Use of vegetated agricultural drainage ditches to decrease pesticide transport from tomato and alfalfa fields in California, USA
Abstract
Irrigation and storm water runoff from agricultural fields has the potential to cause impairment to downstream aquatic receiving systems. Over the last several years, scientists have discovered the benefit of using edge-of-field practices, such as vegetated agricultural drainage ditches, in the mitigation of pesticides and sediment. After demonstrating this practice's feasibility in California, field trials were initiated to document irrigation runoff pesticide mitigation in California alfalfa and tomato fields. In the alfalfa field, chlorpyrifos concentration was decreased by 20% from the inflow to the ditch outflow. Thirty-two percent of the measured chlorpyrifos mass was associated with ditch plant material. In the tomato field, permethrin concentration was decreased by 67% and there was a 35% reduction in suspended sediment concentration from inflow to the ditch outflow. When surface water was not present in the ditch systems, the sediment was a significant repository for pesticides. Based on the field trials, vegetated agricultural drainage ditches can be successfully used as part of a suite of management practices to reduce pesticide and sediment runoff into aquatic receiving systems. Environ. Toxicol. Chem. 2011; 30:1044–1049. © 2011 SETAC
INTRODUCTION
Agriculture is the single largest user of freshwater, consuming some 70% of the global surface water supply 1. Adequately feeding an ever-growing global population requires the use of pest control and fertilizers to maximize yield on a limited amount of production acreage. However, nonpoint source pollution attributed to agriculture has sometimes affected the water quality of both groundwater and surface water resources. Olle et al. (2; http://www.environmentcalifornia.org/uploads/1A/3S/1A3SQHgTmKwLJ4zdz8kpsg/Water_Woes.pdf) reported pesticides were detected in 96% of the 133 California waterway locations tested, with the most frequent detections being diuron (CAS 330–54–1), diazinon (CAS 333–41–5), simazine (CAS 122–34–9), chlorpyrifos (CAS 2921–88–2), and molinate (CAS 2212–67–1).
In response to nonpoint source pollution concerns, agriculture has invested heavily in development of management practices to alleviate agricultural runoff. Use of practices individually or in suites depends on physical land factors, as well as the willingness of a farmer to try new methods. Although traditional management practices have focused mainly on tillage, the CORE 4 program, developed for the U.S. Department of Agriculture Natural Resource Conservation Service (USDA NRCS), emphasizes four management components to address reduction of agricultural pollution: conservation tillage, crop nutrient management, pest management, and conservation buffers (3; http://www.nrcs.usda.gov/technical/ecs/agronomy/core4.pdf).
Conservation buffers may include contour buffer strips, field borders, filter strips, windbreaks, or wetlands. In particular, the use of constructed wetlands as an edge-of-field management practice for agricultural runoff has been promoted for the last two decades 4. Although several studies have reported wetland mitigation efficiency 5-11, removing sufficient land from production acreage for wetland development is not always a viable option. Utilizing in-place, edge-of-field features of the agricultural production landscape, such as drainage ditches, allows for an economically efficient alternative 12-15. Moore et al. 16 demonstrated that vegetation in similar-shaped ditches in California reduced pesticide half-distances 2 to 3 times as compared to unvegetated ditches. A pesticide half-distance is the distance required in the ditch for the pesticide concentration to be decreased 50% from inflow concentrations.
The objective of the present study was to provide data on the ability of vegetated agricultural drainage ditches (VADD) to mitigate the organophosphate insecticide chlorpyrifos (CAS 2921–88–2) and the pyrethroid insecticide permethrin (CAS 52645–53–1) in runoff from normal crop production settings in California (Table 1). Chlorpyrifos is the most used organophosphate pesticide in California, with nearly 614,000 kg applied in 2008 (17; http://www.cdpr.ca.gov/docs/pur/pur08rep/tables/table6a.htm). Almonds were the top California crop receiving chlorpyrifos applications in 2008, with 131,140 kg applied (18; http://www.cdpr.ca.gov/docs/pur/pur08rep/chmrpt08.pdf). Permethrin, as a representative of the broader pyrethroid pesticide class, has been used to replace the older organophosphates, including chlorpyrifos. As a third-generation pyrethroid, permethrin was the most widely used pyrethroid in California in 2005 19, and in 2008, approximately 155,700 kg were applied in the state, with pistachio applications (16,168 kg) receiving the most input per crop 18.
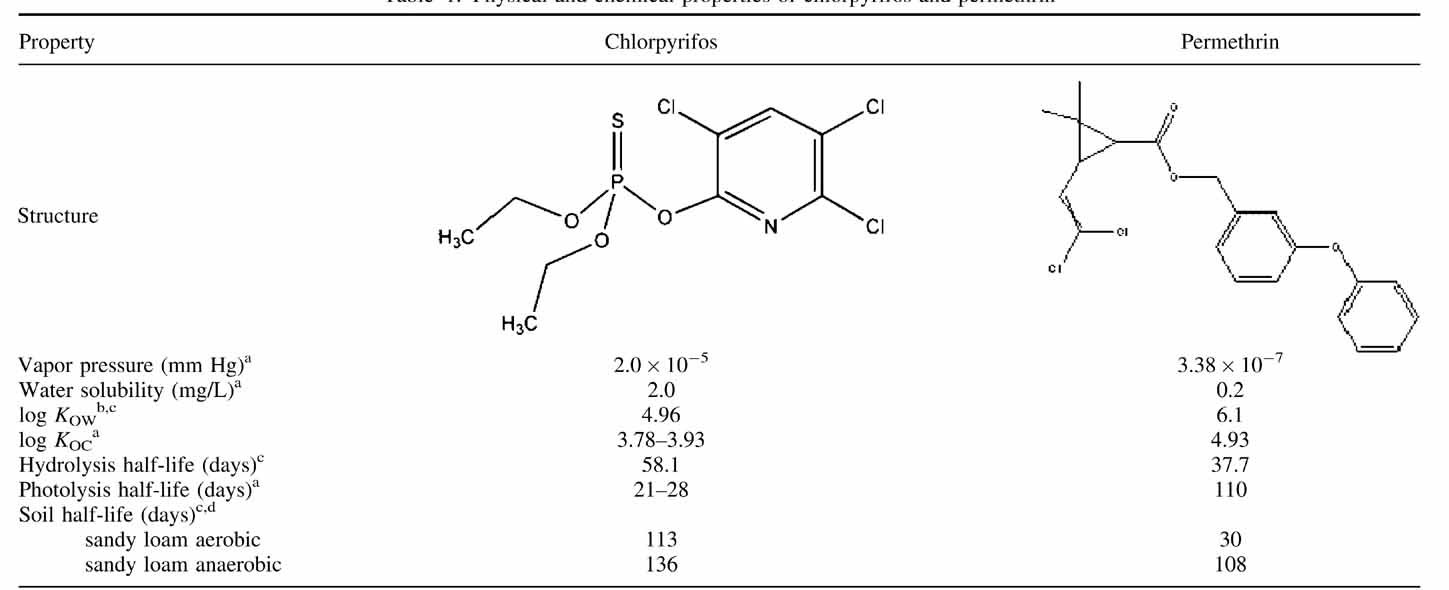
- a EXTOXNET 1996 (36; http://extoxnet.orst.edu/pips/ghindex.html)
- b Howard 1999 37
- c USDA 1995 (38; http://www.ars.usda.gov/Services/docs.htm?docid = 14199)
- d Kegley et al. 2007 (39; http://www.pesticideinfo.org)
MATERIALS AND METHODS
Field information
A 7-ha portion of an alfalfa field and a 6-ha portion of a tomato field in Yolo County, California, were used for farm trials. Each field had existing drainage ditches in place that were reshaped for this research project. The alfalfa field drainage ditch was 1.53 m (width) × 0.61 m (depth) × 402 m (length). Sampling locations were established at 30.8 m (inflow), 185 m (middle), and 326 m (outflow). Tomato field drainage ditch dimensions were 3.29 m (width) × 0.52 m (depth) × 389 m (length). Sampling locations were established at 44.2 m (inflow), 204 m (middle), and 361 m (outflow).
Both ditches, one in each field, were planted with plugs of creeping wildrye, Leymus triticoides (Buckley) Pilger, and slender sedge, Carex praegracilis W. Boott, on 46-cm centers in 30-m alternating blocks from 13 to 20 December 2006. This allowed approximately five months for the plugs to mature prior to irrigation season. These two native grasses were chosen because of their availability and common use within California ditches. A 10-m unplanted buffer was left between each vegetated block and each block was replicated four times (Fig. 1). One day prior to the irrigation events (11 July 2007), plant density and percent plant cover were recorded in each ditch. Three replicate quadrants (0.25 m2) were analyzed in each planted section of Leymus, Carex, as well as the originally unplanted buffers, which subsequently contained various weeds.
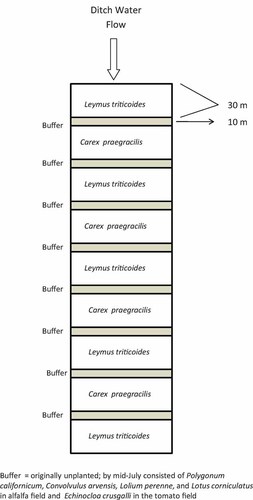
Schematic of vegetation planted in drainage ditch. Buffers were originally bare; however, emergent weeds were present by the time irrigation runoff was initiated.
Soils data
The alfalfa field soil type was Myers clay (fine smectitic, thermic Aridic Haploxererts). Soil analyses conducted by the University of California Agriculture and Natural Resources Analytical Laboratory indicated a soil pH of 7; particle size distribution of 21:35:44 (% sand:silt:clay); cation exchange capacity of 35.5 meq/100 g; 0.6% organic carbon; and 27.6% soil moisture. A mixture of Sycamore silt-clay loam (fine-silty, mixed, superactive, nonacid, thermic Mollic Endoaquepts) and Brentwood silt-clay loam (fine, smectitic, thermic Typic Haploxerepts) were the soil types found in the evaluated tomato field. Soil analyses reported a pH of 7.6; particle size distribution of 13:52:35 (% sand:silt:clay); cation exchange capacity of 31.2 meq/100 g; 0.47% organic carbon; and 32.8% soil moisture.
Pesticides
Lock-On™ (chlorpyrifos; Dow AgroSciences) was applied via an all-terrain vehicle rig to the ground on the alfalfa field at a rate of 1.75 L/ha in July 2007. This corresponded to 0.42 kg of chlorpyrifos/ha. Field (furrow) irrigation was initiated approximately 24 h after application. Although not completely uncommon, irrigation initiation is not usually done within 1 day of pesticide application. However, pest development levels can increase rapidly during summer temperatures under the plant canopy, resulting in the need for insecticide application. Twelve hours after irrigation initiation, runoff water began entering the vegetated ditch, lasting for approximately 14 h. The tomato field was treated aerially with Perm-Up 3.2 EC™ (permethrin; United Phosphorus) at a rate of 0.015 L/L of water dilution per hectare in August 2007. This corresponded to 0.006 kg of permethrin/ha. As with the alfalfa field, furrow irrigation was initiated 24 h after application, with runoff water entering the vegetated ditch 5 h later. Runoff water flowed in the vegetated ditch for a total of 6 h.
Water quality, sediment, and plant samples
Water quality and flow data were collected at each sampling station a minimum of five times during irrigation runoff events in the VADDs. In addition to flow, parameters measured included pH, dissolved oxygen (mg/L), temperature (°C), conductivity (µS/cm), and total suspended solids (TSS; mg/L). Water quality measurements were collected using calibrated, hand-held YSI™ field meters (Yellow Springs Instruments).
Spatial and temporal water, sediment, and plant samples were collected from VADDs using the same protocol reported by Moore et al. 16. Briefly, grab samples of water were collected in precleaned, certified 1 L amber Boston round, narrow-mouth glass bottles with Teflon™-lined closures at 0, 0.5, 1, 4, 8, 16, and 24 h after runoff entry into the sampled ditches. Sediment samples were collected in 120-ml wide-mouth glass bottles with Teflon-lined closures using an identical time sequence as water samples. Only the top 1 cm of sediment was collected from ditches sampled, using solvent-rinsed spoons. Plant samples, taken at identical times as water and sediments, were collected from plant material exposed in the water column, using solvent-rinsed scissors, wrapped in aluminum foil, and placed in prelabeled 3-L freezer bags. All samples were preserved on wet ice from collection through transport to the Aquatic Toxicology Laboratory (ATL) at the University of California, Davis. Prior to next-day shipment to the California Department of Fish and Game Water Pollution Control Laboratory (DFG-WPCL), aqueous samples were kept in the dark at 4°C. Sediment and plant samples were frozen immediately upon receipt at the ATL. Sediment samples were shipped next-day to the DFG-WPCL, whereas plant samples were shipped overnight to the USDA Agricultural Research Service National Sedimentation Laboratory (NSL) for sample preparation. After immediate drying and grinding, plant samples were placed in glass vials and shipped overnight to the DFG-WPCL for pesticide analyses. All pesticide analyses followed protocols outlined in Moore et al. 16 using liquid–liquid extraction (water samples) or pressurized fluid extraction (sediment and plants). Final extracts were analyzed using methods U.S. EPA 8141B and U.S. EPA 8081B on a dual column high-resolution gas chromatograph equipped with electron capture detectors.
RESULTS AND DISCUSSION
Alfalfa field ditch
Mean density of L. triticoides in plots was 223 ± 42 plants/m2, with a 46 ± 15% mean cover. In C. praegracilis plots, the mean density was 163 ± 15 plants/m2, with a 93 ± 6% mean cover. By mid-July, the originally bare buffers now had a mixture of Polygonum californicum, Convolvulus arvensis, Lolium perenne, and Lotus corniculatus at a mean density of 130 ± 59 plants/m2, and a 97 ± 5% mean cover.
Water quality variables in the ditch differed slightly through the duration (14 h) of the runoff collection. Ditch pH values were essentially neutral, ranging from 7.6 to 7.7 (± 0.1). These conditions would place the hydrolytic and photolytic half-lives of chlorpyrifos at approximately one month; however, degradation has been known to significantly increase in natural waters, such as in ponds and canals (20, 21; http://www.dowagro.com/PublishedLiterature/dh_0039/0901b80380039661.pdf). Mean ± standard error (SE) values for temperature, dissolved oxygen, and conductivity were 23.2 ± 5.3°C, 3.4 ± 1.0 mg/L, and 372 ± 76 µS/cm, respectively. Although TSS never exceeded 10 mg/L in any sample, a 5% overall reduction in TSS was noted between the ditch inlet and outlet. The mean flow rate at the top (inlet) of the ditch sampling area was 25 ± 13 L/s, while mean flow rate at the ditch outlet was 27 ± 10 L/s.
Inflow aqueous chlorpyrifos concentrations from irrigation runoff were variable, ranging from 2.8 µg/L (at 0 min) to a maximum of 4.5 µg/L after 90 min of runoff (Fig. 2). Outflow chlorpyrifos concentrations at the bottom of the ditch ranged from 2.6 µg/L (625 min) to a maximum of 3.1 µg/L (265 min). These field concentrations were slightly higher than the 0.22 µg/L to 1.67 µg/L reported by Gill et al. 22; however, temporal sampling methods between the two studies also differed. Gill et al. 22 collected samples at intervals ranging from 30 min to 1 h, with a target of six collected samples at each site per irrigation set. Using a t test, a statistically significant difference (α = 0.05) was noted between the mean aqueous chlorpyrifos concentration at the ditch inlet (3.45 µg/L) versus the ditch outlet (2.75 µg/L) in the present study. This represented a 20% mean reduction in aqueous chlorpyrifos concentration through the ditch. Gill et al. 22 reported a 38% chlorpyrifos reduction after traveling through a 200-m vegetated ditch, as opposed to only 1% reduction after exposure to a nonvegetated ditch. Chlorpyrifos concentrations measured in the sediment and plants were variable with time and sample location. Inflow sediment concentrations ranged from below detection to 1,700 µg/kg, whereas plant concentrations ranged from 272 to 782 µg/kg. Sediment and plant concentrations at the middle sampling station ranged from 4.35 to 103 µg/kg and 204 to 1,361 µg/kg, respectively. Outflow sediment and plant chlorpyrifos concentrations ranged from 8.74 to 3,610 µg/kg and 360 to 1,522 µg/kg, respectively.
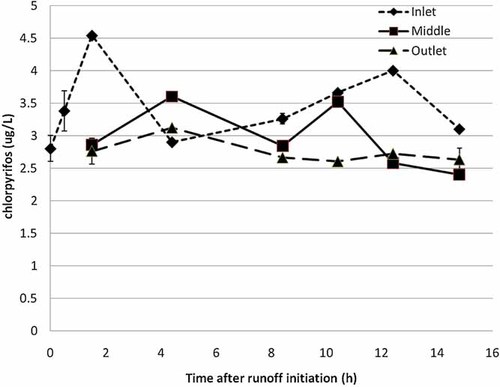
Mean aqueous chlorpyrifos concentration (µg/L) at ditch inlet, middle, and outlet for a 14-h alfalfa irrigation event.
Other environmental systems utilizing the concept of pesticide–vegetation sorption have reported successful results with chlorpyrifos mitigation. A vegetated treatment system with duckweed (Lemna sp.), watercress (Nasturtium sp.), and pennywort (Hydrocotyle ranunculoides L.f.) was reported to reduce chlorpyrifos by 52% according to Hunt et al. 23. The system used by these researchers was more pond-like, allowing for more settling and contact time for potential pesticide removal, as compared to the present study. Poletika et al. 24 discovered chlorpyrifos removal efficiency with uniform runoff flow in vegetated filter strips (4.6 m in length) to be 85%. Filter strips comprised 90% smooth brome (Bromus inermis Leyss.) and 10% bluegrass (Poa pratensis L.). Similar findings by Arora et al. 25 reported 83.1% chlorpyrifos retention in vegetated buffer strips composed of 81% B. inermis, 12% P. pratensis, 5% Festuca arundinacea Schreber, and 2% other with a density of 8.82 M tillers/ha. Schulz and Peall 26 reported essentially 100% chlorpyrifos retention in a South African constructed wetland over a five-month period (inflow concentration was 31 µg/kg on suspended particles; no detection on outflow suspended particles). The constructed wetland (100 m × 36 m) was vegetated with Typha capensis (Rohrb.) N.E.Br. (60% coverage), Juncus kraussii Hochst. (10% coverage), and Cyperus dives Delile (5% coverage). Variable results of pesticide–vegetation sorption can result from differences in hydraulic retention times of vegetated systems, water quality factors such as temperature, pH, and TSS, as well as the type of vegetated system being utilized (constructed wetland versus vegetated filter strip).
When water was present at all sampled sites (inlet, midway, and outlet) within the ditch site, 52% of the measured chlorpyrifos mass was associated with the water column, whereas 16% was associated with sediment. The remaining 32% of measured chlorpyrifos mass was associated with ditch plant material. With regard to plant versus soil sorption, Rogers and Stringfellow 27 reported chlorpyrifos sorption to whole plant stems was 10 times higher than to soil. Their study used batch equilibrium laboratory exposures of either chlorpyrifos plus sediment or chlorpyrifos plus plant stems instead of in situ field collections, as in the present study. In addition, Rogers and Stringfellow 27 had a considerably longer exposure period than the present study. Of the measured masses in the present ditch study, 26% were located at the inlet of the ditch, 34% were in the middle of the ditch, and 40% were from samples near the outlet. After draining, only 1% of measured chlorpyrifos mass was found in the inlet (84% in sediment; 16% in plants). Samples collected in the middle of the ditch held 8% of the measured mass (34% in sediment; 66% in plants). The majority (91%) was located at the outlet of the ditch (96% in sediment; 4% in plants). By examining the estimated mass balance of chlorpyrifos in the ditch, there was a 19% reduction between the inlet (4.45 g) and the outlet (3.63 g).
Tomato field ditch
Mean density of L. triticoides in plots were 358 ± 40 plants/m2, with a 50 ± 22% mean cover. No C. praegracilis plugs survived into the irrigation season. By mid-July, the originally bare buffers now had Echinocloa crus-galli (L.) Beauv at a mean density of 1 ± 1 plants/m2, and a 1 ± 1% mean cover.
Ditch pH values were slightly more alkaline than that of the alfalfa field ditch (mean 8.2 ± 0.27) for the 6 h event duration. At acidic and neutral pH ranges, permethrin is relatively stable; however, hydrolysis begins slowly when alkaline conditions are present 28. Mean ± SE values for temperature, dissolved oxygen, and conductivity were 31.5 ± 3.0°C, 6.5 ± 0.8 mg/L, and 380 ± 35 µS/cm, respectively. Sharom and Solomon 29 reported temperature, pH, and microorganisms can influence permethrin's rate of chemical degradation in aqueous systems. In their laboratory studies, when incubated at 30°C, the initial rate of permethrin degradation was more rapid than at 15°C 29. In the present study, a 35% reduction in TSS concentration overall from inlet to outlet was observed, with maximum inflow TSS of 566 mg/L and maximum outflow of 146 mg/L. Permethrin, as with many of the synthetic pyrethroids, has an affinity for suspended solids and dissolved organic matter present in runoff water 30, 31. With relatively high TSS concentrations present in the tomato field runoff, it is expected that much of the permethrin would be bound to such material. This would also affect the pesticide's bioavailability to aquatic organisms 31. The mean flow rate at the top (inlet) of the ditch sampling area was estimated to be 8.9 ± 2.9 L/s, while mean flow rate at the ditch outlet was 9.5 ± 3.1 L/s.
Inflow aqueous permethrin concentrations from irrigation runoff were variable, ranging from 0.23 µg/L (415 min) to a maximum of 0.67 µg/L at the initial (time 0) sample (Fig. 3). Outflow permethrin concentrations at the bottom of the ditch ranged from below detection (1380 min) to a maximum of 0.26 µg/L (60 min). A statistically significant difference (α = 0.05) existed between the mean permethrin concentration at the top of the ditch (0.67 µg/L) versus the mean permethrin concentration at the bottom of the ditch (0.26 µg/L). This represented a 62% mean reduction in aqueous concentration from top to bottom. When water was present at all sampled sites (inlet, middle, and outlet) within the ditch, 18% of the measured permethrin mass was associated with the water column, while 67% of the measured permethrin mass was associated with sediment. Based on its chemical profile, permethrin is expected to prefer sorption to sediment or other forms of organic carbon. The remaining 15% of measured permethrin mass was associated with ditch plant material. As with chlorpyrifos, mitigation of permethrin by use of vegetation has been documented. Schmitt et al. 32 reported that filter strips comprising trees, shrubs, and grasses at widths of 7.5 m and 15 m reduced permethrin-associated contaminants by 27 to 83%. Plant-specific mitigation of cis-permethrin in aquatic mesocosms ranged from 67% reduction with Typha latifolia L. to 71% reduction with Leersia oryzoides (L.) Sw. 33. Within this same study, however, there was no significant difference in permethrin mass mitigation between different plant species and the unvegetated control 33. The short hydraulic retention time (4 h) was likely the main factor for this lack of discrepancy. In larger-scale studies from Yolo County, California, Moore et al. 16 reported that permethrin half-distances in vegetated drainage ditches were 21 to 22 m, whereas unvegetated drainage ditches had a half-distance of 50 to 55 m. Inflow sediment and plant permethrin concentrations ranged from below detection to 110.4 µg/kg and 489 to 1232 µg/kg, respectively. Sediment and plant concentrations in the middle sampling location ranged from below detection to 77.2 µg/kg and 58.9 to 463 µg/kg, respectively. Outflow sediment and plant permethrin concentrations ranged from below detection to 12.3 µg/kg and 38.9 to 216 µg/kg, respectively.
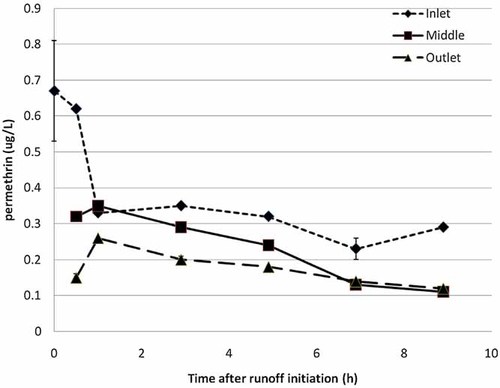
Mean aqueous permethrin concentration (µg/L) at ditch inlet, middle, and outlet for a 6-h tomato irrigation event.
Of the measured permethrin masses in the present study, 13% were located at the inlet of the ditch, 33% were in the middle of the ditch, and 54% were from samples at the end of the ditch. After water had drained out of the ditch, only 9% of measured permethrin mass was in the inlet of the ditch (92% in sediment; 8% in plants). Samples collected in the middle of the ditch held 69% of the measured mass (94% sediment; 6% in plants). The 22% remainder was located at the outlet of the ditch (98% in sediment; 2% in plants). The estimated permethrin mass balance indicated a 44% reduction from ditch inlet (0.091 g) to the outlet (0.051 g). With the increased TSS concentrations, mass percentages of measured permethrin indicate a significant portion of the pyrethroid likely sorbed to the TSS and settled out in the sediments as they traveled through the ditch.
CONCLUSION
Results of the present study demonstrate the ability of VADDs to assist in pesticide mitigation of irrigation return flows. Chlorpyrifos concentrations were decreased 20% from inflow to outflow, whereas permethrin concentrations were decreased 63% from inflow to outflow. This translated into a 19% mass reduction of chlorpyrifos and a 44% mass reduction of permethrin from ditch inflow to outflow. Chemical structure, presence of TSS, and microbial activity are just some potential reasons permethrin was decreased by a greater extent than chlorpyrifos. Mean chlorpyrifos concentration in water leaving the alfalfa field ditch was 2.75 µg/L, which is still above the U.S. EPA aquatic life acute criteria of 0.083 µg/L (34; http://www.epa.gov/oppefed1/ecorisk_ders/aquatic_life_benchmark.htm). In the tomato field ditch, mean permethrin concentration in outflow water was 0.26 µg/L. This value falls within the acceptable range of U.S. EPA's aquatic life benchmark for fish (acute); however, it is still above the acute aquatic life benchmark for invertebrates 34. It is imperative to remember that no single management practice is continuously 100% effective. Using suites of in-field (no-till, reduced till, cover crops, etc.) and edge-of-field (vegetated drainage ditches, stiff grass hedges, conservation buffers, etc.) management practices, landowners will have more effective overall control of agricultural runoff. Future research should examine impacts of multiple management practices, while paying special attention to the microbial aspect of pesticide degradation.
The California Department of Water Resources reports that 50% of the state's irrigated crops uses surface irrigation (flood and furrow water application) systems, which results in surface return flows that contain nutrients and chemicals 35. Although the use of flood and furrow systems is decreasing, the degradation of surface and ground water by irrigation return flows is well documented, and the federal and state governments are implementing programs to reduce future impacts. Implementation of VADDs to reduce nutrients and chemicals in return flows will become an effective tool in these programs.
As a result of this field study, the USDA Natural Resource Conservation Service state office in California made VADDs a cost-share practice under the Environmental Quality Incentives Program (EQIP). Under EQIP, farmers can receive up to 75% cost-sharing for installing conservation practices that improve or benefit natural resource conditions. Evaluation and approval by the state office was obtained in 2008, and the practice is now incorporated in the electronic field office technical guide for California. The practice standard for VADDs was categorized under the current practice standard 607 (surface drain, field ditch).
Acknowledgements
Funding for this project has been provided in full or in part through an agreement with the California State Water Resources Control Board (SWRCB). Contents of this document do not necessarily reflect the views and policies of the SWRCB. Mention of trade names or commercial products in this document is solely for the purpose of providing specific information and does not imply recommendation or endorsement by the California SWRCB, U.S. Department of Agriculture, or U.S. Environmental Protection Agency (Gov. Code 7550, 40 CFR 31.20).