [19]. This may indicate a consistent source, predominantly combustion related, of PAH inputs into the study site. However, the AHD (Fig. 2i) also showed possible petrogenic PAH inputs, at least for low molecular weight PAHs. On the other hand, the PCD (Fig. 3i) in the sample was similar to those in sediments contaminated with pyrogenic PAHs [5]. Apparently, PAHs in this sediment may have derived from both petroleum and combustion sources. The microlayer particulate sample was clearly contaminated with PAHs of combustion origin, as indicated by the similar PCD patterns in Figure 3j and reported by Lake et al. [5] for sediments containing predominantly combustion-derived PAHs. The AHD in this sample (Fig. 2j) also pointed to a similar conclusion. The microlayer filtrate sample contained extremely low PAH concentrations and therefore neither AHD nor PCD was determined. The only PAHs detected in the filtrate sample were fluoranthene and pyrene, indicating a combustion-related origin for PAHs.
The major sources of PAH inputs into the SDB site may be a large storm runoff outfall draining the San Diego International Airport, engine exhausts from boats and ships, and accidental oil spills. Without detailed analyses of samples collected from these sources, it is impossible to quantify the contributions of PAHs from each of these sources. Because the proportion of two-, three-ring PAHs was less than 20% in all the SDB sediment and microlayer samples (Table 2), PAHs from combustion sources appeared to prevail. This might effectively disqualify oil spills from contributing significant amounts of PAHs. Automobile exhausts, probably similar to boat engine exhausts, are known to contain both petroleum residues and incomplete combustion products [20, 21].
It may be concluded from the above discussion that PAHs in the PLWTP effluent and Tijuana River runoff were predominantly derived from petroleum-related sources. Sediments and water column particulates near the PLWTP outfall received PAHs mostly from combustion sources, with minor inputs of low molecular weight PAHs from petrogenic sources, probably the PLWTP outfall and natural oil seepage. Despite heavy shipping activities in San Diego Bay, oil spills did not appear to be the important source for the PAH contamination in sediment and microlayer. Instead, combustion-related sources, for example, engine exhausts and urban surface runoff, seemed to contribute significant amounts of PAHs to San Diego Bay.
To corroborate the above conclusions, we examined the ratios of MP/P, P/A, FL/PYR, and BZ[a]A/CHR in the same samples (Fig. 4). In general, petroleum-contaminated samples contain higher MP/P, FL/PYR, and P/A and lower BZ[a]A/ CHR than samples containing combustion residues [4].
For MP/P, the highest ratios were found in the PLWTP effluent particulates and Tijuana River runoff filtrates. In addition, the PLWTP effluent filtrates also showed a fairly high MP/P ratio. Nondetectable methylphenanthrene in MTR microlayer particles and sediments yielded a zero MP/P ratio for these samples. The SDB microlayer particulates also had a small MP/P ratio. Similarly, the highest P/A ratio occurred in the PLWTP effluent particulates. Because anthracene was not detectable in the PLWTP effluent filtrates, Tijuana River runoff filtrates, and MTR microlayer particulates, a very high P/A ratio would be expected for these samples. The SDB micro-layer particles and sediments had the smallest P/A ratios.
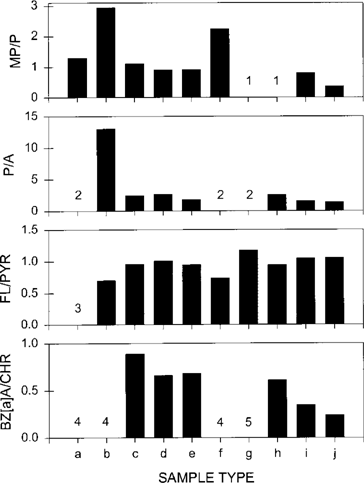
Ratios of methylphenanthrene to phenanthrene (MP/P), phenanthrene to anthracene (P/A), fluoranthene to pyrene (FL/PYR), and benzo[a]anthracene to chrysene (BZ[a]A/CHR) in selected samples collected off San Diego, California. The sample type follows the same order as used in Figures Fig. 2., Fig. 3.. (1) Concentrations of methylphen-anthrenes were below detection limits. (2) Concentrations of anthracene were below detection limits. (3) Concentrations of both fluor-anthene and pyrene were below detection limits. (4) Concentrations of both benz[a]anthracene and chrysene were below detection limits. (5) Concentration of benz[a]anthracene was below detection limit.
For BZ[a]A/CHR, both benz[a]anthracene and chrysene were not detectable in the PLWTP effluent (both filtrates and particulates) and Tijuana River runoff filtrates. Benz[a]anthracene was not found in the MTR microlayer particulates. Because benz[a]anthracene and chrysene are normally abundant in samples contaminated with combustion-derived PAHs, the low levels of these compounds indicated a predominantly petroleum-related origin. As a comparison, the A-17 and MTR sediments and A-17 sediment trap particulates displayed relatively large BZ[a]A/CHR ratios. The trend of the BZ[a]A/CHR ratios was consistent with those observed for MP/P and P/A. These results are generally in agreement with the conclusions drawn above based on the patterns of AHDs and PCDs.
In contrast, the FL/PYR ratio did not show any consistent trend among the samples examined. In particular, the PLWTP effluent particulate and Tijuana River runoff filtrate samples had relatively low FL/PYR ratios, whereas the SDB microlayer particulate and sediment samples possessed high FL/PYR ratios. As observed by Colombo et al. [4], the range of the FL/PYR ratio did not vary much (0.5-0.9) for a variety of sediments.
It should be noted that the generally low levels of PAHs found in the sample media limit the applicability of compositional indices in the identification of PAH sources. Many other factors, such as solubility and extent of biodegradation, may also contribute to the alteration of PAH assemblages after they are released into the environment. In this sense, the above compositional analyses are qualitative at best. If possible, other molecular indices should be combined to enhance the confidence of source identification. For instance, alkanes have also been found useful for source identification [4].
Assessment of PAH inputs from point and nonpoint sources
There are two major point sources of PAHs in the study area: the PLWTP sewage outfall and Tijuana River. Nonpoint sources may include aerial fallout, inadvertent oil spills, and marine oil seepage. The PAH inputs from the PLWTP outfall and Tijuana River may be estimated from this study and relevant information in the literature. Contributions from non-point sources, however, are difficult to quantify; only a qualitative assessment can be made.
From the PAH concentrations in the PLWTP effluent (Table 2) and the average daily effluent flow of 171.7 million gallons (6.50 × 108 L) in 1994 [22], the average mass emission of total PAHs from the PLWTP outfall is estimated to be 395 kg in 1994. Estimation of the PAH mass emission from the Tijuana River is somewhat difficult, as the samples were collected during low flows and the total water discharge in 1994 is unavailable. If a long-term annual mean flow of 4.29 × 1010 L from 1955 to 1988 [8] is used, the average mass emission of PAHs during 1994 was roughly 2.2 kg. This estimate may still be in the same order of magnitude even if data during storm events were applied, because PAH concentrations might be slightly lower during storms, as in the case of oil and grease measured in Los Angeles River runoff [23].
Eganhouse and Gossett [24] estimated the mass emission of a similar suite of total PAHs to be 110,500 kg for the Joint Water Pollution Control Plant of the County Sanitation Districts of Los Angeles County in 1979. If a mass emission ratio of 3.4:1 for aromatic hydrocarbons determined in the effluents of this plant and PLWTP in 1979 [25] was used to correct for PAHs, the PAH mass emission from PLWTP would be approximately 32,500 kg in 1979. Thus, it appears that PAH emissions from the PLWTP outfall have been reduced by a large factor over the last 15 years, probably attributable to dramatic source reduction from petrochemical inputs and improved treatment processes. For comparison, our results suggest that the PAH contribution from the Tijuana River appeared to be insignificant.
Because the microlayer reflects recent inputs of contaminants, the microlayer data may be useful in identifying the in situ sources of contaminants. The percentages of fluoranthene + pyrene relative to total PAHs were 100% and 67% in the A-17 and R-61 microlayer, respectively; these were much higher than those found in the PLWTP effluent (Table 2). Because fluoranthene and pyrene are generally considered to be combustion-related PAHs [17, 26], the dominance of fluoranthene + pyrene in the A-17 and R-61 microlayer suggests that aerial fallout possibly has greater impact on the sea surface micro-layer than the inputs from the PLWTP outfall, even though A-17 is close to the outfall. However, without the knowledge of atmospheric PAH deposition rates in the area, the relative importance of the contributions from the PLWTP outfall and aerial deposition cannot be quantified.
By contrast, the PAH compositions were similar in MTR microlayer and Tijuana River runoff (both containing relatively high portions of two-, three-ring PAHs), underscoring the influence of the Tijuana River. The higher PAH concentration (2,830 ng/g) in MTR microlayer particulates, compared to those in TR runoff (158 and 104 ng/g) (Table 2), confirmed a substantial enrichment of PAHs in the sea surface microlayer [27, 28].
The fluxes of particulates at A-17 (both 1-m and 5-m depths) were greater than those at R-61 (Table 3). In addition, the particulate fluxes at both A-17 and R-61 were greater in January 1994 than in June 1994. Fluxes of particulates, TOC, TN, and PAHs at the 5-m depth of A-17 were similar to those at the 5-m depth of R-61 measured in June 1994. As mentioned previously, discharge from the PLWTP outfall extension began in November 1993, which increases the distance between the discharge site and station A-17. All these suggest that the PLWTP sewage outfall was an important source of particulates and organic matter in the surrounding water column. It should be noted that the particulates may also carry materials from nonpoint sources into the sediment traps (more discussions below), thus amplifying the organic matter fluxes.
Baker et al. [29] estimated that 3% of sewage particles rose to the surface in a laboratory experiment within 24 h, whereas 22% settled onto the bottom and 75% remained in suspension. If these results are applicable in the coastal marine environment, then the water column dynamics must be evaluated to better understand point and nonpoint inputs.
Water column dynamics
Sedimentation processes in the water column can be extremely complex. As noted previously [24], petroleum-related PAHs, enriched on the surfaces of sewage-derived particles, were more vulnerable to biodegradation and/or desorption in the water column during deposition. Therefore, sinking organic materials may undergo rapid degradation or desorption (especially for low molecular PAH compounds), leading to depleted TOC and PAH concentrations in sediments compared to water column particulates. On the other hand, resuspension of selective fractions of sediment particulates may magnify or compromise this effect.
From the results presented thus far, several observations can be summarized: (1) PAH compositions in the sediments and sediment trap particulates were similar, but substantially different from those of the PLWTP effluent; (2) the A-17 sediment trap particulates contained higher TOC, TN, and PAH concentrations (dry weight) than the A-17 sediments, but the TOC-normalized PAH concentrations were higher in sediments than in the sediment trap particulates; (3) the R-61 sediment trap particulates contained higher TOC, TN, and PAH concentrations (both dry weight and TOC normalized) than the R-61 sediments; (4) there was a trend of increasing TOC and TN contents from 1-m trap to 5-m trap particulates for both A-17 and R-61; (5) PAH concentrations (both dry weight and TOC normalized) decreased from the 1-m trap to the 5-m trap at A-17, but they were similar in the 1-m and 5-m traps at R-61. These observations may reflect the water-column dynamics related to a number of processes, for example, mixing and transport of organic matter from point and nonpoint sources, bottom sediment resuspension, and biochemical degradation.
Observation 1 implies that sources other than the PLWTP outfall contribute considerably to the PAHs present in the water column and sediments, or that degradation and/or desorption of PAHs may occur rapidly in the water column. The following information supports the first mechanism. The sediment dry weight-based PAH concentrations in A-17 sediments and trap particulates were about 20 to 150 times lower than those found in the PLWTP effluent particulates, whereas the TOC-normalized concentrations in these samples were much less different (Table 2). If PAHs in the water column or sediments were solely from the PLWTP effluent, degradation or desorption would yield lower TOC-normalized concentrations of PAHs in the water column particulates and sediments, assuming TOC is the main component for partitioning of PAH in particles. On the other hand, the similar PAH compositions in A-17 and R-61 sediments and sediment trap particulates may underscore the influence on the depth dependence of particle fluxes from sediment resuspension by near-bottom currents (Table 3). Resuspension of sediment particulates occurs over a short vertical distance, magnifying the flux of sinking particles near the bottom [11].
Observation 2 suggests that at station A-17, the sediment traps collected sinking sewage-derived, high-density particulates. Organic materials settling with particulates from the upper water column may undergo rapid degradation, resulting in depleted TOC and TN concentrations in the sediments [11]. The higher TOC-normalized PAH concentrations in the sediments were mainly a result of much lower TOC contents in the sediments than in the sediment trap particulates. This might indicate that PAHs degraded much slower than sewage-derived organic carbon.
Observation 3 probably points to a long-range transport mechanism carrying sewage-derived organic matter to remote locations, such as R-61. Low-density sediment particulates highly enriched with PAHs [30, 31] (presumably also enriched with TOC) may tend to stay in suspension and are transported by currents to adjacent areas. These particulates may eventually aggregate with each other or with high-density particulates from other sources and finally sink to the sea floor. Because the sedimentation process is relatively slow for low-density particles, organic materials may experience biodegradation, fractionation, and transformation.
Observation 4 further supports the hypothesis of rapid degradation for organic materials during sedimentation, or that the settling particles normally contain low contents of organic matter. Prahl and Carpenter [30] suggest that combustion-derived PAHs do not preferentially stay in finer grained sediment particles of greater surface area and are not easily biodegraded or desorbed from particles. Hence, observation 5 might be due to a combination of slow degradation of sinking combustion-derived PAHs and resuspension of low-density particles enriched with PAHs from the bottom sediments.
Acknowledgements
We are indebted to Harold Stubbs, Dario Diehl, Andrew Jirik, Larry Cooper, Tim Rothans, Jack Russell, Richard Mange, and Mike Kelly for sample collection; the City of San Diego Metropolitan Wastewater Department for the use of R/V Monitor III; M. Indira Venkatesan and Dave Young for critically reviewing the manuscript; and Azra Khan, Charlie Yu, and Kim Tran for technical assistance.




