Measurements of pesticide spray drift deposition into field boundaries and hedgerows: 2. Autumn applications
Abstract
Tractor spray applications of a pyrethroid insecticide combined with a fluorescent tracer were applied to cereal field edges on four separate occasions in the autumn. Spray drift measurements were made in field boundaries adjacent to conventionally sprayed and 6-m-wide unsprayed “Conservation Headlands,” which act as buffer zones designed to keep the outermost edge of the crop unsprayed with certain pesticide groups. The volume of drifting spray reaching field boundaries was dependent upon wind conditions and was significantly reduced by the use of these buffer zones. Comparisons of spray drift are made with comparable experiments carried out in summer cereals (reported in an accompanying paper). In a separate experiment, high mortalities of Spodoptera littoralis Boisd. larvae were recorded when they were exposed to grasses collected from hedge bottoms adjacent to conventionally sprayed headlands, and from additional spray applications made with a hand-held sprayer (used to simulate the levels of drift recorded in field trials). Larval mortalities were reduced, but still detectable, when grasses contaminated with drift adjacent to Conservation Headlands were bioassayed. In light of these findings, the current recommendation of these 6-m-wide buffer zones in cereals is discussed.
INTRODUCTION
Previous investigations have measured pesticide drift from tractor-mounted spray booms [1, 2] and shown that the drifting volume is dependent upon wind speed and distance downwind from the sprayer. The effects of the driftable fraction of pesticide on certain lepidopteran species that can be found inhabiting arable field boundaries have been studied under both laboratory and field conditions and have been shown to pose an important risk to these populations [2-5]. The factors determining the extent of damage caused by this misapplication of agrochemicals to hedgerow flora and fauna have been reviewed by Freemark and Boutin [6] and Longley [7].
Under a normal “conventional” spraying regime within cereal crops, the end of a tractor spray boom can pass within 1 m or less from the front edge of a field boundary. The previous study [2] found that the level of pesticide drift resulting from spraying cereal crops in the summer can cause up to 100% mortality of the large cabbage white butterfly larvae (Pieris brassicae [L.]) exposed to residual deposits of pesticides drifted onto adjacent hedge-bottom grasses. However, the volume of spray drift entering the field boundary was shown to be significantly reduced when a “Conservation Headland” regime (consisting of leaving the outer 6-m-wide strip of crop unsprayed) was employed. This work led to the conclusion that the mature crop and tall hedge-bottom vegetation filtered out drifting droplets, thereby minimizing hedgerow contamination. This filtering effect has important consequences for the conservation of nontarget plants and invertebrates within field boundaries. In order to compare the environmental risks posed by spray applications during different times of year, this paper reports an investigation of pesticide drift during an autumn spray campaign when no mature crop or tall hedge-bottom vegetation existed. The pattern and level of drift deposition into field boundaries adjacent to unsprayed Conservation Headlands and into hedgerows that were not protected by an unsprayed strip were investigated, with the addition of bioassays involving the exposure of lepidopteran larvae to contaminated grasses.
MATERIALS AND METHODS
On four separate occasions during November and December 1995, pesticides and chemical tracers were applied by tractor-mounted sprayers when the wind was detectable and blowing directly towards a hedgerow. The range of wind conditions spanned those likely to be encountered during commercial spraying. The methodology for the four experiments remained the same, but different field boundaries (all similar in physical structure) were used.
Spray application
On each occasion, the tractor made a single pass along the outer edge of the field delivering spray at a volume rate of 200 L/ha with a 8.5-kph forward speed and a tank pressure of 42 psi. The boom was approximately 55 cm above the ground and its end was 1 m from the hedge bottom. The field was covered by wheat stubble at an approximate height of 5 cm. This represented a similar vegetation cover to that found with cereal crops during this time of year. The hedgerow was divided into eight plots of 15-m lengths, separated by 10-m gaps. Spray was delivered from the full 24-m boom adjacent to four randomly allocated plots to give the “conventionally sprayed” headland treatment. Adjacent to the remaining four plots, spray from the end 6 m of boom (next to the field boundary) was switched off to give 6-m-wide unsprayed buffer zones. The hedge bottom, used here to describe the bank of vegetation extending out from the hedge, was approximately 1.5 m wide, consisting of a grassy sward reaching a maximum height of 20 cm. In the center of each plot, three bamboo canes, spaced 30 cm apart, were placed against the hedge and three additional canes were placed at the front edge of the hedge bottom. Plastic drinking straws (6-cm length, 0.37-cm diameter), used for collecting spray droplets, were attached vertically and slightly separated in groups of four on canes, facing the crop, at heights of 0, 0.5, 1.0, and 1.5 m above the ground. The method of collection relied upon the impaction of droplets on the outer straw surface. Records of wind speed were kept at 5-s intervals using an anemometer held at boom height. Mean air temperature was recorded at 1 m above the ground.
Volumetric analysis of spray deposition
The pyrethroid insecticide deltamethrin was applied with a formulation of 0.03% (w/v) fluorescein (Acid Yellow 73, Aldrich, UK) in 0.05% (v/v) wetting agent (Farmon Blue, 900 g/L, nonionic wetting agent, Farm Protection Limited, Cambridge, UK). Such fluorescent pigments are reported to be very stable and are recommended for the quantitative interpretation of spray deposits on any substrate [8]. Tracer was recovered from the straw samplers within 1 h of application by washing them in phosphate buffer solution (pH = 6.9) (technique fully described in Čilgi and Jepson [9]). The samples were analyzed in a Perkin-Elmer LS3-B fluorimeter with 490 nm and 515 nm excitation and emission wavelengths, respectively. Calibration was against samples from the original spray solution. These readings were converted to the equivalent volume of formulated tracer landing per square centimeter of collector, assuming only one outer side was exposed to drifting spray. The results should therefore be taken as relative and not absolute values, as in reality the air flow around the straw collectors may have deposited small droplets on all sides.
Insect bioassay
After spray application in experiments 3 and 4, approximately 30 stalks of grass were excised at ground level, adjacent to the canes, from the front edge of the hedge bottom in each plot. The cut stalks were placed into labelled 9-cm-diameter pots of damp, fine sand. This substrate provided sufficient support and moisture for the grasses over the following 48 h. The pots were returned to a controlled-environment room (20°C, 16L:8D, 75% RH) and acetate columns (9-cm diameter, 26 cm tall) were placed over the pots. Ten second-instar Spodoptera littoralis Boisd. larvae, previously cultured on an artificial diet, were introduced to each pot and their condition was assessed after 24 h.
Log-dose spray application
An additional experiment involved the direct spraying of grasses at a range of pesticide concentrations followed by the exposure of S. littoralis to this vegetation. This was to verify and further predict mortality of larvae exposed to the doses of drifted pesticide measured during experiments 1 through 4 in the hedge bottom environment.
Grass turves of uniform composition were cut and potted into 9-cm-diameter pots. The grass height was approximately 3 cm. Pots of grass were placed on the ground at 2-m intervals along a 20-m spray transect. Four replicate transects were laid out. In each transect, the spray applications of deltamethrin were made using a Chesterford Miniature Logarithmic Sprayer (MDM Engineering Ltd., Portsmouth, UK) operating at 2 bar, and giving at swath width of 1 m. Walking speed was adjusted to give a precise volume application rate of 200 L/ha following individual calibration of the nozzles. The logarithmic dilution principle of the sprayer involves the chemical concentrate being forced out of a pressurized vessel at a constant rate and being replaced at an equivalent rate by a diluent (water) that is intimately mixed with it. This causes the concentration of the chemical to decrease exponentially. During application, if the operator's walking speed is constant, then the chemical dosage rate is proportional to both time and distance. Calibration of the sprayer was achieved by applying a red dye solution (Kenacid Scarlet, 1%, w/v) to 9-cm-diameter filter discs placed at 1-m intervals along a flat 20-m transect. The deposition of dye on each filter paper was measured colori-metrically. It was calculated that the dose of concentrate halved every 3 m along the transect with a constant walking speed of 1 m/s.
Solutions of deltamethrin, made up initially at the recommended field concentration (6.25 g AI/ha in 200 L of water), were applied along the transects. Walking commenced, and was maintained at a constant speed of 1 m/s, immediately when the spray emerged from the nozzles. The sprayer was set up to logarithmically dilute the initial concentrate to a final concentration of 1/100th of the original over the 20-m transect.
After spraying, the pots were returned to the controlled-environment room and acetate columns, as used in experiments 3 and 4, were placed over the pots. Pots of unsprayed grass were used as controls. Five third-instar S. littoralis were placed in each pot and their condition was assessed after 48 h.
RESULTS
The mean wind speeds recorded for each experiment are indicated in Figure 1. Deposition of spray was measured in the field boundaries during all the experiments. The volumes of drifted pesticide were related to the wind speed, with the highest deposition (0. 16 μ1/cm2) recorded in experiment 3, which was carried out during a mean wind speed of 4.0 m/s (Figs. Fig. 1., Fig. 2.). Higher volumes of deposition were recorded on straw samplers located next to the conventionally sprayed headland compared to those next to unsprayed headlands. However, small volumes of spray drift were still detected in the latter, especially during the higher wind speeds, indicating that the 6-m-wide unsprayed headland was not sufficient to offer complete protection to the adjacent field boundary vegetation. In all experiments, the samplers located at the back of the hedge bottom received lower volumes of spray drift than those positioned at the front.
The volumetric data were converted to a proportion of the maximum theoretical volume deposition rate that could have occurred. This maximum was defined as the volume that would result if the full rate of 200 L/ha was deposited upon a perfectly flat field (i.e., 2 μ1/cm2). During the recorded mean wind speeds of between 1 and 4 m/s, the percentage drift into field boundaries adjacent to fully sprayed headlands was between 1 and 7%. These levels were much greater than those previously recorded during summer spraying [2] at comparable wind speeds (Fig. 2).
Mortalities of 25% and 29% were recorded with S. littoralis larvae exposed to grasses collected from adjacent to fully sprayed headlands in experiments 3 and 4, respectively. These mortalities were significantly greater (one-way ANOVA: F = 17.67, d.f. = 15, p < 0.001; F = 51.69, d.f. = 15, p < 0.001) than the 5% and 2% mortalities recorded in the larval population exposed to grasses adjacent to conservation headlands in the respective experiments (Fig. 3).
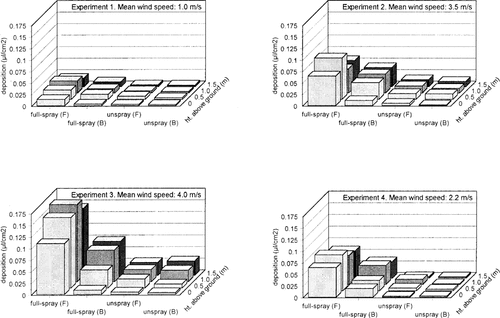
Mean volume deposition rate (μ1/cm2) of spray formulation on straw targets placed at different heights at the front (F) or back (B) of a hedge bottom, adjacent to fully sprayed (full-spray) or unsprayed (unspray) headlands, during four different experiments.
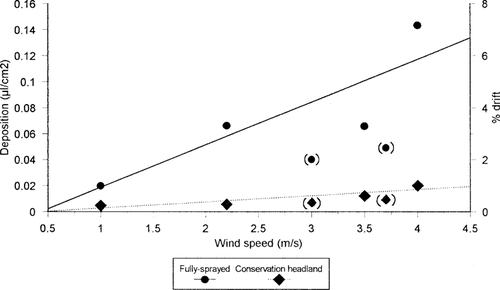
Mean volume deposition rate (μ1/cm2) of spray formulation on the straw targets, at the four different heights, positioned at the front edge of a hedge bottom adjacent to fully sprayed or unsprayed Conservation Headlands. Data are also expressed as percentage drift (see text for explanation). Symbols in parentheses indicate deposition levels recorded during the summer spray campaign (details in Longley et al. [2]), and are not incorporated in the regression line.
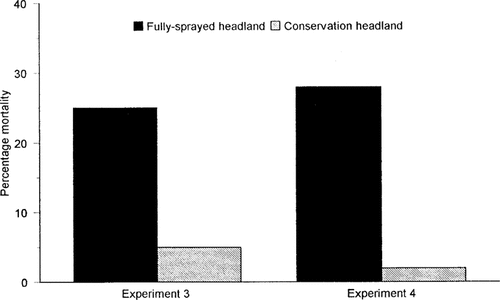
Percentage mortality of Spodoptera littoralis larvae exposed to grasses collected from the hedge bottoms in experiments 3 and 4, bordered by a fully sprayed headland or unsprayed Conservation Headland.
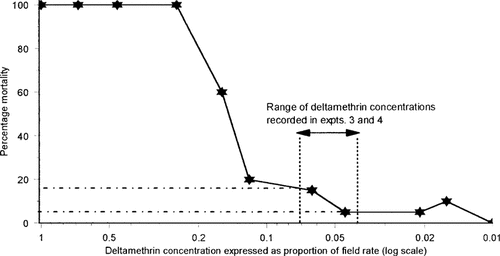
Percentage mortality of Spodoptera littoralis larvae exposed to grasses sprayed with a range of deltamethrin concentrations. The area between the dotted lines represents the predicted range of concentrations recorded in field boundaries adjacent to fully sprayed headlands in field experiments 3 and 4.
With the log-dose sprays, larval mortality reached 100% after exposure to grass sprayed with deltamethrin concentrations greater than 1/4 of the recommended field rate (Fig. 4). The range of deltamethrin concentrations equivalent to those contained in the percentage volumes of drift recorded at the front edge of the hedge bottoms adjacent to fully sprayed headlands in experiments 3 and 4 were predicted. From the log-dose sprayer data, these concentrations (equivalent to between 6 and 7% of field rate) would result in a predicted larval mortality of between 5% and 15%. (Fig. 4). These values are lower than the actual observed mortalities of 25 to 29% recorded for larvae exposed to these concentrations in experiments 3 and 4 within the band of wind speeds observed (Fig. 3).
DISCUSSION
The sampling method used in this study relied primarily upon the process of droplet impaction on the outer vertical surface of the straw collectors. Some additional spray may have entered the straw openings through sedimentation; however, this process remained unquantified and the straws were left open at the bottom. The collection efficiencies of a wide range of drift collectors have been demonstrated to commonly be in the order of 50% or less [10]. However, small-diameter vertical plastic rods (similar in dimension to the collectors used in the present study) were shown to be efficient collectors of droplets of a wide spectrum, especially of small droplets that are likely to be deposited on narrow plant stems and insects [10, 11]. At present, the measured levels of drift can only be taken as relative and not absolute values. The sampling efficiency of the passive collectors needs to be investigated and validated with actual deposition measurements on vegetation, thus allowing future work on drift deposition rates to be translated to quantities of pesticide active ingredient.
For the purpose of comparisons between treatments in the current work, the levels of drift have been expressed as percentages of the maximum theoretical volume deposition rate that could have occurred directly under the spray boom. Further work is required to validate the correlation of deposition levels occurring on both vertical and horizontal substrates, as the majority of deposition on the straw collectors was likely to have occurred through lateral impaction of droplets rather than direct fallout from above.
Higher volumes of spray drift in field boundaries were measured in the current investigation compared to identical experiments conducted during a summer spray campaign [2] during comparable wind speeds. The major differences between the seasons were the absence of a mature cereal crop and tall vegetation in the hedge bottom during the autumn sprays, and the heights of the spray boom, which were at 1.5 m (summer) and 0.55 m (autumn) above the ground. Wind speed and turbulence could have been assumed to have been less at the lower boom height, resulting in reduced spray drift. However, the absence of any “filtering” of drifting spray droplets by crop vegetation is likely to have been the major factor leading to the higher levels of field boundary contamination during autumn sprays.
At present, the Game Conservancy Trust's guidelines on Conservation Headlands are that no insecticides are to be used on the outer 6 m of crop after March 15. However, insecticides may be used on these headlands in autumn to control aphid vectors of Barley Yellow Dwarf Virus [12]. As the current research indicates that large volumes of pesticide drift can occur into field boundaries during autumn spraying, overwintering populations of beneficial invertebrates may be at risk. However, the adoption of unsprayed headlands significantly reduced the volume of field boundary contamination, resulting in lower mortality of exposed S. littoralis larvae. It would therefore seem rational to adopt such a management regime during autumn spraying in windy conditions, especially near the downwind field boundaries or adjacent to sites requiring protection, such as nature reserves or waterways. However, even with the unsprayed headlands, spray drift was still measured in field boundaries during both the summer spraying [2] and to a greater extent during the autumn sprays reported here. This therefore raises the question as to whether these buffer zones are of optimum size. Historically, The Game Conservancy Trust's recommendation of leaving unsprayed headlands of 6-m width was not based on research findings, but chosen purely as a pragmatic approach, whereby the outermost section of a spray boom (in most cases, the outermost 6 m) could be switched off when spraying around headlands. Within an average-sized field (16-ha square) this involves carrying out the guidelines on about 6% of the cereal area. These buffer zones have been suggested as adequate for the protection of semi-natural vegetation [13, 14] and honeybees [15] downwind of the spray boom. However, recent studies made with larvae of P. brassicae have estimated that buffer zones need to be increased to between 16 and 24 m to limit mortality to below 10% during moderate wind conditions [16]. These large buffer zones were estimated from bioassays involving larvae openly exposed on potted seedlings with no surrounding vegetation. This represents an unrealistic scenario for predicting conditions during summer sprays, but more closely matches the reality found during autumn spray campaigns where no mature crop or tall hedge-bottom vegetation exist.
Higher mortalities of S. littoralis larvae were recorded with exposure to grasses collected from the field experiments compared to those exposed to log-dose spray applications of equivalent concentration. These differences may have resulted from using third-instar larvae in the latter bioassays as opposed to second instars, as it has been shown that older, larger lepidopteran larvae are less susceptible to insecticides than younger instars [17, 18].
It is evident that field boundary contamination by drifting pesticides can be avoided or at least minimized if spraying is carried out at low wind speeds and by adopting 6-m-wide Conservation Headlands. However, further studies are needed to determine the optimum width of buffer zones needed to protect field margins during autumn spraying in cereal fields with wind speeds below 3 m/s, as recommended by the Ministry of Agriculture, Fisheries and Food, UK [19]. Any additional extension of the buffer zones would prove beneficial. For example, the consistently lower deposition levels of pesticide recorded during the current study at the back of the hedge bottom, compared to the front, indicated that the additional 1.5-m distance from the spray boom was important in reducing the volume of airborne droplets.
The toxicologic risks posed by the levels of pesticide drift recorded in this study upon other beneficial invertebrates overwintering in field boundaries (e.g., linyphiid spiders) need to be investigated. It must be borne in mind, however, that before producing and issuing revised guidelines to farmers about the implementation of Conservation Headlands, the management, husbandry, and fiscal costs, as well as environmental benefits, must first be considered.
Acknowledgements
We would like to thank the management and farm staff of the Manydown Company for their spraying expertise, and Sue Thomas for field work assistance.




