Toxicity of oxyanions of selenium and of a proposed bioremediation intermediate, dimethyl selenone
Abstract
The relative toxicity of selenate, selenite, and dimethyl selenone were assessed by determining growth inhibition and growth rate inhibition of Pseudomonas fluorescens K27 and using the Microtox® bioassay method at varying concentrations of these three selenium species. The results of EC50 vary depending on the organism tested and the method used; for P. fluorescens K27, EC50 increased in the order of dimethyl selenone < selenate < selenite. However, for Vibrio fisheri, EC50 increased in the order of selenite < dimethyl selenone < selenate. The possibility that different reduction mechanisms may dominate the remediation of the two selenium oxyanions with different oxidation states by P. fluorescens K27 is discussed, as is whether dimethyl selenone is a possible intermediate in selenium reduction and methylation.
INTRODUCTION
The reduction and biomethylation of selenate (SeO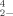 ) and selenite (SeO
) and selenite (SeO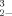 ) by microorganisms is not clearly understood. Proposed mechanisms suggest that dimethyl selenone [(CH3)2SeO2] is an intermediate of this reduction pathway [1-3]. Selenium-resistant microbes reducing selenium oxyanions to form elemental selenium and/or methylated, volatile selenide products can decrease the concentration of selenium compounds in solution and thus decrease the toxicity of these compounds in their immediate environment [4, 5].
) by microorganisms is not clearly understood. Proposed mechanisms suggest that dimethyl selenone [(CH3)2SeO2] is an intermediate of this reduction pathway [1-3]. Selenium-resistant microbes reducing selenium oxyanions to form elemental selenium and/or methylated, volatile selenide products can decrease the concentration of selenium compounds in solution and thus decrease the toxicity of these compounds in their immediate environment [4, 5].
The toxicity of selenium oxyanions and some of the volatile products of bioconversion has been examined, and though the results are somewhat variable depending on the organism tested, the general consensus has been that selenate is less toxic than selenite and that dimethyl selenide, (CH3)2Se, is less toxic than both of these oxyanions [4-9]. Recently the comparison of organoselenium production by selenium-resistant bacterial cultures of Pseudomonas fluorescens K27, amended with either selenate, selenite, or dimethyl selenone, showed that the cultures produced significantly more dimethyl selenide and dimethyl diselenide when amended with dimethyl selenone compared to those with added selenate or selenite [3]. This suggested to us that dimethyl selenone might indeed be viable as an intermediate in this reduction process from Se6+ or Se4+ to elemental Se or selenide, and if it were produced in a bio-remediation mechanism, would therefore aid in the detoxification of the immediate environment.
The discovery of biological reduction of dimethyl selenone by anaerobic bacteria sparked our interest in the relative toxicity of this proposed intermediate compared to the oxyanions of selenium most commonly found in the environment, selenate and selenite. To examine this toxicity, tests with these three chemical species were performed (1) with a commercial bioassay (Microtox®) that uses the bioluminescent bacteria Vibrio fisheri [10-13] and (2) by comparing growth inhibition and growth rate inhibition using P. fluorescens K27, a selenium-resistant bacterium isolated from Kesterson Reservoir in the San Joaquin Valley of California, USA [14]. A selenium-resistant bacterium was used for the growth inhibition and change in growth rate experiments because previous experience with this microbe [3, 15] has shown that it has some ability to grow in the presence of all three reagents, SeO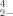 , SeO
, SeO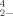 , and (CH3)2SeO2, and therefore could be examined in this way. Also, we wanted to compare the toxicity of (two of) these chemical species on a selenium-resistant microbe to determine whether the relative toxicity of these compounds was significantly different from what has been reported in the literature from studies that used toxicity assays with non-selenium-resistant organisms. As far as we know this is the first report of toxicity experiments using dimethyl selenone.
, and (CH3)2SeO2, and therefore could be examined in this way. Also, we wanted to compare the toxicity of (two of) these chemical species on a selenium-resistant microbe to determine whether the relative toxicity of these compounds was significantly different from what has been reported in the literature from studies that used toxicity assays with non-selenium-resistant organisms. As far as we know this is the first report of toxicity experiments using dimethyl selenone.
MATERIALS AND METHODS
Reagents and synthesis of dimethyl selenone
Sodium selenate (Na2SeO4) and sodium selenite (Na2SeO3) were purchased from Strem Chemicals (Newburyport, MA, USA) and from Aldrich Chemical Company (Milwaukee, WI, USA). Reagents used in the Microtox tests were supplied by the manufacturer (Microbics, Carlsbad, CA, USA) and include diluent and osmotic adjustment solutions (NaCl solutions in highly purified H2O).
Dimethyl selenone (hereafter called DMSeO2) was synthesized following the method of Krief et al. [16] with slight modifications. In our early synthetic procedures [3], 3 Meq of 3-chloroperoxy benzoic acid (65% purity, Aldrich) were added to 1.41 g of dimethyl selenide (DMSe) (Strem Chemicals) that had been dissolved in methylene chloride (high-performance liquid chromatography [HPLC] grade) (Sigma Chemical Co., St. Louis, MO, USA). In our later syntheses of DMSeO2 these amounts of these two reactants were combined in the reverse order after we determined that adding dissolved DMSe to the oxidant increased the yield of DMSeO2. After the mixture was stirred for 2 h, it was taken to dryness and washed three times with ether to remove unreacted DMSe and the 3-chlorobenzoic acid by-product. Finally, the crude DMSeO2 was recrystallized twice from HPLC-grade methanol (Sigma). Recrystallized DMSeO2 had a melting point (m.p.) of 147 to 149°C (m.p. reported in the literature, 147-148°C [17]). The nuclear magnetic resonance (NMR) spectrum taken using deuterated water (Aldrich) as the solvent and 2,2-dimethyl-2-silapentane-5-sulfonate (Aldrich) as the internal standard showed a singlet at 3.57 ppm (chemical shift, δ). This datum has not been reported in the literature as far as we know but can be compared to the proton resonance for dimethyl sulfone at 3.03 ppm (Aldrich Library spectrum). Dimethyl selenone was also characterized by elemental analysis and Fourier transform infrared spectroscopy (FTIR) [3].
Dimethyl selenone is somewhat unstable at room temperature, possibly disproportionatly to the methyl methaneseleninate, CH3Se(O)OCH3, and was recrystallized and completely dried immediately before it was used in all toxicity tests.
Microorganisms, media, culture growth, and amendment
Vibrio fisheri (NRRL B-11177) was supplied by the manufacturer of Microtox. The freeze-dried bioluminescent bacteria were reconstituted approx. 15 min before the test using a simple manufacturer-supplied sodium chloride solution in high-purity distilled water. Pseudomonas fluorescens K27 was obtained from Ray Fall, University of Colorado (Boulder, CO, USA). Growth and inhibition of P. fluorescens K27 were studied using a modified dimethyl (DM) minimal medium [18] that contained the following compounds: potassium phosphate dibasic, K2HPO4, 7 g/L; potassium phosphate monobasic, KH2PO4, 3 g/L; ammonium sulfate, (NH4)2SO4, 1 g/L; magnesium sulfate, MgSO4·7H2O, 0.1 g/L; sodium citrate, 0.5 g/L; and glycerol, 10 g/L. Glycerol was added as a 50% water solution. The pH was adjusted to 7.4. DM medium containing 0.1% of potassium nitrate, KNO3 (hereafter called DM-N) was used for anaerobic cultivation of P. fluorescens K27.
Three selenium toxicant stock solutions were prepared in DM-N medium. While Na2SeO4 (1 M) and Na2SeO3 (0.1 M) stock solutions were sterilized by autoclaving, DMSeO2 stock solutions (1 M) were freshly prepared immediately before each experiment and sterilized by sterile filtration (0.2 μm).
Preculture I (aerobic) of P. fluorescens K27 was prepared by inoculating 20 ml of DM medium in a 50-ml Erlenmeyer flask with one colony from an agar plate. After cultivation in a 30°C water bath shaken at 105 rpm for 24 h, 2.5 ml of preculture I were added to 250 ml of DM-N medium in a screw-cap flask to produce (anaerobic) preculture II. After 10 h of incubation at 30°C, preculture II reached the early exponential phase of growth and was used to run growth and growth inhibition experiments. Different amounts of the three selenium toxicants were added to 16-ml screw-cap test tubes (used to measure culture growth in a Klett meter; see below). Preculture II was added to each tube until the final volume was 10 ml in each. Control samples only contained 10 ml of preculture II. All concentrations of the three compounds tested were replicated at least three times.
Growth in test tubes was followed at a wavelength of 526 nm (optical density, hereafter OD526) with a Klett-Summerson Photoelectric Colorimeter (Klett Manufacturing, New York, NY, USA).
Growth inhibition
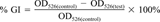
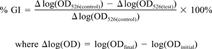
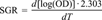
Microtox
The Microtox toxicity experiments were carried out following the manufacturer's procedures in a sodium chloride solution with pH of approx. 7 [23]. All tests were performed at 15°C using the Microtox dedicated spectrophotometer (λ = 490 nm), which provides subambient cooling for 15 sample and control cuvettes during the testing period (Model 500 Analyzer). Selenate and DMSeO2 experiments were performed at 5-, 10- and 15-min exposure times. Selenite was so toxic to this bacterium at the concentrations tested that only 5-min tests were carried out [24]. For longer tests the final amount of light measured at the test's end was so small that it introduced unacceptable amounts of error into the subsequent calculation of EC50s. Lower selenite concentrations were not examined in the longer time tests but would probably have solved this problem. Following the manufacturer's protocol, each test used four different toxicant concentrations and a reagent blank. These five bioluminescence readings were used to determine the concentration at which the light output dropped to 50% of that of the control.
The result of this acute toxicity test is also an EC50, defined as the concentration of each compound (calculated as ppm Se) necessary to cause a decrease in bioluminescent light output of 50% at the final time of each test. All EC50 values were calculated as means at a 95% confidence level using the Student's t test [11].
| Compound | EC50 (ppm Se) | 95% CI | Time (min) | n |
|---|---|---|---|---|
SeO |
110 | ±13.6 | 5 | 7 |
| (CH3)2SeO2 | 914 | ±77.6 | 5 | 8 |
| 490 | ±29.5 | 10 | 8 | |
| 375 | ±29.7 | 15 | 8 | |
SeO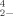 |
7,725 | ±1,591 | 5 | 5 |
| 5,762 | ±1,620 | 10 | 5 | |
| 5,669 | ±2,811 | 15 | 5 |
- a EC50 is the concentration of each compound necessary to cause a decrease in bioluminescent light output of 50% at the final time of each test.
Microbics' protocol [23] suggests determining the toxicity of a standard toxicant with a well-established EC50 to check the performance of the Microtox system. Phenol (100 ppm C6H5OH in H2O; provided by Microbics and double-checked with our own reagent, from Matheson, Coleman, and Bell, Norwood, OH, USA) was used for that purpose in 5-min tests.
RESULTS
Microtox
The EC50 values at the 95% CI for the Microtox experiments involving all seleniferous compounds studied are reported in Table 1. Detailed are the average EC50 values for replicate selenite, selenate, and DMSeO2 experiments, the time of each test, and the number of experiments involved. An EC50 for n = 8 is an average of the EC50 calculations of eight independent experiments.
The average EC50 for all the phenol tests that we ran (n = 10) was 26.7 ppm (±3.4 95% CI), and this average includes experiments using the manufacturer's phenol standard as well as our aged (>5 years) laboratory reagent. Reports of the EC50 of V. fisheri exposed to phenol range from 13 [23] to 42 ppm in 5-min tests [25].
Growth inhibition
Figure 1 shows the overall results of growth inhibition experiments with P. fluorescens K27 amended with selenate, selenite, and DMSeO2. Linear regressions of growth inhibition versus log(concentration) were used to determine the EC50 values recorded in Table 2; linear range, number of data points (n), and linear regression coefficients (r) are also given in Table 2.
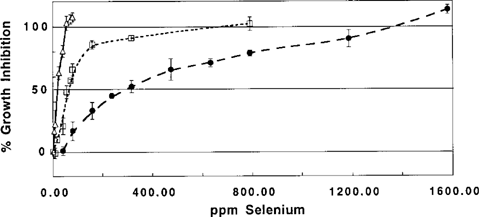
Growth inhibition (GI) of Pseudomonas fluorescens K27 by increasing concentrations of dimethyl selenone (δ), selenite (•), and selenate (□). Error bars represent the standard deviation of three or more replicates (55.3 and 71.1 ppm selenate, only two replicates). Curves were generated by a nonparametric smooth function.
| Method | (CH3)2SeO2 | SeO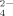 |
SeO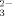 |
|---|---|---|---|
| Growth inhibitiona | |||
| EC50 (ppm Se) | 21 | 61 | 276 |
| r | 0.999 | 0.988 | 0.998 |
| range (ppm Se) | log(7.9-39.5) | log(39.5-79) | log(39.5-1,185) |
| n | 6 | 4 | 9 |
| Growth rate inhibitionb | |||
| EC50 (ppm Se) | 13 | 53 | 412 |
| r | 0.962 | 0.970 | 0.967 |
| range (ppm Se) | 3.95-39.5 | 15.8-158 | 323.7-1,580 |
| n | 6 | 8 | 12 |
- a The EC50 for growth inhibition (GI) was determined as the toxicant concentrations at which GI = 50% calculated in the linear range of growth inhibition plotted versus log(concentration).
- b The EC50 for growth rate inhibition was calculated as the concentration of toxicant that causes a 50% decrease in specific growth rate as compared to growth of the unpoisoned control calculated in the linear range of growth rate plotted versus concentration.
Specific growth rate
Following the growth of P. fluorescens K27 in anaerobic cultures amended with varied concentrations of selenate, selenite and DMSeO2 allowed the determination of the corresponding SGRs as displayed as an overview in Figure 2. The average SGR of unpoisoned cultures of P. fluorescens K27 grown anaerobically at 28°C in DM-N medium was determined to be 0.126 ± 0.017 h−1 (n = 50) and is represented by the upper labeled horizontal line in Figure 2. The EC50 values (Table 2), where the organism is growing at only half the SGR of the control, were determined by linear regression of SGR versus concentration. The plot in Figure 2 gives an indication of the effects of increasing concentrations of the selenium species on SGR. Observing SGRs also revealed that selenite amendment in the range of 10 to 100 ppm slightly enhanced the growth of this bacterium in this medium. Neither at concentrations of ⩾79 ppm Se (1.0 mM, DMSeO2) nor at concentrations of ⩾3,950 ppm Se (50 mM, selenite) was any K27 bacterial growth ever observed. In contrast, even at 15,800 ppm Se (200 mM, selenate), P. fluorescens K27 was still able to grow, but with extended lag phases of 4 to 8 weeks.
It must be noted that all reported selenium concentrations in this work are the nominal, that is, actual amended, selenium concentrations. No tests were performed to determine whether the bioavailability of these toxicants in solution had been affected by other components in the media; however, our use of very simple, well-defined media (DM-N medium for work with K27 or a simple salt solution for the V. fisheri assay) was an effort to minimize these effects [26].
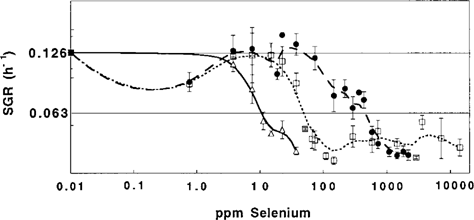
Effect of increasing concentrations of dimethyl selenone (δ), selenite (•), and selenate (□) on the specific growth rate (SGR) of Pseudomonas fluorescens K27. Error bars represent the standard deviation of three or more replicates. The average SGR of 50 unpoisoned control cultures was 0.126 h−1. The EC50 was determined as the toxicant concentration causing a 50% drop of the SGR down to 0.063 h−1. Curves were generated by a nonparametric smooth function.
| Subject | Time | Selenite (ppm Se) | Selenate (ppm Se) | Dimethyl selenone (ppm Se) | Reference |
|---|---|---|---|---|---|
| Vibrio fisheri | 5 min | EC50 110 | EC50 7,725 | EC50 914 | This work |
| 5 min | EC50 215 | — | — | [36] | |
| 15 min | EC50 33.4 | — | — | [30] | |
| 20 min | EC50 49.1 | EC50 > 1,100 | — | [37] | |
| Daphnia magna | 48 h | LC50 3.02 | LC50 4.07 | — | [6] |
| Chironomus riparius | 48 h | LC50 7.95 | LC50 16.2 | — | [6] |
| Pseudomonas fluorescens K27 | EC50 21 | ||||
| growth inhibition | 10 h | EC50 276 | EC50 61 | This work | |
| Pseudomonas fluorescens K27 | EC50 13 | ||||
| growth rate inhibition | up to 60 d | EC50 412 | EC50 53 | This work | |
| Pseudomonas fluorescens wild | — | ||||
| type growth rate inhibition | 2 h | EC50 52 | — | [30] | |
| Green alga | 24 h | EC50 143 | EC50 61.5 | — | [24] |
| Chironomus decorus | 48 h | LC50 48.2 | LC50 23.7 | — | [32] |
| Chick embryos | 17 d | LD50 0.3 | LD50 0.13 | — | [29] |
DISCUSSION
The advantages of using short-term bacterial motility [27, 28] or bioluminescence bioassays [10, 11] for toxicity are obvious: quick results and high throughput, high sample population (∼106 bacteria per sample), and cost efficiency. Uncertainty with this method may stem from turbidity and sample color complications because the actual measurement is spectrophotometric. Procedural protocols have been developed to allow colored or turbid samples to be evaluated [23]. Because the dilution volumes are on the microscale, small errors in the pipetting reagents involved may have large consequences, unlike with more conventional large-scale bioassays. For the acute tests carried out in this study, no information can be gained on the effects of the toxicant on growth and cell division of the microorganism.
The data in Table 1, determined using V. fisheri, show that selenite had the lowest EC50 among the three compounds tested (110 ppm Se; n = 7; t = 5 min). Selenate is substantially less toxic to this organism, with EC50 of 7,725 ppm Se for the same 5-min test (n = 5). Dimethyl selenone's 5-min average EC50 was 914 ppm (n = 8) and falls between that of the two more oxidized species. Selenium toxicity data from the literature and our results are listed in Table 3. One additional point is that the Microtox assay experiments, which used a manufacturer-supplied unbuffered culture “medium,” may be affected at the highest selenite concentration by a substantial pH change from neutral. For V. fisheri this is considered to be unimportant at a pH as high as 8 [23]; however, a test showed that the highest selenite concentration of the four used in each run had a pH of 8.8. (Selenite anion is the strongest base of the three toxicants examined.) Therefore, although the relative toxicity order of the three selenium species examined is certainly valid, a small deviation from the absolute value of the 5-min selenite EC50 is possible.
The lower LC50 data reported by Ingersoll et al. [6] and the other data in Table 3 are in line with the increasing toxicity seen in Table 1 with increasing assay times (>5 min). The longer the organisms were exposed, the lower the calculated EC50 [24]. Interpreted in this way, our much shorter V. fisheri tests appear reasonable and in accord with 24- and 48-h data and even the 17-d data of one research group [29].
Incubation times were also long for growth and growth inhibition experiments with P. fluorescens K27. As Figure 1 shows, the growth inhibition method led to a typical inhibition graph [20] and allowed the determination of EC50 values with good linear regressions (Table 2). However, unlike many other organisms reported in the literature, selenate was more toxic than selenite for P. fluorescens K27. This strain, isolated from a selenium-contaminated site (Kesterson Reservoir, San Joaquin Valley, CA, USA) [14] tolerates more selenite than the wild type (EC50 = 52 ppm, determined by growth rate inhibition in the first 2 h after the addition of the toxicant) [30]. It is surprising that selenate is more toxic to this microbe than selenite because the main selenium species in the San Joaquin Valley is selenate [31]. The selenium concentrations reported for that site are, however, much lower than the EC50 values for selenate and selenite and P. fluorescens K27 reported here: as selenium these naturally occurring amounts range from less than 0.2 ppm [31] to 1.4 ppm [14].
Unlike for the V. fisheri assays, growth inhibition experiments, and the growth rate inhibition method suggested by Paran et al. [30], no time limit was set to study the effect of varied concentrations of seleniferous compounds on the specific growth rate of P. fluorescens K27 in the research reported here. Following growth until the cultures had reached the stationary phase showed a drastic decrease in growth rate for all three selenium compounds in the same concentration ranges where growth inhibition occurred (Fig. 2 and Table 2) but also revealed that even at 15,000 ppm Se (200 mM, selenate), K27 was still able to grow (Fig. 2). It has to be mentioned, though, that beyond the selenate concentrations causing the steady decrease of the specific growth rate, longer and longer lag phases were observed with those increasing selenate concentrations. At 200 mM selenate, the apparent lag phase was between 4 and 8 weeks. In sterile control experiments no bacterial growth was ever observed, showing that the cultures growing in the presence of 100 and 200 mM selenate were P. fluorescens K27 and not simply contaminations. The long lag phases indicate that at such high concentrations, highly resistant strains of K27 were enriched. This was verified by streaking the 200 mM amended cultures and unpoisoned cultures of P. fluorescens K27 on plates containing 200 mM selenate. Colonies appeared within days when inoculated with cultures previously grown in the presence of 200 mM selenate, whereas on plates inoculated with original, unpoisoned cultures, it took weeks for the first colonies to grow. Neither for selenite nor for DMSeO2 was the development of such high resistance ever observed.
Comparing growth inhibition and growth rate inhibition of P. fluorescens K27 (Table 2), the EC50 values lie in the same order of magnitude. However, only for selenate were the numbers relatively close. Determined by growth rate inhibition, the EC50 of DMSeO2 was almost 40% lower than the value obtained by using the growth inhibition method, whereas for selenite it was almost 50% higher. In comparison, the observation of a negative and a positive deviation indicates that the two methods indeed lead to different results and not just to a systematic difference between the two methods involved. Considering the results of our growth rate inhibition experiments, the term growth inhibition was found to be somewhat misleading: 100% growth inhibition was determined at concentrations of 10 mM selenate and 15 mM selenite, even though growth occurred far beyond these concentrations. The limitation in this method is the definition of growth inhibition, which depends on the relative growth of an unamended control and does not, in any way, take an extended lag phase into account.
In addition, comparing the highest concentrations where growth was observed, DMSeO2 (39.5 ppm Se) is still the most toxic to P. fluorescens K27 among the three selenium compounds investigated; however, selenite (2,370 ppm Se) is more toxic than selenate (15,800 ppm Se), as could generally be expected from the literature. On the other hand, the 50% growth inhibition concentrations determined by Ibrahim and Spacie [24] and the LC50 and LD50 values for these two selenium oxyanions, as reported by Maier and Knight [32] and Palmer et al. [29] respectively, show that selenate was more toxic than selenite in their tests.
In their work with green algae, Ibrahim and Spacie [24] recognized the difficulty in comparing their EC50 values with the general toxicity trend of SeO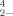 and SeO
and SeO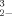 in the literature and specifically with data reported for another algae bioassay [33]. In their discussion they mention the importance of investigating the “mechanism(s) whereby these chemical forms cause bioeffects….” [24]. Our examination of the relative toxicity of DMSeO2, Challenger's [1] proposed intermediate, as compared to SeO
in the literature and specifically with data reported for another algae bioassay [33]. In their discussion they mention the importance of investigating the “mechanism(s) whereby these chemical forms cause bioeffects….” [24]. Our examination of the relative toxicity of DMSeO2, Challenger's [1] proposed intermediate, as compared to SeO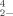 and SeO
and SeO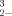 is an effort to do this. Although still combined with oxygen, selenium in DMSeO2 can be assigned a formal oxidation state of zero, whereas that of selenide is more reduced at -2 [34]. Yet, unlike elemental selenium, DMSeO2 is freely soluble in water. The intermediate toxicity of (CH3)2SeO2 in an assay using V. fisheri suggests that if these bacteria do indeed supply electrons to reduce and detoxify oxidized forms of selenium to DMSeO2, then this chemical species as an intermediate would be less toxic than the starting material if that oxyanion were selenite but more toxic than the initial oxyanion if it were selenate. In reference to the following discussion, it must be remembered that the Microtox assay's organism, V. fisheri, is not considered to be selenium resistant because it has been exposed to selenium at levels present in its marine environment (≪1 ppm Se). Furthermore, we have no information on how this microorganism responds metabolically to contact with selenium; therefore, the above discussion is speculation on our part.
is an effort to do this. Although still combined with oxygen, selenium in DMSeO2 can be assigned a formal oxidation state of zero, whereas that of selenide is more reduced at -2 [34]. Yet, unlike elemental selenium, DMSeO2 is freely soluble in water. The intermediate toxicity of (CH3)2SeO2 in an assay using V. fisheri suggests that if these bacteria do indeed supply electrons to reduce and detoxify oxidized forms of selenium to DMSeO2, then this chemical species as an intermediate would be less toxic than the starting material if that oxyanion were selenite but more toxic than the initial oxyanion if it were selenate. In reference to the following discussion, it must be remembered that the Microtox assay's organism, V. fisheri, is not considered to be selenium resistant because it has been exposed to selenium at levels present in its marine environment (≪1 ppm Se). Furthermore, we have no information on how this microorganism responds metabolically to contact with selenium; therefore, the above discussion is speculation on our part.
The results of the growth inhibition and growth rate inhibition with a selenium-resistant bacterium tell a different story. Toward P. fluorescens K27, DMSeO2 is more toxic than both selenium oxyanions using these two assays. Based on these results alone one might rule out DMSeO2 as a possible intermediate of the selenium reduction pathway. However, our recent results with amending bacterial cultures have shown that DMSeO2 is converted into less toxic [8, 9] and less water-soluble volatile organoselenides at much higher rates than selenite and selenate by both P. fluorescens K27 [3] and several strains of purple nonsulfur bacteria (V. Van Fleet-Stalder, unpublished data). This means that if DMSeO2 is an intermediate in the bioreduction process for bacteria that have developed a resistance mechanism, the rate-limiting step in the formation of volatile organoselenides from selenite or selenate would probably lie between selenite (or selenate) and DMSeO2. Thus, relative toxicity aside, high concentrations of DMSeO2 would not be allowed to build up and do damage in the cell because DMSeO2 is so readily converted to more reduced, volatile, and less-soluble forms.
Anaerobic, selenite-amended cultures of P. fluorescens K27 turn brick red, most likely from formation of red elemental selenium, whereas selenate-amended cultures do not, and recent work with this organism has shown that the amounts of Se0 produced are at least 10 to 20 times larger in selenite-compared to selenate-amended cultures for the same incubation time. Furthermore, this same work has shown that selenite-amended cultures of K27 produce organoselenium compounds in the log phase, whereas in selenate-amended cultures this production occurs in the stationary phase of growth [35]. Together these data, relative toxicities, relative production of organoselenium species, and the growth phase in which they are produced, indicate that selenite and selenate are likely to be metabolized through different biochemical pathways by P. fluorescens K27. Currently, we are examining the relative distribution of the entire suite of selenium-containing species, Se0, Se(IV), Se(VI), and organoselenium compounds, in K27 cultures amended with selenium-containing oxyanions. The distribution of each chemical member in selenite- and selenate-amended cultures may shed even more light on the possible mechanisms of selenium toxicity and bioremediation pathways.
Acknowledgements
We appreciate the assistance of D.M. Carpenter with statistical analysis and V.R. Pinney and T.K. Dowe with technical support. This research was supported by a Cottrell College Science Award of Research Corporation and the National Swiss Foundation and by a departmental grant from the Robert A. Welch Foundation. We also wish to acknowledge support from the Sam Houston State University Research Enhancement Fund.




