Survival and precopulatory guarding behavior of Hyalella azteca (Amphipoda) exposed to nitrate in the presence of atrazine
Abstract
Nitrate is one of the most commonly detected contaminants found in aquatic systems with other pesticides such as atrazine. The current study examined potential combined effects of nitrate and atrazine on adults of the freshwater amphipod Hyalella azteca, using survival and precopulatory guarding behavior as toxic endpoints. Although significant differences in acute toxicity with nitrate alone and in binary combination with atrazine (200 µg/L) in water-only tests were not consistently observed for each time point, potential biologically relevant trends in the data were observed. Posttest growth and behavioral observations (10-day period) conducted after 96-hour exposure suggested that atrazine and nitrate at these concentrations did not result in delayed effects on H. azteca. However, when test conditions were modified from standard toxicity tests by feeding amphipods, nitrate was found to be more toxic, with a reduction in median lethal concentration (LC50) values of approximately 80%. We also demonstrated that nitrate exhibits a dose–response effect on precopulatory guarding behavior of H. azteca, suggesting that reproductive effects may occur at environmentally relevant concentrations. Environ. Toxicol. Chem. 2011; 30:1170–1177. © 2011 SETAC
INTRODUCTION
Pesticides and other chemical contaminants are rarely found in the environment as single compounds but rather as mixtures 1, 2. Although an extensive database exists indicating the effects of single toxicants on aquatic organisms, relatively few data have been generated for effects of contaminant mixtures found in most aquatic ecosystems. Atrazine (2-chloro-4-ethylamino-6-isopropyl-amino-S-triazine) is one of the most commonly used corn herbicides in the United States and Canada 3, 4 and the most frequently detected pesticide in a recent report of the U.S. Geological Survey National Water-Quality Assessment Program 1. Atrazine is commonly found in the presence of other contaminants in streams and groundwater 1. The maximum contaminant level of atrazine in drinking water is 3 µg/L (http://www.epa.gov/waterscience/criteria/atrazine). The Environmental Protection Agency (U.S. EPA) is currently developing new aquatic life criteria for atrazine to protect sensitive species of plants and animals. However, concentrations of atrazine in rivers can reach and exceed 20 µg/L during the spring months 3. In addition, concentrations as high as 200 µg/L have been detected in isolated ponds and water bodies during pulsed rain events 5. Previous studies have shown that atrazine at these concentrations (200 µg/L) is not acutely toxic to invertebrates but has chronic sublethal and acute potentiating effects on the toxicity of other pesticides 6, 7. Hyalella azteca exposed to atrazine in combination with other organophosphate insecticides increased toxicity, showing some synergistic effects 7-9.
Atrazine application in agricultural areas often coincides with fertilizer use, resulting in the presence of both atrazine and nitrate in surface and groundwaters 10, 11. The drinking water standard in the United States for nitrate is 10 mg/L (http://www.epa.gov/safewater/contaminants/index.html), whereas safe criteria for aquatic life have yet to be established. Measured concentrations worldwide exhibit wide ranges often exceeding 25 mg/L in surface waters adjacent to farmlands 12. Elevated levels of nitrate deteriorate water quality throughout the watershed and increase coastal hypoxia via discharge into estuarine systems, affecting not only freshwater organisms but also the marine food chain 13. Recent research has indicated that some sensitive species of aquatic invertebrates are negatively affected at concentrations below drinking water standards 12. Thus, investigating potential deleterious effects of nitrates on aquatic organisms is important, especially when they are present with other chemical contaminants such as atrazine.
Recent studies with atrazine and nitrate have been reported, primarily with amphibians 14-16. To the best of our knowledge, no studies investigating the combined effect of atrazine and nitrate for invertebrates exist. The present study sought a better understanding of the effects of nitrate on amphipods and the possible interaction between nitrate and atrazine. First, we determined lethal and sublethal effects of nitrate on adult Hyalella azteca in water-only exposures under two testing conditions: with and without the presence of food. Second, we tested whether atrazine would potentiate nitrate toxicity, using mortality and precopulatory guarding behavior as endpoints. Precopulatory guarding behavior has been shown to be a sensitive indicator of toxicant-induced stress and is considered ecologically important because it serves as a prerequisite for successful mating of amphipods 17, 18.
MATERIALS AND METHODS
Culture method
Hyalella azteca has been used extensively for standardized water and sediment toxicity testing 19, 20. Test organisms were obtained from Arkansas State University Ecotoxicology Research Facility (Jonesboro, AR, USA). Amphipods were acclimated to dechlorinated (0.075 ± 0.007 SD mg/L Cl2) Conway city tap water (pH 7.4 ± 0.1, hardness 35.2 ± 4.7 mg/L CaCO3, alkalinity 18.1 ± 2.5 mg/L CaCO3 and 7.27 ± 0.17 mg/L dissolved oxygen) adjusted to a hardness of 61.6 ± 3.9 mg/L CaCO3 by addition of magnesium sulfate and calcium sulfate. A photoperiod of 16:8h (light/dark) with an intensity of approximately 600 lux was maintained to promote successful reproduction. Long-term cultures were maintained under static conditions in 10-gallon glass aquaria with a constant temperature of 23 ± 1°C and aeration. Half the volume of overlying water in aquaria was changed weekly by siphoning and adding freshwater. The culture diet consisted of a mixture of 2.5 g Tetramin fish food and 2.5 g wheat grass blended in 62.5 ml distilled water. Amphipod cultures were fed approximately 2.5 ml of this mixture three times per week. Sterile, 100% cotton gauze was also added for culture substrate.
Test organisms were isolated from the culture by transferring cotton gauze substrate containing amphipods into a no. 40 sieve (425-µm mesh) 20. Test water was sprinkled over the gauze to wash juveniles through the no. 40 sieve into a no. 60 sieve (250-µm mesh). Adults (at least four weeks old based on size) remaining on the no. 40 sieve were acclimated to test water for a minimum of 2 d before initiating tests 19. Borosilicate glass beakers (250 ml) were used as test chambers containing 200 ml test solution. Measurement of all water quality parameters was performed following standard methods 21.
Source of water and preparation of test solutions
Reconstituted moderately hard water was used as exposure water in all tests and prepared following EPA standard methods 20. Sodium nitrate (72.687 g NaNO3; CAS 7631-99-4; Sigma Aldrich) was dissolved in 1 L reconstituted moderately hard water to make a stock solution of 12,000 mg/L NO3-N. Technical-grade atrazine with a purity of 96.0% (CAS 1912-24-9) provided by Syngenta was used to prepare stock solution of atrazine (1.003 g/L) in pesticide-grade acetone.
Binary mixture bioassays
Reference tests with amphipods were carried out using cadmium chloride (CdCl2) before conducting all definitive acute tests with atrazine and nitrate to verify that each batch of organisms used in different tests reacted similarly. Culture water (dechlorinated Conway city tap water adjusted to a hardness of 61.6 ± 3.9 mg/L CaCO3 with magnesium sulfate and calcium sulfate) was used as dilution water in all reference tests.
Six nominal concentrations (0, 37.5, 75, 150, 300, 600 mg/L) of sodium nitrate were crossed with two nominal concentrations of atrazine (0 and 200 µg/L) in a randomized factorial design for a total of 12 treatment combinations, six concentrations of nitrate alone and six concentrations of nitrate with atrazine. Dilution water without the addition of nitrate and atrazine was used as a negative control. An additional treatment containing 200 µl/L acetone served as a solvent control. Solutions were not renewed during the course of experiments.
Eight test amphipods were placed into three replicate test chambers (250-ml borosilicate glass beakers), each containing 200 ml test solution (24 individuals per treatment, 36 test chambers total). A pilot study was conducted to determine the number of organisms in each beaker to optimize survival and statistical power. Nitex screen (3 × 3 cm, approximately 250 µm) was added to each test chamber to serve as a substrate for amphipod attachment. Static, nonrenewal tests were conducted during which the organisms were not fed. Mortality was documented at 24, 48, 72, and 96 h. A subset of amphipods (n = 30) taken from the test batch before assigning treatments was used to determine average dry weight (0.28 mg) and length (3.28 mm ± 0.6 SD, n = 30) measured from the base of the first antenna to the tip of the third uropod along the curve of dorsal surface. Tests were considered acceptable if control mortality did not exceed 10%. Individuals exhibiting no movement of antennae or limbs after gentle prodding were scored as dead.
After the 96-h exposure, surviving amphipods were transferred by pipet into 500-ml beakers containing 450 ml culture water. Replicate treatments were combined for posttest mortality and behavioral observations. Each beaker received a single whole, well-conditioned silver maple leaf (Acer saccharinum) of similar size to serve as food and substrate. Leaves were air dried and conditioned in distilled water for more than two weeks before use. Amphipods were fed 0.5 ml Tetramin and wheat grass food solution used for culture. Water was renewed completely every 48 hours to maintain optimum dissolved oxygen (no aeration). Dissolved oxygen and pH were measured before each water renewal. This test was conducted from day 0 postexposure to day 10, with mortality and precopulatory mate-guarding behavior recorded at 24-h intervals. After the 10-d period, live individuals were sexed using a dissecting microscope (males can be easily identified by their enlarged second gnathopods), pairing status noted, number of juveniles counted, and dry weight of surviving amphipods determined. Amphipods were dried at a constant temperature of 60°C for 24 hours and desiccated for at least 1 h before weighing to the nearest 0.0001 g. This test was designed to determine whether any toxic hangover or delayed effects in the absence of toxicants occurred after 96-h exposure.
Percent mortality and average weight of test organisms was determined for each treatment. The cumulative number of paired amphipods (amplexus) that were observed throughout the 10-d period was also recorded. Total number of juveniles divided by the number of females in each treatment was indicative of reproductive success of the animals during the study period. The median lethal concentration (LC50) values based on measured concentrations were used to calculate synergistic ratios for atrazine (ATR) by dividing LC50(no ATR) by LC50(ATR) 7.
Binary mixture bioassays, with food
Eight amphipods were placed into four replicate test chambers (250-ml borosilicate glass beakers), containing 200 ml of test solution (32 individuals per treatment and 44 test chambers total). Five nominal concentrations (0, 10, 20, 40, and 80 mg/L) of sodium nitrate were crossed with two concentrations of atrazine (0 and 200 µg/L) in a randomized factorial design with a total of 10 treatment combinations. An additional treatment containing 200 µl/L acetone was used as a solvent control. Static, renewal tests (96-h) were conducted with complete water renewal at intervals of 24 hours. Live amphipods were transferred to new identical sets of exposure containers, with fresh solutions prepared from the stock. Organisms were fed 0.25 ml Tetramin and wheat grass culture food daily, and Nitex screen was added as a substrate.
Precopulatory guarding behavior
Precopulatory pairs were selected randomly from culture tanks and maintained separately in a 1-L glass beaker with sterile cotton gauze and food for 24 h before initiating tests. Five nominal concentrations (0, 20, 40, 80, 160 mg/L) of sodium nitrate were crossed with two concentrations of atrazine (0 and 20 µg/L) in a randomized factorial design. Reconstituted moderately hard water and acetone (200 µl/L) served as control and solvent control, respectively. For each test treatment, a total of 20 precopula pairs were exposed for 24 hours; 10 replicate beakers with two pairs H. azteca each in 200-ml solution. Disruption of precopulatory behavior (direct separation of precopula pair) was recorded at 2-h intervals for the first 8 h and then at 19 and 24 h of exposure. At the end of the of the 24-h exposure period, mortality was recorded, and water quality parameters were measured.
Toxicant and water quality analyses
Conductivity, pH, dissolved oxygen, and temperature were measured using an Orion 5-Star Plus Benchtop Multimeter (Thermo Scientific). Total alkalinity and hardness were determined using U.S. EPA buret titration method 8221 and U.S. EPA ManVer 2 buret titration method 8226, respectively. Ammonia was measured using a Hach® DR/5000 Spectrophotometer (Hach) using the TNT plus 830 Hach reagent kit.
Atrazine and nitrate were analyzed in the initial solutions for each test. Nitrate analysis was performed using Hach® DR/5000 Spectrophotometer. When prompt analysis was not possible, samples were stored in clean glass vessels for up to 24 h at 4°C. Standard procedures for the Cd reduction method 8039 were employed using powder pillows (Nitrate HR Reagent, NitraVer, 10-ml sample). The detection limits for nitrate and atrazine analysis were 0.5 mg/L and 50 µg/L, respectively.
Atrazine was isolated from water samples using solid-phase extraction on ENVI-Carb cartridges (Supelco Analytical). The cartridges were sequentially cleaned and conditioned with high-performance liquid chromatography–grade dichloromethane-methanol, methanol, and distilled water. Water samples were passed through the cartridges under constant pressure, using a vacuum aspirator. The retained compounds were eluted with methanol, then dichloromethane-methanol. Extracts were placed into a SpeedVac (Thermo Scientific) until all solvents were evaporated. After addition of phenanthrene-d10 to the extract, the final volume was reconstituted with methanol. The samples were then injected into a gas chromatograph/mass spectrometer. Gas chromatography was performed with a Varian interfaced to a mass-selective detector operating in electron ionization mode and programmed for autosampler operation with splitless injection.
Statistical analysis
The LC50 values with 95% confidence intervals based on measured concentrations were estimated using nonlinear regression with Prism 5.02 (GraphPad). Percent mortality data were arcsine square root transformed before statistical analysis. Dunnett's multiple comparison tests were used to identify significant differences in percent mortality and pair separation between treatments and control, using JMP (SAS Institute). Two-way analysis of variance (ANOVA) was used to compare toxicity between single and mixture treatments. If data did not meet the assumptions of normality and homogeneity of variance, nonparametric tests (Wilcoxon/Kruskal-Wallis test) were used to test for significant effects among the treatment groups. Posttest observations, including survivorship, sex ratio, and reproductive success (number of juveniles/females), were analyzed using the chi-squared test of independence. Statistical significance was established with an alpha level of 0.05.
RESULTS
Amphipod response to CdCl2 among four reference tests was similar, suggesting that adult amphipods used for definitive testing were similar in terms of health and sensitivity. Initial dilution water parameters were similar for all tests. Dissolved oxygen concentration and pH in single and binary mixture experiments were within U.S. EPA standards and did not change over the 96-h test period. In experiments with food, however, both dissolved oxygen concentrations and pH decreased (dissolved oxygen as low as 1.3 mg/L and pH 7.14) between 24-h water renewal periods. Mean total ammonia concentration measured before water renewal was 0.24 (± 0.1) mg/L.
Most mean measured nitrate concentrations did not deviate substantially from nominal values (Table 1). Extraction efficiency of atrazine was 94.5 ± 6.9% (mean ± SD, n = 6). Measured concentrations (±SD) of atrazine varied somewhat from nominal (200 µg/L), averaging 139.6 µg/L (± 15.4, n = 6) and 120.1 µg/L (±41.9, n = 3) in survival tests with and without food, respectively. In the precopulatory guarding behavior test, measured concentrations of atrazine averaged 31.5 µg/L (± 6.6, n = 4). Atrazine was below the detection limit in control water for all tests.
| Binary without foodb | Binary with food | Precopulatory behavior | |
|---|---|---|---|
| Nominal (mg/L) | 0 | 0 | 0 |
| 37.5 | 10 | 20 | |
| 75 | 20 | 40 | |
| 150 | 40 | 80 | |
| 300 | 80 | 160 | |
| 600 | |||
| Measured (mg/L) | NDc | ND | ND |
| 30.1 | 9.3 ± 1.1 | 18.8 ± 1.1 | |
| 107.6 | 21.2 ± 2.8 | 40.4 ± 0.4 | |
| 200.2 | 44.8 ± 10.1 | 59.4 ± 2.2 | |
| 284.1 | 84.0 ± 7.8 | 135.5 ± 14.7 | |
| 738.9 |
- a Chemical analyses in all tests were performed before test initiation.
- b Values based on a single measurement.
- c Not detected.
Binary mixture bioassays
All LC50 calculations were based on initial measured values (Table 2). At both 48 and 96 h, mortality increased with increasing nitrate concentration (Fig. 1). Percent mortality between single and binary mixture experiments was significantly different only at 72 hours (F11,24 = 4.94, p = 0.04). No significant differences were seen in percent mortality at 24 h (F11,24 = 0.29, p = 0.60), 48 h (F11,24 = 0.22, p = 0.65), or 96 h (F11,24 = 0.01, p = 0.93).
| 24 h | (95 % CI) | 48 h | (95 % CI) | 72 h | (95 % CI) | 96 h | (95% CI) | |
|---|---|---|---|---|---|---|---|---|
| Binary bioassays | ||||||||
| NO3-N alone | 173.5 | (136.4–220.6) | 161.0 | (137.0–189.2) | 142.4 | (128.5–157.9) | 124.2 | (112.0–137.7) |
| NO3-N +ATR | 190.5 | (165.0–220.0) | 152.1 | (132.0–175.3) | 117.9 | (96.1–144.7) | 98.1 | (81.8–117.6) |
| SRa | 0.91 | 1.06 | 1.21 | 1.27 | ||||
| Binary bioassays (foodb) | ||||||||
|---|---|---|---|---|---|---|---|---|
| NO3-N alone | NAc | NA | NA | NA | 26.8 | (16.9–42.7) | 14.5 | (6.9–30.7) |
| NO3-N +ATR | NAc | NA | 43.4 | (34.6–54.3) | 31.4 | (23.5–41.9) | 27.2 | (13.5–54.8) |
| SR | NAc | NA | 0.85 | 0.53 | ||||
- a
The synergistic ratio (SR) is calculated as SR =
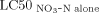 /
/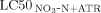 .
.
- b 0.25 ml Tetramin and wheatgrass solution.
- c Not available; LC50 value could not be determined in the absence of partial mortality in higher treatments.
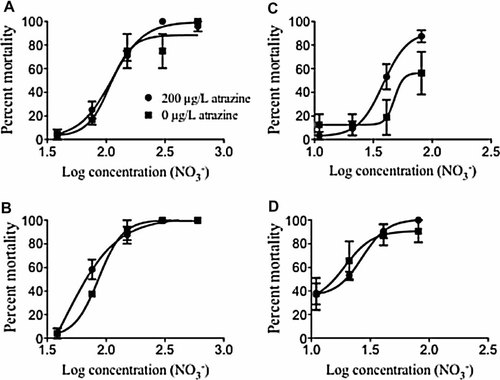
Concentration–response curves for binary mixture assays at 48 h no food (A), 96 h no food (B), 48 h food (C), and 96 h food (D). Error bars represent 1 standard deviation.
Posttest growth and behavioral observations suggested that nitrate and atrazine at these concentrations do not result in toxic hangover (delayed effects in the absence of toxicant) on H. azteca under our experimental conditions (Table 3). Survivorship in the 10-d study period was not significantly different between treatments (χ26 = 10.52, p = 0.09). The number of juveniles/female (χ26 = 10.81, p = 0.10) and sex ratio (χ26 = 10.81, p = 0.10) did not differ between the treatments. However, the total number of amphipods paired up in the control treatment was much greater than that in the contaminant treatments (Table 3). Increases in weight and reduced amplexus were observed in the nitrate 75/ATR treatments.
| ATR (µg/L) | NO3 (mg/L) | No. of amphipods | Percent survival | Weight (mg) | Total number in amplexus | No. juveniles/female | Sex ratio (male/female) |
|---|---|---|---|---|---|---|---|
| 0 | 0 | 22 | 100 | 0.286 | 21 | 1.5 | 1.2 |
| 0 | 37.5 | 24 | 91.3 | 0.290 | 13 | 1.38 | 1.63 |
| 0 | 75 | 15 | 100 | 0.333 | 11 | 0.375 | 0.88 |
| 200 | 0 | 24 | 100 | 0.271 | 10 | 2.67 | 1.33 |
| 200 | 37.5 | 23 | 91.7 | 0.277 | 14 | 0.917 | 0.92 |
| 200 | 75 | 10 | 90 | 0.444 | 4 | 0 | 8b |
| 200 | 150 | 3 | 66.7 | NAc | 0 | 0 | 1 |
- a Atrazine (ATR) 0 and nitrate 150 treatment not included; 100% mortality occurred during 96-hour exposure.
- b Higher sex ratio was observed only in this treatment with small sample size of 10. Possibly, higher number of males were used in the 75/ATR treatment, because the ratio of male to female was not known at test initiation.
- c Not available because of the low number of surviving amphipods.
Binary mixture bioassays, with food
In all controls (water and acetone), mortality was less than 10%. Mortality increased with increasing nitrate concentration. Average mortalities of 37.5% and more than 90% were observed in the lowest (10 mg/L) and highest (80 mg/L) nitrate treatments, respectively. In treatments with atrazine, average mortality was 37.5% and 100% in 10 mg/L and 80 mg/L, respectively. Median lethal concentration values decreased with increasing exposure duration (Table 2). A 24-h LC50 value could not be determined in the absence of partial mortality in some treatments. Percent mortality between single and mixture experiments was significantly different only at 24 h (F9,30 = 5.72, p = 0.02). No significant differences were seen in percent mortality at 48 h (F9,30 = 2.93, p = 0.10), 72 hours (F9,30 = 0.02, p = 0.90), and 96 hours (F9,30 = 0.31, p = 0.58). The LC50 values in the presence of food were much lower than that without food (Table 2).
Precopulatory guarding behavior
Precopulatory pair separation was higher with increasing nitrate concentration and exposure duration (Figs. 2 and 3). At 24 h, 55% of control precopula pairs were separated, possibly because of natural completion of H. azteca coupling. In nitrate-only tests, higher pair separation was observed in all nitrate treatments compared with control (χ24 = 116, p < 0.01) after 120 min exposure; 15% of pairs separated in the controls, whereas in all nitrate treatments separation was equal to or greater than 50%. The highest concentration (160 mg/L nitrate) caused 100% pair separation after 480 min exposure (Fig. 2).
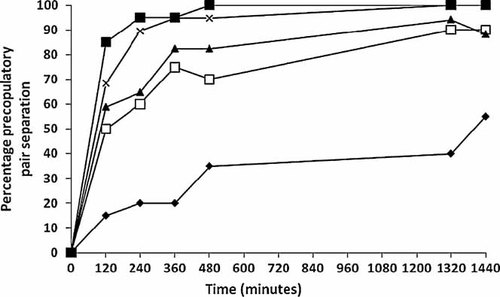
Cumulative percent separation of precopulatory Hyalella azteca during exposure to control water and nitrate (mg/L) treatments; Control (black diamonds), 20 mg/L (white squares), 40 mg/L (black triangles), 80 mg/L (×), 160 mg/L (black squares). All nitrate treatments are significantly different from control after 120 min exposure.
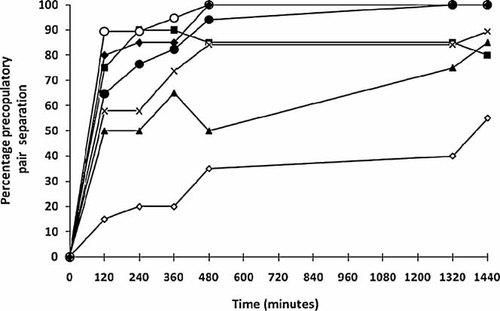
Cumulative percent separation of precopulatory Hyalella azteca during exposure to acetone and nitrate treatments in combination with atrazine. The letter A represents 31 µg/L atrazine; the number indicates the nitrate concentration. Control (white diamonds), acetone (black squares), A0 (black triangles), A20 mg/L (×), A40 mg/L (black circles), A80 (white circles), A160 (black diamonds). All treatments are significantly different from control after 120 min exposure.
In mixture treatments, a higher percentage of pair separation was observed after 120 min exposure (Fig. 3). In 0 mg/L NO3-N and acetone control treatments, percent separation was 50 and 75%, respectively, after 120 min exposure. After 480 min, 100% pair separation was seen in 80 and 160 mg/L nitrate concentrations (Fig. 3). When compared with the water control, acetone control resulted in a higher pair separation (χ21 = 52.51, p < 0.01). Higher pair separation was also seen in mixtures compared with nitrate-only treatments (χ21 = 5.46, P = 0.02).
After 24 hours, mortality was low in each treatment group except in the 160-mg/L NO3-N treatments. Intermittent pairing (pairing and separating repeatedly) was also observed in low concentrations (0 and 20 mg/L NO3-N treatments). As reported previously, single males attempt to take separated females into precopula positions 17.
DISCUSSION
Hyalella azteca has been used extensively in sediment toxicity testing. However, in the current study we conducted water-only exposures as a valid test procedure based on the extent of exposure to contaminants from the water column 22. To the best of our knowledge, no other published data exist on nitrate toxicity in H. azteca, although studies have been conducted with other crustaceans 12, 23.
Binary mixture bioassays
Our results with adult H. azteca are consistent with other studies indicating toxicity increases with nitrate concentration and exposure duration 12, 24, 25. The nitrate 96-h LC50 for adult H. azteca in the current study was 124.2 mg/L, approximately 40 times lower than that reported by Call et al. 26 for juvenile H. azteca in a range-finding test. The reason for the discrepancies in results of these two studies is unclear.
Although a paucity of data are available regarding nitrate effects on H. azteca, data exist for other species of freshwater invertebrates (Table 4). European gammarids (Eulimnogammarus toletanus and Eulimnogammarus echinosetosus) appear to be the most sensitive aquatic invertebrates tested with nitrate to date 12. However, exposure of adult Gammarus pseudolimnaeus to a nitrate concentration as high as 128 mg/L NO3-N showed no effects on mortality, molting, and egestion rate 23.
 N) to selected freshwater invertebrates 12
N) to selected freshwater invertebrates 12| Species | Common name | Developmental stage | LC50 |
|---|---|---|---|
| Echinogammarus echinosetosus | Amphipod | Adult | 62.5 (96-hour) |
| Eulimnogammarus toletanus | Amphipod | Adult | 85 (96-hour) |
| Hydropsyche occidentalis | Caddisfly | Early instar larvae | 97.3 (96-hour) |
| Hydropsyche occidentalis | Caddisfly | Last instar larvae | 109 (96-hour) |
| Cheumatopsyche pettiti | Caddisfly | Early instar larvae | 113.5 (96-hour) |
| Hyalella aztecaa | Amphipod | Adult | 124.2 (96-hour) |
| Cheumatopsyche pettiti | Caddisfly | Last instar larvae | 165.5 (96-hour) |
| Hydropsyche exocellata | Caddisfly | Last instar larvae | 269.5 (96-hour) |
| Ceriodaphnia dubia | Daphnia | Neonates (<24 h) | 374 (48-hour) |
| Daphnia magna | Daphnia | Neonates (<48 h) | 462 (48-hour) |
| Potamopyrgus antipodarum | Snail | Adult | 1,042 (96-hour) |
- a Present study.
Toxicity databases show that atrazine is not acutely toxic to freshwater invertebrates at environmentally relevant concentrations (http://cfpub.epa.gov/ecotox/). Anderson and Lydy 9 reported an LC50 value greater than 10,000 µg/L for H. azteca (14–21-d-old juveniles). Invertebrate LC50 values range from 3,000 µg/L for Hydra sp. to 49,000 µg/L for Daphnia magna 27. Chronic effects of atrazine exposure studied with freshwater invertebrates also indicate that environmental concentrations do not affect growth and reproduction. The growth of Chironomus tentans was reduced at 230 µg/L, a concentration higher than typically found in the environment 27. Although not acutely toxic at these concentrations, previous studies have shown that atrazine at relevant concentrations (up to 200 µg/L) increases the toxicity of other insecticides to freshwater invertebrates 7-9.
Increases in weight and reduced amplexus after 10-day posttest observations in 75/ATR treatments were likely a result of the larger males, because sex ratio was male-biased (Table 3). Although insignificant, a higher sex ratio was observed only in this treatment with a small sample size of 10. Bias in sex ratio may be attributable to a higher number of males used in the 75/ATR treatment, because the ratio of male to female was not known at test initiation. Selective survival of males in this treatment is unlikely a result of endocrine effects, because 96 h could be a short time to cause lethal effects. However, effects of nitrate and atrazine would need to be investigated further before a conclusive assessment of the endocrine effects could be made.
Binary mixture bioassays with food
Nitrate toxicity was more pronounced when the test was conducted in the presence of food, as evidenced by a sharp decline in median lethal concentration (approximately 70 and 80% decrease in 48-h and 96-h LC50, respectively). Because of organic food particles and no aeration of test solutions over the exposure period, dissolved oxygen dropped to as low as 2 mg/L in some treatments, including controls, despite solution renewals at 24-h intervals. Ammonia levels were high because of metabolic waste coincident with feeding activity, and previous studies have shown that ambient nitrate concentrations can significantly increase ammonia excretion in penaeid shrimp 28.
Measured ammonia levels in nitrate treatments were not different from the controls. In all positive and negative controls, mortality was lower than 10%, which renders ammonia being the sole cause of higher mortality in nitrate treatments unlikely. In hypoxic environments rich in organic compounds, detrimental concentrations of nitrite can be formed from nitrate concentrations 29. Although we did not measure nitrite, our test organisms were likely experiencing an environment with mixtures of nitrate, nitrite, and ammonia, which may have interacted synergistically under low dissolved oxygen conditions, eliciting higher observed mortality 30, 31. Beketov 31 reported LC50 values ranging from 5.0 to 37.5 mg/L of total dissolved inorganic nitrogen with exposure to 59.0% ammonia, 1.5% nitrite, and 39.5% nitrate in mixtures 31.
Higher observed mortality in the presence of food also may be attributed to the mechanism of nitrate toxicity. Nitrate converts oxyhemoglobin and oxyhemocyanin, used by crustaceans for oxygen transport, to forms incapable of transporting oxygen 12, 32. Although amphipods have been shown to be very tolerant of low dissolved oxygen in absence of toxicants with a 96-h LC50 of 0.3 mg/L 33, this mechanism of nitrate toxicity possibly promoted inadequate oxygen uptake and oxygen-deprived conditions in amphipod tissues.
Precopulatory guarding behavior
Our results indicated that nitrate at environmentally realistic concentrations exhibits a dose–response effect on precopulatory guarding behavior. Hyalella azteca are more likely to abandon precopulatory pairs when exposed to toxicants because of higher energy cost carrying females 17, 18. Recent studies demonstrated that exposure of aquatic vertebrates to nitrate and nitrite also can cause endocrine toxicity, interfering in important reproductive signal systems 34. Endocrine systems in both vertebrates and invertebrates regulate development and reproductive processes 34, 35. Endocrine disruption of chemicals is generally characterized for vertebrate species. Although nitrate as an endocrine disruptor in invertebrates is poorly understood, one study shows that a metabolite of nitrate can elicit endocrine-disrupting toxicity in daphnids 35. Thus, we cannot exclude the hypothesis that abandonment of precopulatory pairs in the present study also may be the result of endocrine disruption activity of nitrate metabolites.
Atrazine has shown the potential to be an endocrine disruptor in vertebrates interfering with amphibian metamorphosis, sex ratios, and gonadal malformations 15, 16, 36. In fish, atrazine has been shown to reduce egg production in female fathead minnows and produce gonadal abnormalities in both sexes 37. However, effects on the endocrine system of invertebrates are poorly understood at this time. Our results indicate that the presence of realistic levels of atrazine (31 µg/L) may affect mate-guarding behavior of H. azteca in a 24-h test period. However, observed effects of atrazine may be attributed to acetone, because a significant difference was observed between water and acetone controls.
The reason for greater precopulatory pair separation in the acetone control is unclear. Acetone concentrations of 100 µl/L tested on D. magna in a 21-d exposure experiment resulted in offspring with abnormal development of second antennae 38. The authors believe acetone could interfere with ecdysteroid synthesis, important in molting, development, and reproduction in crustaceans 38. The higher concentration of acetone (200 µl/L) in our experiments may have caused a disruption in amphipod mating signal systems. However, further research investigating the sublethal toxicity of acetone and other solvents is needed to confirm this.
Atrazine and nitrate interaction
The 96-h LC50 value of 124.2 (95% confidence interval 112.0–137.7) mg/L for H. azteca exposed to nitrate decreased to 98.1 (95% CI 81.8–117.6) mg/L in the presence of atrazine, an increase in toxicity that could be biologically relevant because of the small overlap in 95% confidence intervals (Table 2). Synergistic ratios for 24-, 48-, 72-, and 96-h durations were 0.91, 1.06, 1.21, and 1.27, respectively. The gradual increase in synergistic ratios over exposure duration and LC50 values at 72 and 96 h suggests that atrazine may potentiate the toxicity of nitrate in long-term tests. Recent studies with atrazine and nitrate have been reported primarily with amphibians 14-16. Allran and Karasov 14 reported that atrazine and nitrate did not appear to have any direct toxic effects on development rate and percent survival of northern leopard frog larvae. Still, other studies have shown evidence that a combination of atrazine and nitrate interfere with metamorphosis, sex ratios, and gonadal maturity during larval development in frog species 15, 16. However, we are unaware of any other studies related to mixture toxicity of atrazine and nitrate on invertebrates, making accurately predicting the combined toxic mechanisms of herbicides and fertilizers on crustaceans difficult.
As agricultural production continues to rise, understanding the impacts of both fertilizers and herbicides on our aquatic systems is critical. Although significant differences in acute toxicity with nitrate alone and in binary combination with atrazine (200 µg/L) in water-only tests were not consistently observed for each time point, potential biologically relevant trends in the data were observed. We also demonstrated that nitrate was toxic at realistic environmental concentrations under altered testing protocols (feeding). Precopulatory guarding behavior tests indicated nitrate and atrazine, although not acutely toxic at relatively higher concentrations, have the potential to elicit sublethal responses, which play vital roles in species survival and reproduction. Further long-term studies investigating sublethal effects on amphipods are needed to better understand how atrazine interacts with nitrate. Expanding similar studies on other invertebrates both in laboratory and field settings is also highly recommended.
Acknowledgements
The authors express their appreciation to Jennifer Bouldin at Arkansas State University for supplying culture organisms and Karen Steelman for assistance with chemical analyses, and to both for critical feedback on experimental design and early drafts of the manuscript. We thank two anonymous reviewers for their comments on the manuscript. We also acknowledge Reid Adams for input on experimental design and statistical analyses. Valuable laboratory assistance was provided by Pyi Thein Kyaw, Richard Walker, Tyler Fox, Martin Sharum, and Michael Uffenbeck. This research was funded by the University Research Council and Biology Department at the University of Central Arkansas.