Influence of pH on the toxic effects of zinc, cadmium, and pentachlorophenol on pure cultures of soil microorganisms
Abstract
In this study the effect of the acidification of soil pore water on the uptake and toxicity of cationic and anionic pollutants was measured in an experimental model system. The influence of pH on the toxic effects of zinc, cadmium, and pentachlorophenol was studied in buffered suspensions of pure cultures of soil microorganisms. In this system the speciation of the toxicant, the pH, and the biomass are defined, constant, and thus easier to study than in a system with the solid soil matrix and pore water. The mineralization of [14C]acetate to 14CO2 was used to measure the toxic effects of pollutants on a fungus (Aspergillus niger CBS 121.49), an actinomycete (Streptomyces lividans 66), two Gram-negative Pseudomonas putida strains (MT-2 and DSM 50026), and a Gram-positive strain (Rhodococcus erythropolis A177). Large differences in sensitivity were observed between the species. For pentachlorophenol the highest EC50 was 81 mg/L for Pseudomonas putida at pH 8, whereas the lowest was 0.13 mg/L for Aspergillus niger at pH 6. Aspergillus niger was not sensitive to 1,000 mg Zn/L, whereas Pseudomonas putida at pH 7.8 showed the lowest EC50, 0.14 mg Zn/L. When pH was increased, pentachlorophenol became less toxic and showed less sorption to the biomass, whereas zinc and cadmium became more toxic and showed more sorption to the biomass. The results indicate that higher pore-water concentrations due to acidification of zinc- and cadmium-polluted soils may not be accompanied by increased toxic effects on microorganisms because of the relatively low toxicity of these metals in pore water at low pH.
INTRODUCTION
Soil pollution can pose a serious threat to the functioning of ecosystems. A pollutant can be sorbed to the soil matrix, interact with dissolved components in pore water, or taken up by the organisms, which can cause a toxic effect. The pore water plays an important role in the uptake of soil toxicants by different organisms. The concentration of chlorophenols in the soil pore water, for example, determines the uptake and toxicity of these compounds for earthworms in soil [1]. The pore-water hypothesis states that the concentration in pore water determines the toxic effect. Sorption to the solid phase is influenced by pH. The influence of the H+ concentration in pore water on sorption, uptake, and toxicity is different for cationic pollutants like metals and anionic pollutants like pentachlorophenolate.
At lower pH levels the solubility of metals and the toxic effects of metals on earthworms increases simultaneously [2]. This is in accordance with the pore-water hypothesis, because a lower pH causes decreased sorption. This causes an increased pore-water concentration, which in turn may increase the uptake of metals by organisms. Therefore, it has been argued that increasing soil pH might alleviate the effects of metal pollution. This may not be the case for microorganisms, however, because the toxicity of 500 mg Zn/kg soil on the dehydrogenase enzyme is lower in acid soils compared to soils with a high pH [3]. Moreover, acetate mineralization in acid soils seems to be resistant to concentrations as high as 1,000 mg Zn/kg [4], whereas the same process is inhibited by 10% at 60 to 300 mg Zn/kg in soils with pH 8 [5]. Hence, at lower pH the toxic effect of metals seems to increase for earthworms and to decrease for soil microbial processes. Aside from microorganisms, other examples indicate increased toxicity of metals at high pH. Zinc, for example, is more toxic to fish in hard water with a high pH and high Ca2+ and CO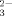 concentrations than in soft water with a lower pH and less salts [6].
concentrations than in soft water with a lower pH and less salts [6].
In addition to zinc, pentachlorophenol (PCP) and cadmium were used as toxicants. Cadmium was selected as an extra metal to confirm the influence of pH on the toxicity of zinc. The pore-water hypothesis was derived with chlorophenols like PCP [1], which in the past was used as a wood preservative and is still present in water and soil [7]. Its mode of action is the uncoupling of oxidative phosphorylation in the cell membrane [8]. The sorption of PCP is dependent on pH because the dissociation constant, pKa, is in the range of 4.74 [9] to 5.25 [10]. At high pH the phenolate form is present and shows decreased sorption to organic material [11]. Pentachlorophenolate also shows less bioaccumulation and toxicity in fish. At low pH there is strong bioaccumulation and a low LC50, whereas at high pH there is less bioaccumulation and a high LC50, resulting in a lethal internal concentration of about 90 mg PCP/kg goldfish (Carassius auratus) at all pH values [12].
The PCP concentration that inhibits 10% of the acetate mineralization rate in 10 soils (EC10) varies from 5 to 800 mg/kg [5]. Variations in sorption due to pH and organic carbon content cannot explain these differences in EC10 concentrations [5]. The variations in toxic effects of pollutants like PCP and zinc on microbial processes in soils with different pH are not easy to explain in complex soil systems because each soil sample has different microflora and its own sensitivity.
In this study the toxic effects of Zn2+, Cd2+, and PCP were measured in dilute cell suspensions of pure cultures of different microorganisms in well-defined buffers. In dilute cell suspensions a number of problems are smaller than in concentrated suspensions; for example, the free toxicant concentration is not significantly lowered by sorption to the biomass or by components excreted by the cells, and the pH is not lowered by large amounts of CO2 produced by the cells. The mineralization of acetate is a common process in soils [13]. In this study the mineralization of [14C]acetate was used as an ecologically relevant monitoring tool to assess the activity of microorganisms in buffers. With this method the modifying effect of pH on the toxicity of zinc, cadmium, and PCP was measured without interference of soil properties and differences in the microbial communities.
MATERIALS AND METHODS
Culture of microorganisms
Aspergillus niger CBS 121.49, DSM 2182 (filamentous fungi) isolated in 1947 from Dutch soil in Wageningen was obtained from the German Collection of Microorganisms and Cell Cultures DSM in Braunsweig, Germany. For storage, 0.1 ml of a spore suspension in 4.8% skim milk with 4% glutamate was pipetted in a tube with 0.5 g of dry and sterilized silica gel. The fungus was grown on nutrient broth (Oxoid CM1, Hampshire, UK) agar plates. The spores were harvested from the agar plate and suspended in water. About 12.5 ml of a spore suspension with A550 = 1 was diluted in 2.5 L of liquid nutrient broth and incubated overnight at 28°C until fungal spheres were formed (1 mm diameter, 0.8 g dry weight/L). The spheres were harvested on a sterilized stainless-steel sieve (38 μm) and washed with the appropriate buffer for toxicity testing.
Streptomyces lividans 66 NCIMB 11416 (actinomycetes) was obtained from the national collections of industrial and marine bacteria in Aberdeen, UK. One colony with fresh white spores was inoculated in 100 ml of liquid nutrient broth, and the actinomyces was grown for 2 d at 28°C. This was used to inoculate 2.5 L of liquid nutrient broth, which was incubated for 24 h at 28°C. The spheres that passed through a 425-μm sieve were harvested and washed on a 38-μm sieve.
Pseudomonas putida MT-2 (Gram-negative bacteria) was originally isolated from Japanese soil. The strain was obtained from P.A. Williams, who stored it in the American Type Culture Collection (ATCC 3301). The closely related strain Pseudomonas putida DSM 50026, obtained from the German culture collection, was isolated from a channel in Berlin and is used as a test strain for water toxicity [14, 15].
Rhodococcus erythropolis A177 (Gram-positive bacteria) was obtained from H.H.M. Rijnaarts [16]. The Rhodococcus and Pseudomonas strains were stored at −80°C, grown on nutrient broth, and washed by centrifugation for a toxicity test.
Zinc sorption
The sorption of zinc to P. putida MT-2 and P. putida DSM 50026 was measured in a 20-mmol/L tris(hydroxymethyl) aminomethane buffer (Tris) (Sigma Chemical Co., St. Louis, USA) neutralized with HC1 (Merck, Darmstadt, Germany). Cells were washed with buffer by centrifugation at 3,300 g for 15 min, and the pellet was resuspended in buffer. For Tris buffer at pH 5.5 and pH 6, nominal zinc concentrations of 30 and 10 mg/L, respectively, were used. For Tris buffer at pH 7.8, three nominal concentrations of zinc were used, 0.3, 3, and 30 mg/L. The suspensions were incubated for 90 min at 28°C. After centrifugation the zinc concentration was measured in the supernatant and the pellet with an atomic absorption spectrophotometer (Perkin and Elmer, type 2380, Ueberlingen, Germany).
PCP sorption
The sorption of PCP (Fluka, Buchs, Switzerland) was measured in a 20-mmol/L potassium phosphate buffer (Merck). Washed microorganisms were incubated in 2.5 ml of buffer with [14C]PCP (Amersham, Buckinghamshire, UK) at a concentration of 3 μg [14C]PCP/ml with 16 Bq/ml for 1 h at 28°C. After centrifugation the PCP concentration in the supernatant and the pellet was measured with a Tricarb liquid scintillation counter (Packard, type 1500 or 1550, Downers Grove, IL, USA).
Toxicity testing
The method used to perform the toxicity tests was described in more detail previously [17]. Washed microorganisms were incubated in a closed 60-ml bottle with 20 ml of buffer containing 1 ng [14C]acetate/ml with 10 Bq/ml. The low amounts of microorganisms and substrate assure that the 40 ml of air in each bottle is not depleted. The bottles were incubated for different time intervals with different toxicant concentrations, and mineralization was stopped with 1 ml of 2 M H2SO4. The 14CO2 was flushed out of the bottles with nitrogen gas, trapped, and counted with a scintillation detector. The remaining substrate was also measured with a scintillation detector.
The tests are designed to measure the toxicity under defined conditions with minimal interaction of the toxicant with the medium. For PCP a 20-mmol/L potassium phosphate buffer (KPi) was used so that potassium was the counter ion of the pentachlorophenolate anion. Pentachlorophenol was added as phenolate anion, which is more soluble, and Zn2+ was added as ZnC12. For Zn2+ a 20-mmol/L Tris buffer neutralized with HC1 or a 20-mmol/L bis(2-hydroxyethyl)imino-tris(hydroxymethyl) methane buffer (BisTris)(Sigma) neutralized with HC1 was used. At 25°C the pKa of KPi, BisTris, and Tris is 7.21, 6.5, and 8.06, respectively. Because BisTris and Tris are both positively charged buffers, the counter ion of Zn2+ was chloride in our experiments.
Statistics
All toxicity and sorption experiments were performed at least in duplicate. The percentage of substrate consumed and CO2 formed are plotted against the logarithm of the toxicant concentration. Two logistic curves were fitted with these data, and the EC50 and EC10 were calculated as described previously [17].
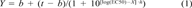 (1)
(1)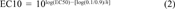 (2)
(2)
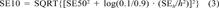 (3)
(3)The standard error in the percentage substrate consumed was larger than the standard error in CO2 production because an increase in the percentage CO2 from 0 to 10% is measured more accurately than a decrease in the percentage substrate from 100 to 90%. The 95% confidence intervals of the log(EC50) were calculated from the log(EC50) ± 1.96 × SE50. Results obtained with this statistical procedure were compared with those obtained using the GenStat 5 (Oxford) and Kaleidagraph 3.0.1 (Abelbeck Software) statistical packages, and all were similar.
| EC50 (mg/L) | EC10 (mg/L) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| pH | Biomass (mg/L) | Half-life (min) | Rate (L/μg min−1) | Time (min) | CO2 | Acetate | CO2 | Acetate | |
| Pseudomonas putida MT2 | 4.5 | 3 | 15 | 15 | 60 | 1.6a | 2.1 | 0.6e | 1.1 |
| 6 | 3 | 29 | 8 | 60 | 1.0a | 0.9 | 0.8e | 0.7 | |
| 6.9 | 6 | 18 | 6 | 30 | 9.6b | 9.9 | 3.6e | 4.5 | |
| 8 | 3 | 31 | 7 | 60 | 81c | 44 | 10e | 9 | |
| Pseudomonas putida DSM 50026 | 6.8 | 6 | 34 | 3 | 30 | 32c | 30 | 26f | 25 |
| Rhodococcus erythropolis | 6.9 | 39 | 31 | 0.57 | 90 | 3.1a | 2.8 | 2.6e | 2.3 |
| Streptomyces lividans 66 | 6 | 58 | 164 | 0.07 | 120 | 0.8a | 0.8 | 0.7e | 0.6 |
| 7 | 58 | 422 | 0.03 | 120 | 2.6a | 1.5 | 2.2e | 0.6 | |
| Aspergillus niger | 6 | 71 | 141 | 0.07 | 200 | 0.13d | 0.15 | 0.05g | 0.07 |
| 7 | 71 | 986 | 0.01 | 1,140 | 0.8a | 1 | 0.4e | 0.4 | |
- a-g Values with the same letter have overlapping 95% confidence intervals. Streptomyces lividans 66 and Aspergillus niger did not mineralize acetate at high pH. The EC50 for CO2 was derived from the 14CO2 production from [14C]acetate. The 14CO2 production of Rhodococcus erythropolis was stimulated at 0.3 to 1 mg PCP/L.
RESULTS
Toxic effects of PCP on acetate mineralization by five microorganisms
The mineralization of low concentrations of substrates by microorganisms can be described by first-order kinetics. When increasing concentrations of a toxicant are added, the mineralization rate decreases.
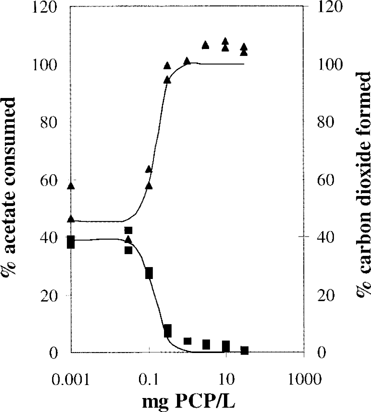
Figure 1 shows the toxic effect of PCP on acetate mineralization by spheres of A. niger mycelium in phosphate buffer at pH 6. At low toxicant concentrations, about 54% of the [14C]acetate is converted to cell material plus 39% 14CO2. The PCP concentration is shown on a logarithmic scale on the X axis. The control is arbitrarily drawn at 0.001 mg PCP/L because zero cannot be plotted on a logarithmic scale. At high PCP concentrations no 14CO2 is formed, and the [14C]acetate is not mineralized during incubation (200 min). The descending 14CO2 production curve was fitted by nonlinear regression and can be described by Equation 1 using a log(EC50) of -0.9 (with a standard deviation of 0.1) and a slope h of -2.4 (with a standard deviation of 1.2). This results in an EC50 of 0.13 mg PCP/L with a 95% confidence interval of 0.08 to 0.2 mg PCP/L and an EC10 of 0.05 mg PCP/L (using Eqn. 2) with a 95% confidence interval of 0.02 to 0.15 mg PCP/L (calculated with Eqn. 3). Note that the confidence intervals are asymmetric because of the 10log transformation of the EC50 and EC10. The EC50 and EC10 from the ascending acetate curve were calculated in a similar way. In Table 1 the data of Figure 1 are summarized at the second row from the bottom. The other EC50 and EC10 values in Table 1 were calculated from dose-effect curves that are not shown.
Table 1 gives an overview of 10 dose-effect curves describing the effect of PCP on different microorganisms of various pH values. Striking differences in sensitivity are evident between species. The Gram-negative P. putida strains are not very sensitive to PCP, whereas A. niger was the most sensitive. Pentachlorophenol becomes less toxic at high pH values with such very different microorganisms as a Gram-negative bacterium Pseudomonas, an actinomycete Streptomyces, and a fungus Aspergillus.
Uptake and sorption of PCP to Pseudomonas putida MT2
 (4)
(4)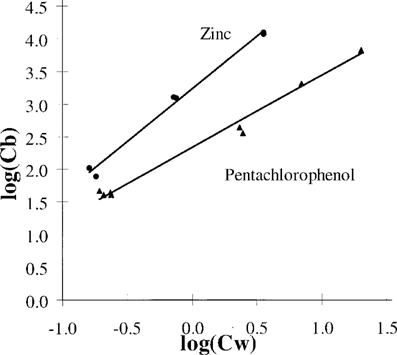
The sorption of pentachlorophenol in phosphate buffer at pH 7 and zinc in Tris buffer at pH 7.8 to Pseudomonas putida MT 2. Cw = the toxicant concentration in water (mg/L), and Cb = the toxicant concentration sorbed to the P. putida cells (mg/kg cell dry weight). The lines are drawn using Equation 4 with n = 1.1 and log(Kf) = 2.3 for PCP and n = 1.6 and log(Kf) = 3.2 for zinc.
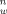 , which can be simplified to
, which can be simplified to
 (5)
(5)| EC50 (mg PCP/L) | EC50 (mg PCP/g dry weight) | |||||||
|---|---|---|---|---|---|---|---|---|
| pH | External | Minimuma | Maximuma | Internal | Minimuma | Maximuma | Kf (L/kg) | Undissociated fraction |
| 4.5 | 1.6 | 0.55 | 4.9 | 2.5 | 0.8 | 7.4 | 1,500 | 0.64 |
| 6 | 1.0 | 0.77 | 1.3 | 0.3 | 0.2 | 0.3 | 260 | 0.053 |
| 6.9 | 9.6 | 7.9 | 12 | 1.6 | 1.3 | 1.9 | 160 | 0.0070 |
| 8 | 81 | 47 | 143 | 12 | 6.9 | 21 | 150 | 0.00056 |
- a Minimum and maximum values indicate the 95% confidence interval.
- b The external EC50 (in mg PCP/L) was taken from Table 1 and was multiplied by the sorption constant K at the proper pH, which was derived from Figure 3. The result was divided by 1,000 to calculate the internal EC50 (in mg PCP/g cell dry weight).
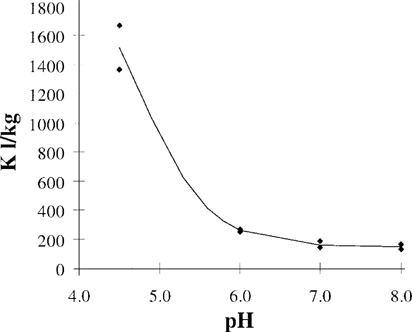
The effect of pH on the sorption of pentachlorophenol to Pseudomonas putida MT 2. The curve is drawn using Equation 6 with Kn = 2,300 (L/kg), Ki = 150 L/kg, and pKa = 4.75.
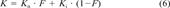 (6)
(6) (7)
(7)Figure 3 shows the effect of pH on the sorption of PCP to P. putida MT 2. The curve describes the K value calculated with Equation 6 and fitted with nonlinear regression with a fixed pKa of 4.75.Kn = 2,300 L/kg dry weight with a standard error of 103, and Ki = 150 L/kg dry weight with a standard error of 40.
Because the sorption constant is now known at all the test pH values, the “internal dose” sorbed onto or into the bacteria can be calculated from the effect concentrations. This internal dose is the sum of the amount of toxicant that is sorbed onto the cell and the intracellular dose. The PCP concentration that is present in the water is referred to as the “external dose.” Table 2 shows the calculation of the internal dose of PCP that inhibits acetate mineralization by 50%. Columns 2 through 4 show the EC50s from Table 1 converted to the “internal” doses using Kf values from Figure 3. The “minimum” and “maximum” columns indicate the 95% confidence intervals that were calculated from the nonlinear regression of the dose-effect curves. The internal EC50 (in mg PCP/g cell dry weight) is not constant for all pH values. Therefore, the differences in the sensitivity to PCP of P. putida MT 2 at various pH values cannot be attributed solely to differences in sorption.
| EC50 (mg/L) | EC10 (mg/L) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| pH | Biomass (mg/L) | Half-life (min) | Rate (L·μg−1.min−1) | Time (min) | CO2 | Acetate | CO2 | Acetate | |
| Pseudomonas putida MT2 | 5.5 | 6 | 36 | 3.3 | 30 | 9a | 1.7ik | ||
| 6.0 | 6 | 34 | 3.4 | 30 | 4.0acg | 2.6 | 0.2i | 0.2 | |
| 7.8 | 6 | 82 | 1.4 | 30 | 0.14b | 0.18 | 0.005j | 0.09 | |
| Pseudomonas putida DSM 50026 | 6.0 | 6 | 21 | 5.5 | 30 | 6.8a | 1.8k | 2.2 | |
| 7.9 | 6 | 23 | 5.0 | 30 | 2.0c | 2.0 | 0.3i | 0.3 | |
| Rhodococcus erythropolis | 5.5 | 39 | 65 | 0.3 | 90 | 113d | 88 | 10l | 10 |
| 5.9 | 44 | 16 | 1.0 | 60 | 53e | 19 | 1.6k | 1.1 | |
| 7 | 49 | 28 | 0.5 | 60 | 21f | 16 | 12l | 10 | |
| 7.9 | 44 | 25 | 0.6 | 60 | 4.5g | 3.8 | 2.3km | 2.5 | |
| Streptomyces lividans 66 | 6 | 374 | 283 | 0.007 | 120 | 12a | 12 | 2.5m | 3.3 |
| 7 | 490 | 201 | 0.007 | 120 | 5.5h | 4.6 | 2.5m | 1.8 | |
| Aspergillus niger | 6 | 148 | 46 | 0.101 | 60 | >1,000 | >1,000 | >1,000 | >1,000 |
| 7 | 148 | 659 | 0.007 | 1,170 | >1,000 | >1,000 | >1,000 | >1,000 | |
- a-m Values with the same letter have overlapping 95% confidence intervals. Streptomyces lividans 66 and Aspergillus niger were inhibited at pH 8; therefore, a BisTris buffer with a pKa of 6.5 was used.
The internal EC50 concentrations of PCP in P. putida can be compared with the internal EC50 concentration in goldfish (0.27 mg PCP/g dry weight [8], assuming that a goldfish contains 80% water). This value is in the same range as the internal EC50 of PCP in P. putida (see Table 2).
Toxic effect of zinc on acetate mineralization by five species of microorganisms
A cationic buffer like Tris or BisTris was used for the experiments with Zn2+ to avoid the strong interactions like precipitation and complexation that can occur between an anionic buffer and zinc. The counter anion was chloride for both the buffer and the toxicant. A cationic buffer might show some competition with Zn2+ on the uptake system of the cells, and therefore it is not possible to select a buffer without any possible interaction with Zn2+. Table 3 shows the toxic effects of zinc on acetate mineralization by five microorganisms in Tris or BisTris buffer at different pH values. Large differences in sensitivity to zinc are evident between the different species. Pseudomonas putida MT2 was the most sensitive to zinc, whereas A. niger was not sensitive at all.
At higher pH the toxicity of zinc increased considerably. This is in agreement with earlier observations that zinc was more toxic to microorganisms in alkaline soils (see Introduction). Acid soils are known to contain more fungi that are relatively tolerant to metals [20]. Both the decreased toxicity and the increased proportion of fungi might add to the relative insensitivity of the microflora in acid soils to zinc.
| EC50 (mg/L) | EC10 (mg/L) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| pH | Biomass (mg/L) | Half-life (min) | Rate (L/μg min−1) | Time (min) | CO |
Acetate | CO2 | Acetate | |
| Zn | 6 | 6 | 406 | 0.28 | 110 | 11b | 11 | 3d | <1 |
| Zn | 7 | 6 | 315 | 0.37 | 110 | <1 | <1 | <0.3 | <0.3 |
| Zn | 8 | 6 | 741 | 0.16 | 110 | 0.09c | <0.3 | 0.02e | <0.1 |
| Cd | 6 | 6 | 406 | 0.28 | 110 | 122d | 43f | ||
| Cd | 7 | 6 | 315 | 0.37 | 110 | 5.9e | 3.6 | 2.9g | 2.9 |
| Cd | 8 | 6 | 741 | 0.16 | 110 | 0.1e | 0.3 | 0.03e | 0.2 |
- a The EC50 for CO2 was derived from the 14CO2 production from [14C]glucose.
- b-g Values with the same letters have overlapping 95% confidence intervals.
Toxic effect of zinc and cadmium on glucose mineralization by P. putida MT2
Acetic acid has a pKa value of 4.75 [21], which might also influence the acetate mineralization and the toxic effect of a metal like zinc at different pH values. Tables 1 and 3 show that the half-lives of the acetate mineralization increase at higher pH with P. putida MT2 and A. niger but not with P. putida DSM 50026 or R. erythropolis. An indirect effect of the cation Zn2+ on the mineralization of the anion acetate could not be excluded from the above experiments. Therefore, the toxicity tests were also performed with [14C]glucose as a substrate for P. putida MT2.
Table 4 shows that the toxicity of both Zn2+ and Cd2+ to glucose mineralization also increases at higher pH. The sensitivities to zinc of acetate and glucose mineralization by P. putida MT2 are very similar. The toxicity of cadmium increases sharply at increasing pH. One might speculate that the increased toxicity of zinc and cadmium at higher pH is not a peculiarity of these metals but a more general phenomenon that occurs with many metals.
Sorption of Zn2+ to P. putida MT−2 in Tris buffer at different pH values
 (8)
(8)| pH | Total concn. (mg/L) | Supernatant conc. (mg/L) (Cw) | Solid-phase concn. (Cb) (mg Zn2+/g cell dry weight) | Ka (L/g) |
|---|---|---|---|---|
| 5.5 | 34 | 6.4 | 12 | 1.9 |
| 5.5 | 34 | 8.7 | 11 | 1.3 |
| 6.0 | 11 | 1.4 | 4.2 | 3.0 |
| 6.0 | 12 | 1.6 | 4.5 | 2.8 |
| 7.8 | 0.40 | 0.16 | 0.10 | 0.7 |
| 7.8 | 0.36 | 0.18 | 0.08 | 0.4 |
| 7.8 | 3.6 | 0.72 | 1.3 | 1.7 |
| 7.8 | 3.6 | 0.76 | 1.2 | 1.6 |
| 7.8 | 32 | 3.6 | 12 | 3.4 |
| 7.8 | 30 | 3.6 | 11 | 3.2 |
The constants n = 1.6 and log(Kf) = 3.2 were derived by linear regression of the sorption data shown in Figure 2. Table 5 shows that sorption is not very dependent on pH for this Gram-negative bacterium. At pH 5.5 the sorption constant K was 1.9 L/g, while at pH 7.8 it was 3.2 (see the upper and lower rows of Table 5). The EC50 at pH 5.5 was 64 times higher than the EC50 at pH 7.8 (see Table 3). Hence, no single internal dose corresponds to the EC50 of zinc at all pH values.
| Toxicant | Effect | Concn. (mg/l) | Organism | Medium | Reference |
|---|---|---|---|---|---|
| PCP | EC50 growth | 6 | Pseudomonas putida DSM 50026 | Synthetic medium (pH 7) | [15] |
| EC10 growth | 0.7 | ||||
| EC50 growth | 85 | Pseudomonas fluorescens | Organic medium | [24] | |
| EC20 growth | 4.7 | ||||
| EC50 growth | 142 | Pseudomonas fluorescens | Organic medium | [25] | |
| EC20 respiration | 75 | Pseudomonas fluorescens | Phosphate buffer (pH 7) + glucose | [26] | |
| 8 h lag growtha | 25 | ||||
| EC14 growth | 25 | Pseudomonas fluorescens | Phosphate buffer (pH 7) | [27] | |
| MIC growthb | 133 | Aspergillus niger PC6 | Mueller-Hinton Agar | [28] | |
| Zn | EC50 respiration | 74 | Pseudomonas fluorescens | Nutrient broth | [29] |
| EC50 growth | >650 | Pseudomonas aeruginosa | Phthalate buffer (pH 6) | [30] | |
| EC44 respiration | 5 | Pseudomonas fluorescens | Maleate buffer (pH 7) | [31] | |
| EC35 growth | 10 | Aspergillus flavus | Nutrient medium (pH 5) | [23] | |
| EC50 growth | 650 | Aspergillus niger | Sabouraud dextrose agar (pH 5.6) | [30] | |
| Cd | EC50 respiration | >100 | Pseudomonas fluorescens | Maleate buffer (pH 7) | [31] |
| EC50 respiration | 53 | Pseudomonas fluorescens | Nutrient broth | [29] |
- a The exponentially growing culture showed an increased lag time before the start of measurable growth.
- b MIC = minimal inhibitory concentration.
DISCUSSION
Sorption and toxicity of metals to R. erythropolis A177
The sorption of zinc and cadmium on the cell walls and intact cells of R. erythropolis A177 was decreased at lower pH [22] and higher Ca2+ concentrations due to competition of H+ and Ca2+ with Zn2+ or Cd2+ on the bindings sites on the cell wall. Rhodococcus erythropolis A177 is a Gram-positive bacterium that has a thick cell wall that is not covered with a membrane [22]. In contrast, Gram-negative bacteria like P. putida have a thin cell wall covered with a membrane that forms a barrier for the sorption of ions. For R. erythropolis A177, the large increase of the EC50 for zinc and cadmium at lower pH could be explained by decreased sorption to the cell wall [22], whereas for P. putida MT-2 this was not the case.
Data comparison
The toxic effects of PCP, zinc, and cadmium were measured on different pure cultures of microorganisms. A detailed comparison is possible only when identical strains are used. Table 6 shows the reported toxic effects of PCP, zinc, and cadmium on the growth or respiration of pure cultures of strains similar to those used in this study. Glutamate respiration by P. putida DSM 50026 in continuous culture was five times more sensitive for PCP than [14C]acetate mineralization by the same strain (Table 1). In both tests a low substrate concentration was present, which is normal in natural environments. Growth of A. niger PC6 on agar medium was 1,000 times less sensitive for PCP than [4C]acetate mineralization by A. niger CBS 121.49 (Table 1). The EC50 of zinc for P. putida ranged from 0.14 to 9 depending on the pH and the strain (Table 3), whereas higher values have been reported for P. fluorescens (Table 6).
Also, the EC50 of cadmium on [14C]glucose mineralization by P. putida MT2 was lower (Table 4) than the EC50 at pH 7 for other Pseudomonas strains (Table 6). Thus, in general assessing the mineralization of low concentrations of 14C-labeled acetate or glucose in buffer seems to be a sensitive method for the measurement of toxic effects. In contrast, growth of A. flavus or A. niger on media (Table 6) was more sensitive to zinc than [14C]acetate mineralization by A. niger CBS 121.49 (Table 1). This might be due to differences in the sensitivity of the strains because the toxicity of zinc to the growth of fungi in medium decreases at higher pH because of precipitation of the Zn2+ with components of the medium [23]. The presence of the Tris+ cation in our experiments at low pH also may have led to decreased toxicity because cations may compete with metal ions for the available anionic sites on the surface of microorganisms.
Influence of acidification on toxic effects of metals for soil animals and microorganisms
The toxicity of metals to soil animals can increase with soil acidification [2], whereas an opposite effect occurs with zinc on soil microorganisms [3-5]. Three factors can contribute to this difference: (1) acid soils contain fungi that are more resistant to metals [20]; (2) the toxicity of metals like zinc and cadmium to microorganisms is decreased at low pH values, (shown in this article); and (3) soil animals can control the level of pH in their intestines, which makes metal uptake via the consumption of pore water and food less dependent on the external pH.
CONCLUSIONS
The pore-water hypothesis predicts that acidification of metal-polluted soils leads to an increase in metal toxicity in soil as a result of an increase in metal concentrations in pore water. The predicted increased metal toxicity on microbial activity in acid soils was not supported by results reported in the literature. This study shows that the toxicity of metals to microorganisms in water can decrease at lower pH values and that the fungi that are predominant in acid soils are more resistant to zinc.
For anionic pollutants like pentachlorophenolate, a decreased level of pH leads to an increase in sorption and therefore to a lower pore-water concentration. This study shows that the uptake and therefore the toxicity of PCP in water increases at lower pH.
At a lower soil pH there is a higher concentration of cationic pollutants like zinc and cadmium and a lower concentration of anionic pollutants like pentachlorophenolate. These concentration changes are counteracted by the decreased toxicity of the cations and the increased toxicity of the anion at low pH.
Acknowledgements
This study was carried out on behalf of the Directorate-General for Enviromental Protection, for the Directorate of Soil Protection, and the Directorate of Chemicals, Safety, and Radiation Protection of the Dutch Ministry of Housing, Physical Planning, and Environment. We thank R.P.M. van Veen for metal analysis and W.J.G.M. Peijnenburg, L. Posthuma, J. Notenboom, and J. Struijs for critical reading of the manuscript.




