Evaluation of temporal and age-related trends of chemically and biologically generated 2,3,7,8-tetrachlorodibenzo-p-dioxin equivalents in lake Ontario lake trout, 1977 to 1993
Abstract
Levels of selected non-, mono-, and di-ortho-substituted polychlorinated biphenyl (PCB) congeners, polychlorinated dibenzo-p-dioxins (PCDDs), and polychlorinated dibenzofurans (PCDFs) were determined in 4-year-old lake trout from the eastern basin of Lake Ontario, collected from 1977 to 1993. Results confirm that overall levels of contaminants have decreased steadily in lake trout since 1977, and that coplanar PCB levels do not appear to be increasing over time in relation to levels of other PCBs. Contaminant levels in lake trout from 3 to 9 years old, collected in 1988 from the western end of Lake Ontario, show the body burden of contaminants increases with age. Relative levels of coplanar PCBs to other PCBs for the age study samples showed no change, except for PCB 77, which exhibited a slight decrease in relation to total PCB levels. Toxic equivalents (TEQs) were calculated from fish contaminant concentrations measured for the time study using toxic equivalence factors (TEFs) from both mammalian and teleost studies. The relative contributions of PCBs, PCDDs, and PCDFs to total TEQs were examined. When TEFs used for risk assessment are applied to temporal trend data, 15 to 20% of the total TEQs were due to mono-ortho-substituted PCBs; 40 to 50% to non-ortho coplanar PCBs; and 20 to 30% to 2,3,7,8-substituted PCDD and PCDFs. The TEQs determined from lake trout extracts by an H4IIE cell bioassay technique are compared to those determined by chemical analyses, using a variety of TEFs.
INTRODUCTION
Polychlorinated biphenyls (PCBs), polychlorinated dibenzo-p-dioxins (PCDDs), and polychlorin dibenzofurans (PCDFs) are highly stable lipophilic contaminants that have been the focus of a wide variety of analytical and environmental studies over the last two decades. Polychlorinated biphenyls, PCDDs, and PCDFs have been found throughout the Great Lakes environment in significant quantities at all trophic levels over the last two decades [1-6]. Although it is accepted that there has been a significant decline in concentrations of these chemicals from the 1970s to the present, comparing data generated during this period is difficult due to changes in analytical methodologies and protocols. Knowledge of the toxicological effects of specific PCB, PCDD, and PCDF congeners has led to a shift from reporting concentrations as “total” levels, to reporting congener-specific PCB, PCDD, and PCDF levels. The wide differences in the relative toxicities of these congeners makes it essential that congener-specific results be used to calculate toxic loading of these chemicals to fish and wildlife species. These numbers are not historically available. As the knowledge of the biological and toxicolgical effects of specific PCB, PCDD, and PCDF congeners continues to increase, the availability of contaminants data for individual species will be of great benefit.
The Great Lakes basin represents one of the world's largest freshwater lake systems, and the lakes receive a complex input of chemical contaminants from a variety of sources within the heavily populated basin. In addition to the input of trace organic compounds such as PCBs, dichlorodiphenyltrichloroethane (DDT), and dieldrin from industrial and agricultural sources, there are two specific sources of chlorinated dioxins and furans into the basin. One source originates from the historical production of chlorophenols and the storage of waste by-products in landfill sites, and the other major source is the effluent from kraft pulp mills that employ chlorine during the bleaching process [1, 7-10]. Losses from waste dumps are the primary source of dioxins in the lower Great Lakes, whereas bleached kraft pulp mills are concentrated in the upper portion of the Great Lakes system. Significant differences in the dioxin and furan isomer profiles from fish exposed to the two different sources have been found [6]. Lake Ontario fish have a higher relative proportion of 2,3,7,8-tetrachlorodibenzo-p-dioxin (2,3,7,8-TCDF) to 2,3,7,8-tetrachlorodibenzofuran (2,3,7,8-TCDF) than fish in any of the other Great Lakes.
To date, the most biologically active PCDD or PCDF congener known is the planar compound 2,3,7,8-TCDD. Other PCDDs and PCDFs, and some PCBs have been shown to elicit a number of common toxic responses similar to those observed for 2,3,7,8-TCDD [11]. Evidence exists that these toxic responses are mediated through binding to a specific protein called the aryl hydrocarbon (Ah) receptor. Structure-binding relationships for PCBs have shown that the most toxicologically active PCBs are those with no ortho substitution, and those that are substituted on both para and at least two meta positions, namely, 3,3′,4,4′-tetrachlorobiphenyl, 3,4,4′, 5-tetrachlorobiphenyl, 3,3′,4,4′,5-pentachlorobiphenyl, and 3,3′4,4′,5,5′-hexachloro-biphenyl (BZ numbers 77, 81, 126, and 169, respectively) [12-14]. This group of non-ortho PCBs are commonly referred to as “coplanar” PCBs, because their substitution pattern allows the two phenyl rings to lie in the same plane.
Although concentrations of PCBs have decreased significantly in the Great Lakes environment over the last two decades [5, 15, 16], the adverse symptoms observed in populations of some fish-eating waterbirds have persisted, and specific PCB congeners are believed to be the cause [17-19]. Evidence has suggested that a large portion of the toxicity associated with PCBs is due to small amounts of coplanar PCBs [17, 20]. Recent analytical data show that several of these coplanar PCBs are present in Great Lakes fish and birds at concentrations orders of magnitude higher than concentrations of 2,3,7,8-TCDD [2, 17]. Because the toxic activity of one of the non-ortho PCBs, BZ 126, is considered comparable to that of the most acutely lethal compound, 2,3,7,8-TCDD, it has been suggested that non-ortho PCBs are of greater toxicological significance than TCDD in Lake Ontario salmonids [20, 21] due to their higher concentrations.
Estimates of toxic loadings of chemicals to fish and wildlife species are complicated, due to the complexity of the mixtures of these compounds. The toxic equivalents (TEQs) approach is being used more frequently, where the toxic potency of dioxinlike chemicals is related to that of 2,3,7,8-TCDD, and toxic equivalency factors (TEFs) are used to generate a total TEQ for a mixture of compounds [13]. Estimates of TEFs are available based on both in vivo and in vitro studies in mammalian and aquatic species. At present, TEFs used for risk assessment purposes are derived from mammalian studies [13, 22], but the effects of contaminants differ from species to species [23, 24], and the relative contribution to total TEQ by individual compounds can change significantly depending on the choice of TEF. Due to the difficulties and costs associated with the chemical analyses of congener-specific PCBs, PCDDs, and PCDFs, the use of alternative methods of analysis to determine total TEQs has been explored, with an example being the H4IIE rat hepatoma cell bioassay.
A study of the changes in levels of contaminants over time and age, and the effect of these changes on TEQs, was undertaken. The samples analyzed in this study were obtained from the Department of Fisheries and Oceans Great Lakes Laboratory for Fisheries and Aquatic Sciences, which as part of the Great Lakes Contaminants Surveillance Program has compiled a tissue archive that contains frozen homogenized tissue samples of a number of aquatic species collected since the mid 1970s. Four-year-old lake trout of both sexes, collected from 1977 to 1993 at Main Duck Island (MDI) in the eastern basin of Lake Ontario, were analyzed for a variety of PCB, PCDD, and PCDF congeners. Three- to 9-year-old lake trout collected in 1988 from Port Credit in the western basin of Lake Ontario were analyzed for the same suite of compounds. The samples were analyzed in a random order from the available sample pool over a 3-year period between 1992 and 1994. Levels of contaminants in the tissues were measured, and TEQs were calculated for temporal trend data using TEFs generated from both mammalian and teleost studies, and the significance of the choice of TEF was examined. The TEQs generated based on the sum potency of the measured inducing compounds, as calculated using both mammalian- and fishspecific TEFs, were compared to TEQs for selected fish from the temporal trends study generated using an H4IIE cell bioassay analysis. The correlation between the TEQs was poor, although it was slightly better when TEFs generated from a rainbow trout bioassay were used to calculate the chemical TEQs.
MATERIALS AND METHODS
Lake trout were collected from the eastern basin of Lake Ontario, north of Main Duck Island, and from Port Credit in western Lake Ontario, in the fall of the years 1977 to 1993, and 1988, respectively. Samples were stored frozen as whole fish prior to homogenization, using methods described elsewhere [25]. Fish were weighed, measured, sexed, and aged prior to homogenization. Aliquots of whole fish homogenates were stored frozen at −20 to −25°C until removed and thawed. A detailed outline of the methodologies used in this study is available in Huestis et al. [26]. A brief outline of the methods follows.
Sample extraction and cleanup
Congener-specific PCBs. Aliquots of whole fish homogenates were ground with anhydrous sodium sulfate (Na2SO4), packed into chromatography columns, and eluted with meth-ylene chloride. Sample extracts were concentrated and passed through a gel permeation chromatography (GPC) column to remove bulk lipids and other biogenic molecules. A series of silica gel columns was used to separate PCBs from other organic contaminants. Gas chromatography with electron capture detection was used for the total PCB analyses. Total PCBs were quantitated against a standard containing a 1:1:1 mixture of Aroclors 1242, 1254, and 1260. Gas chromatography-mass selective detection was used for congener-specific PCB analyses. Quantitation of PCB congeners was via an external standard composed of PCB congeners. The PCB congener levels have been previously reported in Huestis et al. [27].
PCDDs, PCDFs, and coplanar PCBs. The samples were extracted as per the congener-specific PCB method. Surrogate spiking solutions, consisting of 13C-PCDD and 13C-coplanar PCB standards, were added prior to elution of the samples from the chromatography columns. Sample extracts were passed through a GPC column, followed by an alumina column to further clean and separate coplanar PCBs, PCDDs, and PCDFs from possible interferences. A semiautomated high-performance liquid chromatography (HPLC) system was fitted with a carbon column, and the extracts were further cleaned-up and fractionated to separate the coplanar PCBs from the PCDD and PCDFs.
High-resolution gas chromatography-mass sprectroscopy (GC/MS) analyses of coplanar PCBs, PCDDs, and PCDFs were carried out on a VG AutoSpec-Q mass spectrometer. For all analyses the resolving power of the analyzer was 10,000: 1. Compounds were detected in the selected ion monitoring (SIM) mode, using ions characteristic of the analyzed compounds. Chlorinated diphenylethers (CDPEs), which interfere with the determination of PCDFs, were monitored as well, although the method used is known to remove CDPE interferences [28]. An internal standard quantitation method was used, and native coplanar PCB, PCDD, and PCDF concentrations were corrected for calculated 13C-PCB, PCDD, and PCDF surrogate recoveries. The acceptable recovery range was from 40 to 120%. Instrument performance was monitored by the addition of a performance standard immediately before sample injection. Detection limits were defined as three times the background noise in the region of the 13C-surrogate quantification peaks.
| N | Weight | Lipid % | 2,3,7,8-TCDD | 1,2,3,7,8-PCDD | 1,2,3,4,7,8-H6CDD | 1,2,3,6,7,8-H6CDD | 1,2,3,7,8,9-H6CDD | 1,2,3,4,6,7,8-H7CDD | OCDD | 2,3,7,8-TCDF | 1,2,3,7,8-PCDF | 2,3,4,7,8- PCDF | 1,2,3,4,7,8-H6CDF | 1,2,3,6,7,8-H6CDF | 2,3,4,6,7,8-H6CDF | 1,2,3,7,8,9-H6CDF | 1,2,3,4,6,7,8-H7CDF | OCDF | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Year | ||||||||||||||||||||
| 1977 | 6 | Mean | 2,170 | 26.8 | 79.1 | 11.2 | 0.53 | 6.50 | 0.54 | 1.61 | 14.0 | 36.6 | 9.42 | 50.3 | 24.4 | 2.93 | 2.34 | 0.17 | 1.54 | 2.27 |
| SD | 1,510 | 7.20 | 17.9 | 3.92 | 0.25 | 3.22 | 0.41 | 0.48 | 5.81 | 18.8 | 8.18 | 19.0 | 22.5 | 1.70 | 1.18 | 0.11 | 0.96 | 2.26 | ||
| 1978 | 7 | Mean | 1,190 | 20.9 | 48.0 | 7.66 | 0.44 | 4.52 | 0.58 | 1.32 | 13.4 | 36.9 | 8.82 | 36.2 | 17.0 | 3.77 | 1.84 | 0.11 | 1.19 | 1.42 |
| SD | 190 | 3.90 | 9.65 | 2.43 | 0.31 | 1.82 | 0.28 | 0.42 | 4.51 | 15.4 | 4.61 | 10.1 | 10.3 | 1.96 | 0.85 | 0.05 | 0.61 | 1.43 | ||
| 1980 | 7 | Mean | 2,050 | 25.4 | 47.5 | 6.46 | 0.39 | 3.35 | 0.51 | 0.78 | 6.14 | 38.4 | 7.44 | 29.4 | 12.4 | 2.02 | 1.23 | 0.11 | 0.81 | 0.83 |
| SD | 730 | 7.10 | 15.3 | 3.63 | 0.32 | 1.85 | 0.22 | 0.38 | 3.99 | 20.0 | 3.97 | 10.3 | 7.74 | 1.61 | 0.75 | 0.09 | 0.22 | 0.30 | ||
| 1981 | 6 | Mean | 2,210 | 29.5 | 30.0 | 4.38 | 0.26 | 2.74 | 0.39 | 1.29 | 9.85 | 34.3 | 4.33 | 19.4 | 6.32 | 1.30 | 0.95 | 0.12 | 0.54 | 1.07 |
| SD | 710 | 5.20 | 6.65 | 2.29 | 0.21 | 0.86 | 0.16 | 0.56 | 7.57 | 17.3 | 2.78 | 6.59 | 4.95 | 1.93 | 0.57 | 0.07 | 0.47 | 0.67 | ||
| 1982 | 7 | Mean | 2,190 | 26.6 | 47.6 | 6.59 | 0.26 | 4.09 | 0.54 | 1.16 | 8.99 | 44.8 | 8.43 | 30.6 | 13.5 | 2.52 | 1.70 | 0.14 | 0.96 | 2.04 |
| SD | 810 | 5.10 | 8.69 | 3.32 | 0.27 | 1.45 | 0.19 | 0.53 | 6.00 | 16.1 | 4.12 | 7.83 | 7.10 | 1.68 | 0.73 | 0.07 | 0.47 | 1.69 | ||
| 1983 | 5 | Mean | 1,950 | 21.2 | 44.7 | 7.09 | 0.31 | 3.61 | 0.42 | 2.40 | 26.4 | 31.2 | 6.94 | 31.1 | 9.83 | 2.35 | 1.58 | 0.09 | 1.28 | 2.41 |
| SD | 520 | 2.80 | 13.1 | 4.41 | 0.39 | 1.32 | 0.32 | 2.09 | 25.6 | 13.4 | 3.51 | 10.3 | 4.79 | 2.15 | 0.79 | 0.04 | 0.71 | 1.97 | ||
| 1984 | 8 | Mean | 1,810 | 19.2 | 42.9 | 6.58 | 0.16 | 4.18 | 0.34 | 0.93 | 13.0 | 20.9 | 6.43 | 29.8 | 16.4 | 3.07 | 1.79 | 0.18 | 1.08 | 2.50 |
| SD | 530 | 6.90 | 20.2 | 2.27 | 0.13 | 2.17 | 0.31 | 0.78 | 12.6 | 6.48 | 3.12 | 17.2 | 15.0 | 2.12 | 1.39 | 0.12 | 0.86 | 3.08 | ||
| 1986 | 7 | Mean | 2,620 | 26.9 | 31.1 | 6.15 | 0.39 | 2.69 | 0.51 | 0.75 | 5.75 | 22.0 | 4.59 | 20.1 | 4.70 | 1.48 | 1.05 | 0.11 | 0.60 | 0.95 |
| SD | 650 | 4.70 | 8.00 | 1.71 | 0.29 | 0.61 | 0.26 | 0.42 | 3.21 | 7.73 | 1.82 | 6.62 | 1.49 | 0.78 | 0.37 | 0.06 | 0.25 | 0.32 | ||
| 1987 | 8 | Mean | 1,420 | 18.6 | 28.6 | 6.78 | 0.34 | 3.31 | 0.61 | 0.96 | 6.16 | 20.9 | 6.30 | 21.9 | 7.49 | 2.76 | 1.51 | 0.09 | 0.74 | 0.92 |
| SD | 340 | 2.60 | 10.0 | 2.72 | 0.32 | 1.25 | 0.26 | 0.54 | 6.15 | 8.91 | 2.85 | 8.56 | 3.56 | 1.84 | 0.85 | 0.06 | 0.24 | 0.69 | ||
| 1988 | 12 | Mean | 2,200 | 22.2 | 32.5 | 8.27 | 0.58 | 4.54 | 0.77 | 1.31 | 7.11 | 34.2 | 9.29 | 30.2 | 12.3 | 3.48 | 1.98 | 0.26 | 1.09 | 1.18 |
| SD | 400 | 3.20 | 6.39 | 1.76 | 0.51 | 1.47 | 0.38 | 0.63 | 6.51 | 13.3 | 3.89 | 7.30 | 8.27 | 1.26 | 0.51 | 0.34 | 0.50 | 0.58 | ||
| 1989 | 7 | Mean | 2,600 | 24.3 | 33.4 | 7.25 | 0.47 | 3.82 | 0.63 | 0.97 | 11.1 | 32.5 | 7.95 | 28.2 | 7.13 | 4.13 | 1.32 | 0.19 | 0.67 | 0.72 |
| SD | 740 | 3.00 | 10.1 | 3.71 | 0.26 | 1.55 | 0.33 | 0.31 | 14.3 | 10.5 | 4.85 | 14.5 | 3.68 | 5.15 | 1.03 | 0.10 | 0.42 | 0.45 | ||
| 1990 | 9 | Mean | 2,060 | 24.4 | 29.1 | 7.07 | 0.47 | 4.57 | 0.66 | 1.67 | 7.52 | 36.8 | 10.5 | 31.0 | 13.8 | 3.87 | 1.72 | 0.16 | 1.19 | 1.36 |
| SD | 400 | 5.30 | 6.79 | 1.88 | 0.38 | 1.43 | 0.30 | 1.88 | 8.73 | 13.1 | 3.89 | 12.5 | 10.0 | 2.67 | 0.79 | 0.12 | 0.86 | 1.36 | ||
| 1991 | 6 | Mean | 2,310 | 22.7 | 23.6 | 5.57 | 0.55 | 3.70 | 0.56 | 0.78 | 1.42 | 28.0 | 6.68 | 23.5 | 10.1 | 2.97 | 1.65 | 0.13 | 0.75 | 0.42 |
| SD | 670 | 2.90 | 3.77 | 0.73 | 0.33 | 1.11 | 0.27 | 0.26 | 0.40 | 10.6 | 3.18 | 4.75 | 6.70 | 1.23 | 0.51 | 0.18 | 0.29 | 0.10 | ||
| 1992 | 7 | Mean | 2,010 | 17.1 | 19.5 | 4.98 | 1.26 | 3.06 | 1.08 | 1.73 | 5.13 | 37.6 | 6.54 | 19.6 | 5.33 | 2.94 | 1.80 | 1.06 | 1.89 | 6.12 |
| SD | 330 | 2.30 | 2.72 | 1.96 | 0.74 | 1.36 | 0.45 | 0.98 | 5.33 | 8.63 | 1.79 | 3.10 | 3.24 | 1.31 | 0.56 | 0.64 | 1.44 | 8.03 | ||
| 1993 | 7 | Mean | 2,020 | 17.3 | 20.9 | 4.36 | 0.47 | 2.17 | 0.45 | 0.88 | 6.30 | 29.6 | 5.13 | 16.0 | 5.29 | 2.71 | 1.15 | 0.37 | 0.78 | 1.70 |
| SD | 620 | 3.00 | 4.95 | 1.98 | 0.17 | 1.02 | 0.15 | 0.39 | 3.02 | 6.59 | 1.33 | 5.50 | 3.48 | 1.22 | 0.40 | 0.43 | 0.51 | 1.50 | ||
| Age | ||||||||||||||||||||
| 3 | 3 | Mean | 1,160 | 21.3 | 20.6 | 4.69 | 0.16 | 2.56 | 1.63 | 0.82 | 3.87 | 25.4 | 5.85 | 22.5 | 1.36 | 0.02 | 5.05 | 0.64 | 0.49 | 0.23 |
| SD | 620 | 8.50 | 8.91 | 1.76 | 0.16 | 1.32 | 2.21 | 0.21 | 0.47 | 14.8 | 3.16 | 9.91 | 1.55 | 0.02 | 8.14 | 0.72 | 0.29 | 0.06 | ||
| 4 | 4 | Mean | 1,880 | 22.0 | 30.7 | 6.52 | 0.61 | 3.72 | 0.68 | 0.85 | 1.40 | 27.8 | 7.49 | 42.3 | 9.64 | 2.79 | 1.66 | 0.14 | 0.78 | 0.67 |
| SD | 310 | 3.40 | 10.5 | 2.20 | 0.36 | 1.70 | 0.27 | 0.43 | 0.32 | 14.8 | 3.98 | 16.2 | 7.07 | 1.53 | 0.75 | 0.08 | 0.47 | 0.21 | ||
| 5 | 5 | Mean | 2,380 | 25.0 | 30.0 | 5.86 | 0.44 | 2.68 | 0.54 | 0.57 | 1.23 | 23.8 | 5.45 | 37.3 | 6.02 | 1.93 | 1.26 | 0.07 | 0.55 | 0.45 |
| SD | 340 | 3.90 | 3.62 | 0.68 | 0.11 | 0.36 | 0.03 | 0.07 | 0.70 | 6.1 | 1.01 | 4.44 | 1.34 | 0.23 | 0.19 | 0.02 | 0.13 | 0.18 | ||
| 6 | 5 | Mean | 3,290 | 27.4 | 30.6 | 6.82 | 0.63 | 4.44 | 0.69 | 0.84 | 0.98 | 26.1 | 6.87 | 36.6 | 10.5 | 2.78 | 1.49 | 0.09 | 0.76 | 0.42 |
| SD | 840 | 5.30 | 3.86 | 2.73 | 0.65 | 4.19 | 0.60 | 0.86 | 0.57 | 19.1 | 7.67 | 30.6 | 12.2 | 2.54 | 1.35 | 0.03 | 0.70 | 0.12 | ||
| 7 | 5 | Mean | 4,220 | 29.8 | 41.10 | 9.41 | 0.46 | 6.05 | 0.57 | 1.23 | 4.44 | 34.4 | 9.12 | 49.3 | 13.2 | 1.90 | 3.66 | 1.28 | 1.17 | 1.22 |
| SD | 890 | 4.90 | 12.1 | 2.94 | 0.51 | 2.06 | 0.47 | 0.46 | 4.40 | 11.0 | 4.20 | 33.6 | 10.9 | 2.54 | 2.18 | 1.37 | 0.49 | 0.91 | ||
| 8 | 5 | Mean | 5,270 | 32.0 | 46.9 | 9.27 | 0.51 | 4.26 | 0.51 | 0.96 | 8.09 | 36.3 | 8.77 | 50.3 | 9.46 | 2.97 | 2.11 | 1.20 | 1.02 | 1.61 |
| SD | 900 | 3.50 | 7.04 | 1.32 | 1.01 | 0.83 | 0.28 | 0.25 | 8.54 | 14.5 | 1.60 | 18.1 | 3.64 | 2.79 | 1.19 | 0.67 | 0.32 | 0.40 | ||
| 9 | 5 | Mean | 4,900 | 34.8 | 53.2 | 8.27 | 0.27 | 4.41 | 0.62 | 1.00 | 2.66 | 37.6 | 7.93 | 39.0 | 10.6 | 0.07 | 2.14 | 1.45 | 0.63 | 0.44 |
| SD | 660 | 4.50 | 20.5 | 4.68 | 0.41 | 1.10 | 0.13 | 0.34 | 0.81 | 10.7 | 2.56 | 12.5 | 3.83 | 0.05 | 1.34 | 0.41 | 0.21 | 0.20 |
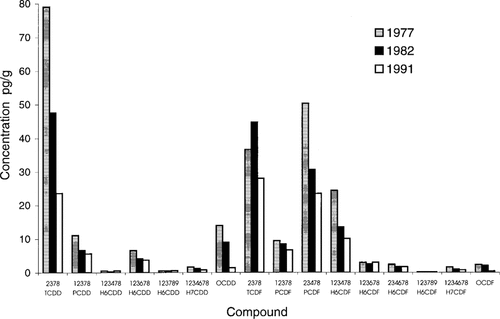
Concentration profiles of polychlorinated dibenzo-p-dioxin and polychlorinated dibenzofuran congeners (pg/g), in lake trout from Lake Ontario, for 1977, 1982, and 1991.
Strict quality control (QC) and quality assurance (QA) measures were followed, with 15 to 20% of the total samples analyzed being QA/QC samples consisting of spikes, blanks, duplicates, and certified reference materials (when available). Instrument performance and stability were monitored on an ongoing basis throughout the study.
H4IIE cell bioassay. Selected lake trout from 1977, 1978, 1982, 1986, and 1988 were analyzed according to established protocols [29, 30]. Homogenized lake trout tissue was extracted with methylene chloride and sodium sulfate, and extracts were cleaned up by GPC and alumina column chromatography as desribed in the previous section. Final extracts were stored in toluene, and extracts were prepared for bioassay analysis by nitrogen evaporation of the toluene and concentration of the sample into 50 μl of dimethyl sulfoxide (DMSO). Serial dilutions were made of the final extract and these samples were analyzed using the H4IIE bioassay.
The H4IIE bioassay was performed using a modification of the methods of Kennedy et al. [31]. Rat hepatoma (H4IIE) cells were cultured using the media described by Tillitt et al. [32]. Median effective doses (ED50s) for the extract dilution curves and dioxin standard curves were determined by fitting a logistic function to the data. Curve fitting of dose-effect curves was performed using a SYSTAT MACRO program (SYSTAT Corp., Evanston, IL, USA) that utilized the nonlinear curve fitting module of SYSTAT. Toxic equivalent concentrations of 2,3,7,8-TCDD in pg/g were derived from the ratio of 2,3,7,8-TCDD standard curve ED50s to sample extract ED50s, using a method similar to Tillitt et al. [32].
Statistical analysis
Calculations of means, standard deviations, standard errors, and linear regressions were performed in Microsoft Excel® (Microsoft, Redmond, WA, USA). Means, standard deviations, and standard errors were calculated for both the temporal and age studies, on an individual compound basis. For compounds that were below the limit of detection, one-half the detection limit was used. Linear regressions permitted the examination of temporal changes in contaminant concentrations from 1977 to 1993. Analysis of variance (ANOVA) testing was performed with the statistical program Minitab, version 8.2.1 (Minitab, Inc., State College, PA, USA). Analysis of variance testing was performed on selected PCB, PCDD, and PCDF compounds to determine whether temporal and age-related trends in the data were statistically significant.
RESULTS
The efficiency of the extraction and cleanup procedures was monitored throughout the study. Recoveries of internally spiked 13C-coplanar PCBs and PCDDs ranged from 72 to 83%, and 58 to 84%, respectively. Recoveries of externally spiked congener PCB samples run concurrently with the sample sets ranged from 83 to 114%. All PCB congener levels have been previously reported [27]. Mean concentrations of 2,3,7,8-substituted PCDDs and PCDFs in samples from both MDI and Port Credit, as well as lipid levels and weights, are listed in Table 1. The PCDD and PCDF levels were highest for 2,3,7,8-TCDD at 80 pg/g in 1977, and ranged down to a low of 0.09 pg/g for 1,2,3,7,8,9-hexachlorodibenzofuran (H6CDF) in 1987. No significant quantities of any non-2,3,7,8-substituted PCDDs and PCDFs were seen in these samples. Results of the age class study at Port Credit showed a similar wide range in levels of contaminants. Statistical analysis of the trends of selected PCB, PCDD, and PCDF congeners over the time period 1977 to 1993 showed significant changes (p < 0.05) from 1977 to 1978 followed by a gradual decline and stabilization for most congeners, with the exception of BZ 169, whose levels showed no significant change from 1977 to 1993. These selected congeners are representative of the data set as a whole.
| ID | Substitution pattern | TEF (mammalian; PCBsa; PCDDs, PCDFsb) | TEF (mammalian)c,d | TEF (teleost)c,d |
|---|---|---|---|---|
| BZ 77 | 3,3′,4,4′- | 0.0005 | 0.00079 | |
| BZ 81 | 3,4,4′,5- | 0.0072 | 0.0034 | |
| BZ 105 | 2,3,3′,4,4′- | 0.0001 | 0.000025 | 0.0064 |
| BZ 118 | 2,3′,4,4′,5- | 0.0001 | 0.00001 | 0.000049 |
| BZ 126 | 3,3′,4,4′,5- | 0.1 | 0.1 | 0.000017 |
| BZ 156 | 2,3,3′,4,4′,5- | 0.0005 | 0.000052 | 0.023 |
| BZ 169 | 3,3′,4,4′,5,5′- | 0.01 | 0.0014 | 0.00003 |
| BZ 170 | 2,2′,3,3′,4,4′,5- | 0.0001 | 0.00016 | |
| BZ 180 | 2,2′,3,4,4′,5,5′- | 0.00001 | ||
| TCDD | 2,3,7,8- | 1 | 1 | 1 |
| PCDD | 1,2,3,7,8- | 0.5 | 1.13 | 2.6 |
| H6CDD | 1,2,3,4,7,8- | 0.1 | 0.49 | 1.1 |
| H6CDD | 1,2,3,6,7,8- | 0.1 | 0.23 | 0.2 |
| H6CDD | 1,2,3,7,8,9- | 0.1 | 0.01 | |
| H7CDD | 1,2,3,4,6,7,8- | 0.01 | 0.09 | 0.2 |
| OCDD | 0.001 | |||
| TCDF | 2,3,7,8- | 0.1 | 0.03 | 0.2 |
| PCDF | 1,2,3,7,8- | 0.05 | 0.16 | 0.2 |
| PCDF | 2,3,4,7,8- | 0.5 | 0.4 | 1.9 |
| H6CDF | 1,2,3,4,7,8- | 0.1 | 0.33 | 1.1 |
| H6CDF | 1,2,3,6,7,8- | 0.1 | 0.1 | |
| H6CDF | 2,3,4,6,7,8- | 0.1 | 0.2 | |
| H6CDF | 1,2,3,7,8,9- | 0.1 | 0.01 | |
| H7CDF | 1,2,3,4,6,7,8- | 0.01 | 0.01 | |
| OCDF | 0.001 |
The PCDD and PCDF contaminant profiles are shown for the years 1977, 1982, and 1991 in Figure 1. There was an overall decrease in the levels from 1977 to 1991. Changes in the relative proportions of 2,3,7,8-TCDD to other PCDDs and PCDFs are seen over the course of the temporal study. Also, an increase in the relative proportion of 2,3,7,8-TCDF is seen as the proportion of 2,3,7,8-TCDD decreases. No large changes in the relative proportions of 2,3,7,8-TCDD and 2,3,7,8-TCDF were seen for the other PCDDs and PCDFs.
As a measure of the potential toxicity of their contaminant burden to the lake trout, TEQs were calculated by taking the mean concentration of the compound of interest, and multiplying by its congener specific TEF. The selected non-, mono-, and di-ortho-substituted PCB congeners, and the PCDD and PCDF congeners, used in the calculations are listed in Table 2, along with their BZ number, structure, and Cl substitution type (non-, mono-, or di-ortho substituted). The TEFs determined from both mammalian and teleost studies (when available) are also listed in Table 2, as well as the source of the TEF.
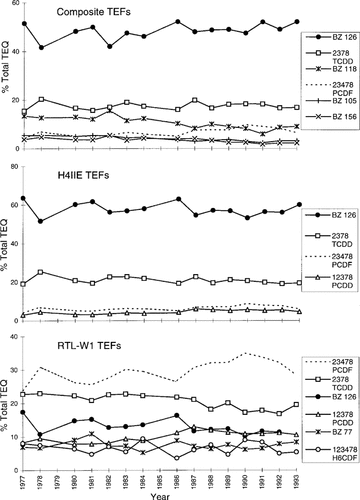
For the temporal trends study, TEQs were calculated using three different sets of TEFs: a set consisting of PCB TEFs from Ahlborg et al. [22] and PCDD/PCDF TEFs from Safe [33], a set consisting of TEFs generated from a rat cell line (H4IIE) [23], and a third set consisting of TEFs derived from a rainbow trout cell line (RTL-W1) [24]. When total TEQs are calculated for the sample set, the totals were highest when the composite TEFs were used, followed by the H4IIE-gen-erated TEFs and the RTL-W1-generated TEFs. Interestingly, in the first year surveyed (1977) the total TEQs were 583, 473, and 396 pg/g TCDD equivalents for the three types of TEFs, respectively, whereas by the last year surveyed (1993) the total TEQs were 124, 108, and 107 pg/g TCDD equivalents, respectively. The differences when TEQs were calculated in the three different ways were large at the beginning of the time study, but the TEQ values were essentially the same in 1993 no matter which TEFs were used.
Contributions of PCB, PCDD, and PCDF to total TEQs as calculated by all three sets of TEFs are illustrated in Figure 2. When risk assessment TEFs were used, the most important contributor was non-ortho BZ 126, which contributed between 40 and 50% of the total TEQ (depending on the year examined). 2,3,7,8-Tetrachlorodibenzo-p-dioxin contributed 15 to 20% and 2,3,4,7,8-PCDF contributed 4 to 10% of the total TEQ. The mono-ortho-substituted PCB congeners BZ 105, 118, and 156 accounted for 14 to 22% of the total TEQ. The TEQs calculated from H4IIE-generated TEFs show BZ 126 contributing 52 to 64% of the total, followed by 2,3,7,8-TCDD (19–25%), 2,3,4,7,8-PCDF (4–9%), and 1,2,3,7,8-PCDD (3–6%). The mono-ortho PCBs were not significant in this set. The TEQs calculated from RTL-W1-generated TEFs show 2,3,4,7,8-PCDF as the most important (24–35%), followed by 2,3,7,8-TCDD (17–23%), BZ 126 (10–17%), 1,2,3,7,8-PCDD (8–13%), BZ 77 (6–11%), and 1,2,3,4,7,8-H6CDF (4–9%). Polychlorinated biphenyl 126 was the most important when mammalian TEFs were used, but the dioxin and furans, specifically 2,3,4,7,8-PCDF and 2,3,7,8-TCDD contributed the most towards total TEQ when teleost TEFs were used. Even though the levels of coplanar PCBs were much higher than those of the PCDDs or PCDFs, the much lower toxicity of PCBs to fish caused the PCBs to not be as significant as the PCDDs and PCDFs when total TEQs were calculated using teleost TEFs. The majority of Ah-active PCB, PCDD, and PCDF compounds did not have any significant impact on total TEQ, due primarily to their low toxicities; some of these compounds are present at quite high levels in lake trout.
The potential enrichment of coplanar PCBs over time was investigated by examining the levels of coplanar PCB congeners 77 and 126 relative to other PCB congeners with the same substitution levels, and to total PCB levels for both the temporal trends and age studies (Figs. Fig. 3., Fig. 4.). The proportion of BZ 77 to other PCBs within the same homologue group (BZs 52 and 66) showed a slight increase from 1977 to 1993 (Fig. 3). There was no change in the relative proportions of BZs 81 or 169 (not shown) to their homologues. The proportions of coplanar PCBs 77, 126, and 169 to total PCBs showed no noticeable trends. These results suggest overall no significant increase in levels of coplanar PCBs relative to the total levels of PCBs, or to other PCB congeners, over the time period 1977 to 1993. For the age classes analyzed, no increase in the relative contribution of coplanar PCBs 126 or 169 (not shown) to their homologues was apparent as lake trout age increased (Fig. 4), but the proportion of BZ 77 relative to PCBs 52 and 66 showed a slight decrease from age 3 to age 9. A decrease in the proportion of BZ 77 to total PCBs was apparent with age. Polychlorinated biphenyls 126 and 169 showed no change relative to total PCB in 3- to 9-year-old lake trout.
A comparison of TEQs generated by an H4IIE cell bioassay technique with the TEQs calculated for non-ortho PCBs, PCDDs, and PCDFs (as measured by analysis of the samples) is shown in Figure 5 for selected samples from 1978, 1982, 1986, and 1991. When TEQs are calculated using two different sets of mammalian TEFs, the resulting r2 values are slightly lower than when teleost TEFs are used to calculate TEQs.
DISCUSSION
The temporal and age-related trends of seven PCDD and nine PCDF congeners are discussed in this study. The PCDD and PCDF levels from the Lake Ontario lake trout analyzed in this study are similar to levels reported in recent studies. DeVault et al. [1] analyzed lake trout collected in 1984 from southern Lake Ontario and found mean levels of 48.9 ng/kg of 2,3,7,8-TCDD, 18.5 ng/kg of 2,3,7,8-TCDF, 20.1 ng/kg of 2,3,4,7,8-PCDF, 8.4 ng/kg of 1,2,3,7,8-PCDD, and 9.7 ng/kg of 1,2,3,4,7,8-H6CDF in whole-fish samples. This study, in lake trout collected at MDI in 1984, found levels of the same PCDD and PCDFs of 42.9, 20.9, 29.8, 6.58, and 16.4 pg/g, respectively, which agree very closely with those reported by DeVault et al. [1]. Zacharewski et al. [4], using fish homogenate samples collected in the early to mid 1980s from Lake Ontario found average levels of 40, 20, 22, 9, and 15 pg/g, respectively, for the same congeners.
Data on temporal trends of PCDDs and PCDFs are lacking in the published literature, which makes it difficult to compare the trends we found with those found by other researchers. Temporal trends of PCBs in lake trout, showing a comparatively large decrease in levels in the late 1970s, followed by a gradual leveling off to present levels, compare favorably with those found in other species throughout the Great Lakes. Mac et al. [15] measured PCB concentrations in lake trout eggs collected from 1979 to 1988, and found total PCB levels in the Great Lakes to be declining. Suns et al. [16] measured contaminants in spottail shiners collected in the 1970s, and in 1990 from Lakes Superior, Huron, Erie, and Ontario, and found significantly lower levels in the 1990 samples than in the 1970s samples. Turle et al. [34] measured levels of PCBs in herring gull eggs collected from 1971 to 1987 in Lake Ontario, and saw the same overall decrease in total levels of PCBs from the 1970s to the present.
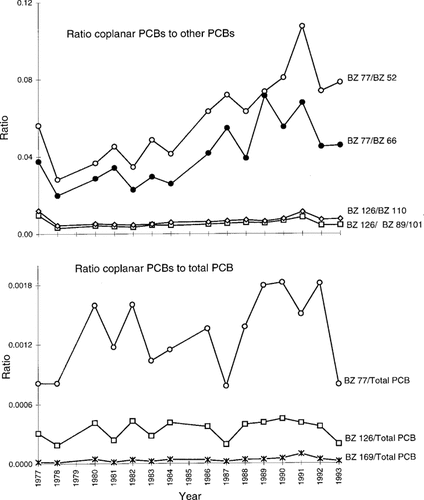
Ratios of coplanar polychlorinated biphenyls (PCBs) 77, 126, and 169 to other PCBs within the same homologue groups, and to total PCB, for Lake Ontario lake trout collected in the years 1977 to 1993.
It has been suggested that selective enrichment of certain PCB homologue groups and specific congeners is occurring in sediments and the aquatic food chain of the Great Lakes [2, 20, 35]. A study by Turle et al. [34] showed that in herring gull eggs from the Great Lakes, levels of mono- through pentasubstituted PCBs declined in importance over time, there was little change in the hexa-substituted PCBs, and hepta- to deca-substituted PCBs increased in importance. Mac et al. [15] found no changes in the PCB pattern in lake trout eggs, and concluded that concentrations of individual congeners were declining at similar rates. Our study found no significant enrichment of non-ortho PCB congeners, relative to total PCB, in any of the samples analyzed (Figs. Fig. 3., Fig. 4.). A small amount of enrichment of BZ 77 relative to other PCBs within the same homologue group occurred in the temporal study samples, and a slight decrease in the proportion of BZ 77 to total PCB occurred in the age class study samples. The magnitude of these changes was very low. A parallel study conducted using these samples looked at the distribution of 61 PCB congeners, and found minimal changes in the PCB congener pattern from 1977 to 1993, although a slight increase in higher chlorinated versus lower chlorinated congeners was observed [27].
A study by Whittle et al. [6] found significant differences in the PCDD and PCDF congener profiles in lake trout samples collected from Lakes Superior and Ontario in 1990. Levels of 2,3,7,8-TCDD were approximately one-half those of 2,3,7,8-TCDF in lake trout from Lake Ontario, whereas the ratio was 1 to 7 in Lake Superior. Another study by Niimi and Oliver [20] also found the proportion of 2,3,7,8-TCDD to 2,3,7,8-TCDF to be about 1.2 to 1 for lake trout collected in 1984 from Lake Ontario. This study found levels of 2,3,7,8-TCDD to be significantly higher than those of 2,3,7,8-TCDF in the earliest year of the study (2:1 in 1977) (Fig. 1), with the proportion dropping to 1 to 1 in 1982, and declining to 1 to 1.5 by 1991. Because only 2,3,7,8-TCDD levels were elevated, a source specific to that congener, such as the production of 2,4,5-trichlorophenol, would appear to be the cause. This chlorophenol was previously manufactured near the Niagara River, and the disposal of associated wastes in landfills adjacent to the Niagara River has been identified as an important source of 2,3,7,8-TCDD to Lake Ontario [7]. The declining proportion of 2,3,7,8-TCDD to 2,3,7,8-TCDF throughout the course of this study would seem to indicate a lessening of the impact of the Niagara River on Lake Ontario, at least with respect to dioxins and furans.
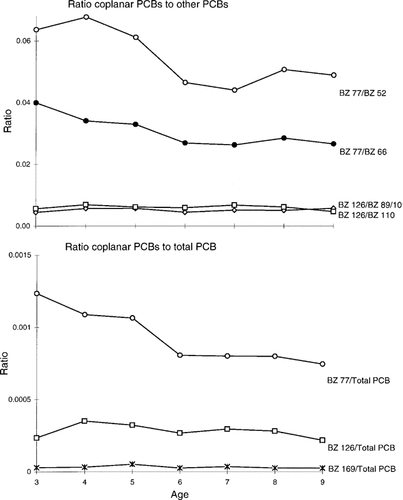
Ratios of coplanar polychlorinated biphenyls (PCBs) 77, 126, and 169 to other PCBs within the same homologue groups, and to total PCB, for Lake Ontario lake trout aged 3 to 9 years.
Many factors influence the levels of chemicals found in fish tissues. The diet of lake trout in Lake Ontario is known to have changed over the time period studied [6, 36], and this could affect both the levels of contaminants ingested by the lake trout if the prey species changes, as well as the growth rates of the fish. Genetics is another factor, as the origin of the fish (native-born versus released into the lake as part of a stocking program) could affect the total body burden of contaminants by exposing the fish to different environments when young. The types of chemical inputs found throughout the Great Lakes basin vary widely depending on how industrialized any given area is. As the basin as a whole becomes more industrialized, the input of contaminants (specifically dioxins) from the atmosphere from sources such as incineration could be changing, and this could also affect levels of contaminants found in lake trout.
Contaminants are always found as mixtures of compounds, and the need to assess these mixtures for their effects on human health has led to the development of TEFs for use in assessing the toxicity of body burdens of PCDDs, PCDFs, and PCBs in fish and wildlife. The TEFs commonly used in risk assessment are derived from mammalian systems, but recent work has shown significant differences between TEFs developed specifically for teleosts and those developed for mammals [14, 21, 23, 33, 37, 38]. Teleosts have TEFs for PCDDs and PCDFs that are as high or higher than those for mammals (Table 2). Some recent studies have found two penta congeners, 1,2,3,7,8-pentachlorodibenzo-p-dioxin (P5CDD) and 2,3,4,7,8-pentachlorodibenzofuran (P5CDF), that are actually more toxic than 2,3,7,8-TCDD to rainbow trout [23, 38]. Teleost TEFs for PCBs are significantly lower than those for mammals. Two possible explanations of lower TEFs for PCBs are lower binding affinity for the fish Ah receptor, or greater elimination of PCB than PCDDs or PCDFs. Zabel et al. [39] did not find that PCB congeners were metabolized or eliminated faster than PCDDs or PCDFs in rainbow trout, but studies have suggested that PCBs have a lower binding affinity for the Ah receptor. Many mono- and di-ortho PCB congeners exhibit no toxicity to fish; this is thought to be due to lower binding affinities [40]. When mono- and di-ortho PCB congeners BZs 4, 28, 52, 105, 118, 128, 138, 156, and 170 were tested on rainbow trout, there was no evidence of blue-sac disease or sac fry mortality [39], although the same congeners do elicit toxic effects in mammals [33]. Similarily, another study showed that BZs 105, 118, and 153 did not cause egg, sac fry, or fry mortality in rainbow trout [37], even when fish were dosed at high levels.
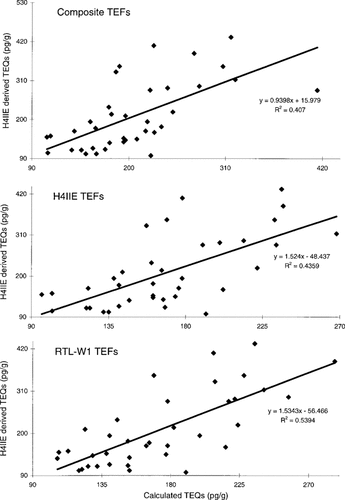
Comparison of H4IIE bioassay-generated toxic equivalents (TEQs) and chemically generated TEQs, for selected Lake Ontario lake trout from 1977, 1978, 1982, 1986, 1988, and 1991.
When total TEQs are calculated, interactions between compounds, which can be additive, synergistic, and/or antagonistic depending on the specific compounds tested and their relative concentrations [41-43], are not considered. Some organochlorine compounds and non-ortho-substituted PCBs have the potential to be embryotoxic to early life stages of Great Lakes fish, but less toxic contaminants can modify this response [40]. Japanese medaka embryos showed inhibited uptake of 14C-labelled BZ 126 in the presence of high levels of other contaminants [40]. Calculations of TEQs also do not generally include PCB, PCDD, or PCDF congeners with fewer than four chlorines (as they do not have substituents at both para and at least two meta positions), and lower chlorinated congeners and congeners having vicinal hydrogen atoms are easily degraded or metabolized in the environment [44]. Thus, tri- and tetra-ortho-substituted PCBs, while potentially having high levels in the environment, are not generally assigned a TEF due to their lack of toxicity (the exceptions to this are the tetrasubstituted coplanar PCBs BZ 77 and BZ 81).
For teleost species, the choice of TEF dictates what the main contributor to the total TEQ would be. When the contributions of non- and mono-ortho PCBs, and PCDDs and PCDFs are summed to generate a total TEQ using risk-assessment (mammalian) TEFs, the top contributors, in order of contribution, are BZ 126, BZ 105, BZ 77, 2,3,7,8-TCDD, and BZ 118 (Fig. 2). The same congeners, in roughly the same proportions, were the most significant in both the temporal and age studies. The total TEQs measured in the late 1970s in Lake Ontario lake trout varied by almost a factor of two, depending on the source of the TEF used in the calculations, but were essentially the same in 1993, regardless of the TEFs used. A comparison of TEQs calculated with both mammalian and teleost TEFs (Fig. 2) has demonstrated that when fish-specific TEFs are used, the main contributors to TEQ in lake trout are the PCDDs and PCDFs (60–70%), and not the PCBs, in spite of the fact that even the non-ortho PCBs are present at levels orders of magnitude higher than the PCDDs or PCDFs. However, using mammalian TEFs causes the most significant compounds to become BZ 77 and BZ 126 (70–75%), which totally changes the interpretation of the TEQ contributors. Thus, the value of using mammalian TEFs in aquatic ecosystems is suspect. The differences between mammalian and teleost TEFs would indicate that species-specific TEFs must be used to ensure valid risk assessment procedures.
Comparisons between bioassay-derived TEQs, and chemically derived TEQs for mammalian species generally show good correlations. Tillitt et al. [19] came to the conclusion that PCBs, and not PCDDs or PCDFs, were the major contaminant influence on cormorant reproductive success in the Great Lakes, and that even though concentrations of total PCBs and other persistent compounds had decreased in the environment, these compounds continued to elicit adverse effects. Tillitt et al. [19] found the correlation between H4IIE bioassay-generated TEQs and egg mortalitiy in double-crested cormorants (using extracts that contained only PCBs, but not PCDD or PCDF-type planar halogenated hydrocarbons) was much stronger than the correlation between total PCB and egg mortality.
Smith et al. [45] found a correlation for pacific salmon from Lake Ontario between H4IIE-derived TEQs and organochlorine residues, but TEQs did not correlate with embryonic mortality. They concluded that although dioxinlike compounds were present in the organisms, these compounds were not directly responsible for the embryonic mortality noted in the species. In this study, a comparison of bioassay-derived TEQs with chemically derived TEQs (using TEFs from a variety of sources) was performed on selected samples from the temporal trends study. When the two methods of generating TEQs were compared to one another (Fig. 5), the results were inconclusive. Relatively poor regression coefficients were obtained, although the correlation when rainbow trout TEFs were used was marginally better. Although the correlations were weak, no obvious trend to over- or underestimate the total TEQ was evident. When the bioassay overestimates the TEQ, a possible conclusion is that some other compound (or compounds) is contributing to the total TEQ, and if the bioassay consistently underestimates the TEQ, then possibly interactions between toxic and nontoxic PCBs are occurring, and having a mitigating effect on the total TEQ.
CONCLUSIONS
The results of this study show that levels of non-, mono-, and di-ortho PCBs, and PCDDs and PCDFs in lake trout from Lake Ontario have declined substantially since the late 1970s. Most chemical levels appear to have reached a steady state, or to be declining very slowly. Non-ortho-substituted PCBs do not appear to be significantly enriched relative to other PCB congeners, or to total PCB, over time, and no enrichment of these PCBs occurs with age. Levels of 2,3,7,8-TCDD have declined in proportion to levels of 2,3,7,8-TCDF, indicating a decline in specific sources of 2,3,7,8-TCDD to Lake Ontario. The choice of TEF used to calculate TEQs dramatically influences the interpretation of which compounds are the most significant for risk assessment purposes. A comparison of H4IIE cell bioassay-generated TEQs with TEQs calculated from TEFs showed a weak correlation between the two techniques.
Acknowledgements
The support of the Canadian Department of Fisheries and Oceans, the University of Waterloo, and of the scientific personnel at the Great Lakes Laboratory for Fisheries and Aquatic Science is greatly appreciated. A special thanks to Cam MacEachen for his invaluable technical assistance.




