Pesticide and toxicity reduction using an integrated vegetated treatment system
Abstract
The California, USA, central coast is one of the most productive agricultural areas in the world, and numerous stakeholders are working there to implement conservation practices to reduce contaminated runoff. Practices include vegetated treatment systems (VTS) designed to promote contaminant reduction and breakdown. The current study evaluated the effectiveness of a vegetated drainage ditch incorporating a sedimentation basin, a vegetated section, and a Landguard organophosphate-A (OP-A) enzyme dosing system. The VTS was constructed on a working farm and was designed to remove organophosphate and pyrethroid pesticides, the primary pesticides causing toxicity in Salinas Valley watersheds. The present study was conducted during five separate irrigation events on tailwater runoff containing mixtures of pesticides and suspended sediments. Water samples were collected at four stations within the system, and these were subjected to chemical analyses and tested for toxicity to Ceriodaphnia dubia. All inflow samples were highly toxic to C. dubia, and this was largely because of diazinon. Treatment of diazinon-contaminated runoff was only partially effective using aquatic vegetation. All diazinon remaining after vegetated treatment was effectively removed after treatment with the Landguard OP-A enzyme. Chemical analysis of the VTS water samples showed that pyrethroid and organochlorine pesticide concentrations in water were greatly reduced in the sedimentation section of the ditch, and these pesticides were further reduced in the vegetated section of the ditch. The overall conclusion from these analyses is that the VTS was effective at reducing the more hydrophobic organochlorine and pyrethroid pesticides from water. The water-soluble pesticide diazinon was not sufficiently removed during the VTS residence times observed in this study; however, residual diazinon was effectively removed using Landguard OP-A. Environ. Toxicol. Chem. 2011; 30:1036–1043. © 2011 SETAC
INTRODUCTION
The coastal valleys of central California, USA, are considered the nation's salad bowl, forming the heart of the most productive vegetable-producing region in the country (http://www.cfbf.com). This region contains year-round, intensively cultivated agricultural land supporting a nearly $5 billion/year industry producing most of the nation's salad greens, artichokes, and crucifer crops. Runoff from irrigated agriculture constitutes a significant portion of stream flow in the region during much of the year, and a number of studies have documented pesticide occurrence, toxicity, and biological impacts in the rivers and estuaries of the Pajaro 1, Salinas 2, 3, and Santa Maria watersheds 4. In the lower Salinas and adjacent Gabilan watersheds, 13 water bodies are currently listed as impaired by pesticides or nutrients under Clean Water Act §303[d], including the lower Salinas River.
Evidence of toxicity and other water quality impacts has motivated a diverse range of stakeholders to begin implementing farm management practices to reduce nutrients, pesticides, and toxicity in agricultural runoff. Based largely on the results of recent research showing effectiveness of grass-lined ditches and constructed wetlands at reducing pesticides in water 5-7, researchers have begun to emphasize these practices for use in California agriculture. Farm owners and managers have worked with technical specialists to design and construct ditch, wetland, and pond vegetated treatment systems (VTS) at a variety of locations in Central California 8, 9. Because studies have shown vegetation to be less effective at reducing concentrations of the more soluble organophosphorus pesticide diazinon to levels nontoxic to Ceriodaphnia dubia 8, 9, use of alternative practices such as the enzyme Landguard organophosphate-A (OP-A) (http://csiro.au/solutions/pesticidebioremediation) is being considered for this class of pesticides 10. The present study was designed to evaluate the effectiveness of an integrated VTS system incorporating a sedimentation basin, a vegetated ditch, and a Landguard OP-A dosing system. The study assessed reductions in water toxicity associated with reduced pesticides in agricultural runoff, and was conducted on a working industrial-scale row crop operation in the Salinas Valley.
MATERIALS AND METHODS
Research was conducted on a vegetated tailwater ditch located on a working farm in the Salinas Valley in Central California. The study site was a 300-m-long V-shaped ditch that drained approximately 120 acres planted in row crops, primarily lettuce, broccoli, and asparagus. The ditch dimensions were height = 1 m, top width = 3.25 m, bottom width = 1.25 m. The ditch was divided into three sections with four sampling points (Fig. 1). The ditch was fitted with a V-notched galvanized aluminum weir at station B and a fiberglass flume at station C. The 45-degree Washington State College-designed extra-large flume dimensions were height = 27 cm from the throat to the top; width = 76 cm; length = 91 cm. The weir (station B) was installed 33 m downstream from the tailwater input (station A), and the flume (station C) was installed 233 m downstream from the weir. The section of the ditch between stations A and B was not planted with vegetation. This section functioned as a sediment settling basin during the trials, although some native grasses began to grow in this part of the system during the trials. The section of the ditch between stations B and C was the vegetated section of the VTS system, and this section was planted with rushes (Juncus phaeocephalus and J. patens) and pennywort (Hydrocotyle spp.), and it was seeded with creeping wild rye and red fescue. As the trials progressed, naturally occurring Bermuda grass also flourished in the ditch. Plant cover during the first trial was approximately 50%, and it was greater than 90% cover during the four subsequent trials. The section of the ditch between stations C and D (downstream of the flume) was 33 m long and served as the Landguard OP-A treatment section (described later).
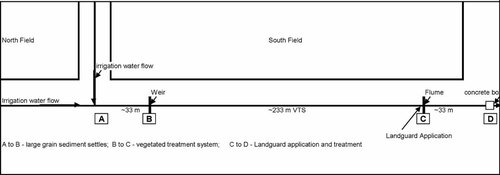
Schematic diagram of VTS ditch and sampling stations. Note that station C (Flume) was sampled upstream of the Landguard application and was not influenced by the enzyme.
Landguard OP-A treatment system
Landguard OP-A is derived from soil bacteria and cultured on a large scale to produce commercial quantities of enzyme for use in treatment of agriculture runoff, contaminated soils, animal pest treatment wastewater, and other applications in which rapid treatment is needed to break down organophosphate pesticides to nontoxic metabolites. The product used in the system is described in more detail in Anderson et al. 10. The OP-A enzyme acts as a catalyst for the rapid hydrolysis of the pesticide, producing metabolites with lower toxicities. The two diazinon breakdown products resulting from the hydrolysis of diazinon are diethyl thiophosphoric acid and 2-isopropyl-4-methyl-pyrimidin-6-ol. Landguard OP-A was added to the tailwater, using a dosing unit designed and constructed by Terry Pritchard, University of California Cooperative Extension, UC Davis. The Landguard Dosing Unit dispenses OP-A solution in relation to the water flowing through the measuring flume at Station C, and it is designed to maintain a constant dosing rate. The dosing unit consists of the following components: a flume (described in the text for station C) that creates conditions in the tailwater ditch that allow water height to indicate water flow rate; a float bucket that receives Landguard OP-A solution from a stock tank and maintains a constant water height, a dispensing solenoid that is attached to the bottom of the float bucket, and a float assembly that attaches to the flume in a polyvinylchloride stilling well. A controller box having cable connections for the solenoid, float, and battery and a 6V battery were incorporated into the system. The stilling well contained a float-operated electrode that sent increasing electrical signal to the solenoid with rising water. As flow increased, the enzyme drip rate from the dispensing solenoid increased. The unit was calibrated to provide a final enzyme concentration of 0.1 mg enzyme/L in the tailwater.
Experimental trials were conducted on five separate irrigation events, which were real-world (i.e., not simulated) irrigations conducted as part of daily operations on the farm. The characteristics of these events are described in later sections. For each of the five trials, the enzyme-OP-A dosing unit was set up on the morning of the trial before water reaching station C. As irrigation runoff reached the flume, it was dosed with Landguard, and this solution was mixed in the final 33 m of the ditch between stations C and D. Landguard-treated water was then sampled at station D. On one occasion (trial 3), water was already flowing through the flume at the start of the trial. In this trial, calibration of the dosing unit relied on the previous calibration conducted during trial 2.
Hydrology
The VTS ditch is divided into three sections: a sedimentation area (reach A-B), a vegetated reach (reach B-C), and a Landguard application reach (reach C-D). When full of water, these reaches hold approximately 5,000 L, 11,000 L, and 2,000 L, respectively. Flow monitoring was conducted at station B by using a 90-degree sharp crested weir constructed from 12-gauge sheet metal, and at station C using a 45-degree Washington State College–designed extra-large flume. Water levels were recorded at the flow monitoring stations and near station D, using pressure transducers and a barometric pressure logger (Solinst Levelogger and Barologger, Solinst).
The residence time of water in the ditch was estimated based on the physical characteristics of the site and observations from previous studies on similar vegetated ditch systems in the Salinas Valley 11. As in the previous studies, the ditch exhibits a very high length-to-width ratio (140), and length-to-water-depth ratio (867), and no visual evidence of dead zones or preferential flow paths. Tracking a pulse of water requires tracking the displacement of water through the system. The reach transit times for water traveling through the three ditch sections was estimated by comparing the average flow rate with this displacement volume. Using the estimated transit times, composite samples were collected for toxicity and chemistry, as described in later sections.
Sample collection
The timing of sampling at each station is given in Supplemental Data Table S1. Sampling for enzyme-linked immunosorbent assays (ELISA) analysis of diazinon in water was conducted every hour (method discussed later). In addition to this, a three-sample composite sample was collected at each station. The composite samples (defined later) were used for toxicity testing (all trials), and gas chromatography-mass spectrometry chemicals analyses (trials 4 and 5 only). Diazinon and chlorpyrifos were also measured in the composite samples, using ELISA.
Although little water was present in the ditch when trials 1 and 5 were sampled, residual water was ponded at several locations in the ditch during trials 2, 3, and 4, and diazinon concentrations in this water often differed from diazinon concentrations entering the ditch from that day's irrigation event. We attempted to differentiate this residual water from that day's new irrigation water by initiating sampling of the water at each station after water had passed through the stations for a prescribed interval. In the first two trials, the composite sample comprised three 0.8-L samples collected every 15 min after irrigation water first began to spill through each of the sampling stations. In the final three trials, the three samples for the composite were collected every 30 min after water first began to spill through each station (Supplemental Data Tables S1 and S2). The hourly ELISA analyses allowed us to evaluate short-term changes in diazinon concentrations in this system. The composite samples allowed us to evaluate associated changes in toxicity, turbidity, and chemistry of the treated pulses of water as they passed through the four sampling stations in the ditch. Water samples were collected by hand in 2.5-L amber glass bottles and were immediately placed on ice in coolers at 4°C during transport to the laboratory.
Sediment collection consisted of Pyrex trays placed upstream of station B (weir) and station C (flume). Suspended sediment from tailwater settled into the trays as it passed by the stations. After six weeks the trays were recovered and emptied into 2-L glass jars for transport to the laboratory. There they were homogenized and subsampled for grain size, total organic carbon, and chemical analysis. Chemistry samples were frozen.
Toxicity testing
Water toxicity was evaluated using the 7-d chronic Ceriodaphnia dubia toxicity test within 48 h of sample collection 12. Each undiluted sample was tested using 10 replicates containing one C. dubia neonate (<24-h-old, obtained from in-house cultures). Survival and reproduction were monitored daily. Samples were defined as toxic if the following two criteria were met: a significant difference (p < 0.05) in mean organism response (e.g., percent survival) between a sample and the negative laboratory control, as determined using a separate-variance t test, and difference in organism response between the sample and control greater than 20% 13. Water quality parameters, including conductivity, hardness, alkalinity, pH, dissolved oxygen, and ammonia, were measured at the beginning of each test. Temperature was measured using a continuously recording thermograph and thermometer. Turbidity was measured in water in the final two trials, trial 4 and trial 5, using a Hach model 2100 P portable turbidity meter.
Chemical analysis
Concentrations of the organophosphate pesticides chlorpyrifos and diazinon were measured using ELISA (Strategic Diagnostics). The ELISA procedures followed those recommended by Sullivan and Goh 14. Readings were compared with a five-point standard curve prepared using standards provided by the manufacturer. Accuracy was determined for each batch using external standards and matrix spikes. Precision was determined by duplicate measurement of one sample per batch. Samples were tested without dilution unless necessary. Lowest detectable concentrations for this procedure were 30 ng/L for diazinon and 50 ng/L for chlorpyrifos. Reporting limits were 60 ng/L for diazinon and 100 ng/L for chlorpyrifos. Whole water samples from trials 4 and 5 were also analyzed for a suite of current use and legacy pesticides by AXYS Analytical Laboratories (Sidney, BC, Canada). These included measures of organophosphate pesticides (EPA method 8141), organochlorine pesticides (EPA method 8081), carbamate pesticides (EPA method 531.1), and pyrethroid pesticides (EPA method 1660). Because of high particle concentrations in the water samples from stations A and B, these samples were first filtered to remove suspended solids, and the water and filtered solids were extracted separately before gas chromatography analysis. Final total concentrations of pesticides in the A and B water samples were derived by adding the aqueous and suspended sediment concentrations. Water samples from stations C and D were not filtered.
Sediment samples from stations B and C were also analyzed for grain size, total organic carbon (EPA Method 9060A), and organophosphate, organochlorine, and pyrethroid pesticides, using the same methods described previously for water analyses.
RESULTS
Effectiveness of the VTS ditch was evaluated from April through August 2008. As the sampling progressed, farming activity increased in the adjacent fields, and this combined with variable weather created variable irrigation schedules. During the first trial, little residual water was present in the ditch at the initiation of the trial, and therefore one could observe the tailwater as it entered the system at the input at station A, then follow this pulse of water as it progressed through stations B, C, and D. The interval for water pulses passing from stations A through D in trial 1 was confirmed by data provided with the in situ level loggers and flow meters and was used to inform sample collection timing in the later trials. Hydrologic information for this ditch is summarized in Supplemental Data Table S1. These data show that irrigation water flow varied within and between each of the five events, with trial 1 receiving the greatest volume of tailwater and trial 5 receiving the least. Outflow volumes also varied, and these were influenced by infiltration of water into the ditch substrate, and by ponding of residual water.
The amount of time a given pulse of water took to pass through each of the four VTS ditch reaches is also summarized in Supplemental Data Table S1 as reach transit time. A range of transit times is presented, because water movement varied with the volume of flow passing through the system: Faster transit times resulted from higher input flows, and as irrigation flows decreased, transit of water took longer. In general, the transit times for each reach coincided with the sampling periods used for collecting the composite toxicity and chemistry samples at each of the four stations (Supplemental Data Table S1). Thus, the average transit time for the first reach (station A to station B) was approximately 0.5 to 1 h during the five trials. The average transit time for the second reach (station B to station C) was about 3 to 4 h, except in trial 5, when flows were lower and the transit time was longer. The average transit time for the third reach (station C to station D) was approximately 1 to 2 h, except in trial 5. Water did not reach station D during trial 5 because of low irrigation flows into the system. The composite sample for station D during trial 5 was therefore collected midway between stations C and D and was collected from a section of the reach that had been dry before water passing through the flume.
Diazinon concentrations varied hourly in the VTS ditch and were apparently influenced by daily variability in tailwater diazinon entering the system. In situations in which ponded water was present in the ditch from previous irrigation events, diazinon concentrations in this water often differed from that entering the system at station A. Trends in hourly diazinon concentrations at the four ditch sampling stations are provided for the five trials in the supplemental data (Supplemental Data Figures S1-S5). These data are discussed later, because they influence results of the composite samples collected during the five trials.
Results from the composite samples demonstrate minimal to moderate reduction of diazinon in the vegetated sections of this system (Table 1). Average (composite) concentrations of diazinon varied at station A, but in all cases sufficient pesticide was present to account for toxicity. The 96-h, 50% lethal concentration (LC50) for diazinon toxicity to C. dubia is 320 ng/L 15, and the 7d LC50 is 110 ng/L 16. Average diazinon concentrations at station B were also variable, but higher than at station A. In most trials, this was apparently because water from station B combined tailwater from that day's irrigation event with residual water from the previous day's irrigation. Average diazinon concentrations were reduced in the vegetated section of the ditch (from B to C), in three of the five trials (Table 1). Reductions in diazinon in this section of the ditch ranged from 10.2% to 55% in the first three trials (average reduction = 32.7% in trials 1–3).
| Station | |||||
|---|---|---|---|---|---|
| A Input | B Weir | C Flume | D Box | Laboratory Control | |
| Trial 1—4/16/08 | |||||
| CD survival | 0 | 0 | 0 | 80 | 100 |
| CD reproduction | 0 | 0 | 0 | 17 | 21.1 |
| [Diazinon] | 237 | 1,008 | 906 | <RL | |
| Trial 2—6/20/08 | |||||
| CD survival | 20 | 0 | 10 | 80 | 100 |
| CD reproduction | 7 | 0 | 6 | 23 | 19.9 |
| [Diazinon] | 116 | 228 | 125 | <RL | |
| Trial 3—6/27/08 | |||||
| CD survival | 0 | 0 | 0 | 89 | 100 |
| CD reproduction | 8 | 0 | 3 | 25 | 24.3 |
| [Diazinon] | 230 | 577 | 387 | <RL | |
| Trial 4—7/10/08 | |||||
| CD survival | 0 | 0 | 0 | 100 | 100 |
| CD reproduction | 0 | 0 | 0 | 19 | 23.5 |
| [Diazinon] | 160 | 385 | 2320 | <RL | |
| [Chlorpyrifos] | 16.6 | 25.1 | 150.0 | 0.34 | |
| Trial 5—8/12/08 | |||||
| CD survival | 67 | 0 | 0 | 90 | 100 |
| CD reproduction | 6 | 0 | 1 | 17 | 22.4 |
| [Diazinon] | 160 | 263 | 299 | <RL | |
- a Organophosphate A. CD = Ceriodaphnia dubia; RL = reporting limit; [Diazinon] = ELISA diazinon concentration in ng/L; [Chlorpyrifos] = ELISA.
In trial 4, a very large increase was seen in the concentration of diazinon measured in the composite water at station C relative to station B. The concentration went from 385 ng/L to 2,320 ng/L, a 600% increase (Table 1). An evaluation of the hourly diazinon concentrations sampled during this trial shows that although low diazinon concentration was seen in the tailwater input at station A, a very high concentration in water ponded in front of the weir was present at the initiation of trial 4. At the start of this trial at 7:00 AM, the background concentration of diazinon in front of the weir was approximately 14,000 ng/L (Supplemental Data Figure S4). A high concentration of diazinon also was seen in water ponded in front of the flume (at 7:00 AM, station C = ∼8,000 ng/L diazinon; Supplemental Data Figure S4). As new tailwater entered the system and passed through station B and then station C, it apparently diluted the residual (ponded) water, so that subsequent diazinon measures after 9:00 AM show considerably lower concentrations. Even with dilution, diazinon measured at station C remained well above toxic concentrations during trial 4. Treatment with Landguard removed all diazinon downstream of station C, and no diazinon or toxicity was detected at station D during this or the other trials.
In addition to diazinon, ELISA and subsequent gas chromatography-mass spectrometry analysis showed that a toxic concentration of chlorpyrifos was present in the composite sample from station C in trial 4 (Table 1 Trial 4; Table 2). The concentrations of chlorpyrifos were 16.6, 25.1, 150.0, and 0.34 ng/L at stations A, B, C, and D, respectively (chlorpyrifos 96-h LC50 = 53 ng/L 15; 7-d LC50 = 20 ng/L 17. Enzyme-linked immunosorbent assay measures indicate the chlorpyrifos was below the detection limit in the tailwater entering the system during trial 4 but was present in residual water ponded from a previous day's irrigation. Although the chlorpyrifos at station C exceeded the C. dubia toxicity threshold, it was effectively treated by the Landguard as it passed through the flume, because only a trace concentration of chlorpyrifos was detected at station D, and no toxicity was observed in this sample.
| Trial 4 | |||||
|---|---|---|---|---|---|
| Analyte | A (Input) | B (Weir) | C (Flume) | D (Box) | % Change A to C |
| Total chlordane | 124 | 39.7 | 5.1 | 3.2 | −95.9 |
| Total DDT | 1,803 | 390 | 27.8 | 13.7 | −98.5 |
| Total OC | 2,139 | 508 | 118 | 106 | −94.5 |
| Chlorpyrifos | 22.7 | 26.9 | 150 | 0.335 | 561 |
| Diazinon | 204 | 331 | 2620 | ND | 1183 |
| Total OP | 271 | 381 | 2802 | 17 | 935 |
| Total carbamate | 3,959 | 1,447 | 2,888 | 1,582 | −27.1 |
| Piperonyl butoxide | 0.253 | ND | ND | ND | −100 |
| Trial 5 | |||||
|---|---|---|---|---|---|
| Analyte | A (Input) | B (Weir) | C (Flume) | D (Box) | % Change A to C |
| Total chlordane | 46.4 | 11.4 | 3.81 | 4.11 | −91.8 |
| Total DDT | 617 | 159 | 37.0 | 48.3 | −94.0 |
| Total OC | 861 | 301 | 109 | 113 | −87.4 |
| Chlorpyrifos | 14.2 | 13.7 | 48.3 | 0.244 | 240 |
| Diazinon | 95.6 | 182 | 200 | ND | 109 |
| Total OP | 2,159 | 10,730 | 346 | 3,348 | −84.0 |
| Cypermethrin | 11.6 | ND | DNQ | ND | −100 |
| Fenvalerate | 1.29 | ND | DNQ | ND | −100 |
| L-Cyhalothrin | 64.7 | 4.27 | ND | ND | −100 |
| Permethrin | 1.18 | ND | ND | ND | −100 |
- GC/MS = gas chromatography–mass spectroscopy; VTS = Vegetated Treatment System; ND = not detected; NA = not analyzed; DNQ = detected not quantified.
- a Laboratory blanks were all below detection limits (with the exception of HCB) and the percent recoveries in the spiked matrix ranged from 48.7 to 167% in trial 4, and from 41.5 to 184% in trial 5.
In trial 5, diazinon increased 13% between stations B and C. Toxic concentrations of diazinon were measured at station C during the entire trial (Table 1). The hourly samples showed no detection of diazinon at station D throughout this trial. The initial samples collected at station D were from ponded water left over from previous irrigation events. The hourly sample data indicate that as the irrigation tailwater from that day's irrigation began to pass through station C at 4:00 PM, this contained approximately 250 to 300 ng/L diazinon (Supplemental Data Figure S5). This was treated by the Landguard, and no diazinon was detected as the water reached station D.
Survival of C. dubia in water collected at the four stations reflected diazinon concentrations in this system. Although some variability was seen in survival, all of the station A samples were toxic, and all of the station B and C samples were highly toxic (Table 1). Average C. dubia survival was 88% at station D, downstream from the Landguard treatment. The average number of neonates produced in samples from this station was 20.2 (average control neonates = 22.2), confirming that these samples were not toxic.
Removal of pesticides other than organophosphates
Although the sedimentation and vegetated reaches of the VTS ditch were less effective at reducing the more soluble organophosphate pesticides, they were very effective at reducing concentrations of organochlorine and pyrethroid pesticides. Analysis of VTS composite water samples using gas chromatography-mass spectrometry during trials 4 and 5 showed large reductions of total dichlorodiphenyltrichloroethane (DDT), total chlordanes, and total organochlorine pesticides between stations A and C (Table 2). In trials 4 and 5, water concentrations of total chlordane and total DDT declined by greater than 90% between stations A and C. In addition, concentrations of pyrethroid pesticides in water were significantly reduced between stations A and C during trial 5 (Table 2; pyrethroids were not analyzed in water during trial 4). Most of the reduction of pyrethroids in water, particularly lambda-cyhalothrin, occurred in the sedimentation section of the ditch between stations A and B (Table 2).
Turbidity
Turbidity was reduced as tailwater passed through the VTS in trials 4 and 5, the two trials in which turbidity was measured. As water passed from station A to station B, the sedimentation reach, the turbidity was reduced by 9% and more than 59% in trials 4 and 5, respectively (Fig. 2). As water passed from stations B to C in the vegetated reach, average turbidity was reduced an additional 94% and 54% in trials 4 and 5, respectively. These results show that even with minimal vegetation present, the 33-m section between the input (A) and the weir (B) facilitated particle settlement from tailwater. By the time the water exited the ditch at station D, turbidity was reduced from 848 to 21 nephelometric turbidity units in trial 4 (=98% reduction), and from greater than 1,000 to 108 nephelometric turbidity units in trial 5 (<91% reduction; Fig. 2).
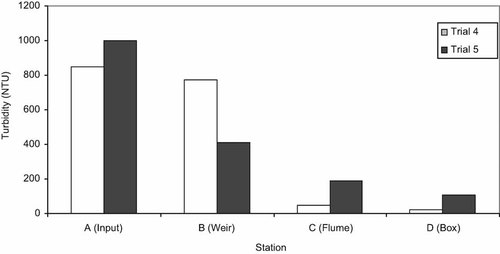
Reduction of turbidity in tailwater at four stations in the Vegetated Treatment System ditch (NTU = nephelometric turbidity unit).
Sediment chemistry
Whole sediment pesticide concentrations were measured in sediments that settled into glass Pyrex dishes placed just above stations B and C (Table 3). The percent total organic carbon was higher at station B (6.82% total organic carbon) than at station C (2.29% total organic carbon; Table 3). The relative proportions of finer-grained sediments were comparable at stations C (93.1% fines) and B (98.7% fines). The organochlorine pesticides chlordane and DDT were detected in sediments from both stations, and slightly higher concentrations of these pesticides were present in the station C sediment, relative to station B (Table 3). Four pyrethroid pesticides were also detected in these sediments, and their concentrations were also slightly higher in the station C sediments (Table 3).
| Station B | Station C | % Change B to C | |||
|---|---|---|---|---|---|
| Silt and clay (%) | 93.1 | 98.74 | NA | ||
| Total organic carbon (%) | 6.82 | 2.29 | NA | ||
| ng/g | µg/g oc | ng/g | µg/g oc | ||
| Total chlordane | 25.4 | 0.372 | 26.3 | 1.148 | 3.8 |
| Total DDT | 230 | 3.37 | 281 | 12.271 | 21.9 |
| Total OC | 268 | 3.93 | 370 | 16.157 | 37.9 |
| Chlorpyrifos | 6.9 | 0.101 | 160 | 6.987 | 2,218 |
| Diazinon | 2.39 | 0.002 | 6.53 | 0.285 | 173 |
| Total OP | 12.9 | 0.013 | 174 | 7.598 | 1,254 |
| Cypermethrin | 3.96 | 0.004 | 7.97 | 0.348 | 101 |
| Delta/tralomethrin | ND | ND | 1.14 | 0.050 | 100 |
| Fenvalerate | 0.539 | 0.001 | 3.63 | 0.159 | 574 |
| L-Cyhalothrin | 2.28 | 0.002 | 3.36 | 0.147 | 47.4 |
| Permethrin | 0.322 | 0.0003 | 7.53 | 0.329 | 2,239 |
| Resmethrin | ND | ND | 2.83 | 0.124 | 100 |
- VTS = Vegetated Treatment System.
- a Laboratory blanks were all below detection limits and the percent recoveries in the spiked matrix ranged from 71.4 to 146% with the exception of resmethrin (21.4%). ND = not detected. OP = organophosphate.
DISCUSSION
Because of its relatively high solubility, research has shown that high proportions of diazinon transported in aquatic systems can be expected to remain in the aqueous phase 18. Previous evaluations of the effectiveness of vegetation to remove diazinon from water have shown mixed results. Watanabe and Grismer 19 evaluated diazinon removal by vegetated filter strips under controlled laboratory conditions and found that most diazinon removal occurred through infiltration into the root zone and sorption to vegetated matter. Although losses of diazinon up to 73% of total mass applied were detected in these experiments, significant loss also occurred through runoff of diazinon. Moore et al. 8 also used a simulated runoff event to evaluate removal of diazinon and permethrin in vegetated ditches in Yolo County, California, USA. Although these authors also described reductions in diazinon runoff using a V-shaped vegetated ditch, significant concentrations of diazinon remained in the system outflow after 5 h. Moore et al. 8 found that vegetation was much more effective at removing the pyrethroid pesticide permethrin, because of its increased hydrophobicity. Hunt et al. 9 evaluated the effectiveness of vegetation (pennywort) at removing diazinon and pyrethroids in a two-pond tailwater system in the Salinas Valley. This study differed from the controlled VTS studies described previously because it evaluated removal of pesticides during actual irrigation events on a large-scale commercial farm. The results demonstrated that the vegetated ponds were effective at significantly reducing pyrethroid concentrations in sediment between the input of the upper pond and output of the lower pond but were less effective at removing diazinon in pond outflow water. This study demonstrated that the primary mechanism of diazinon reductions in these ponds occurred through dilution 9.
A number of studies have described partitioning of organophosphate and pyrethroid pesticides in vegetated ditches and constructed wetlands. In general, these studies show that reduction of pyrethroid, organochlorine, and organophosphate pesticides in water is primarily attributable to their sorption to plant surfaces and secondarily to sediments 7. Studies involving diazinon have also demonstrated removal of this pesticide from water via dilution and its sorption to plant and sediments 8, 9, 20, but all of these studies demonstrated that residual diazinon concentrations in water exiting these vegetated systems remained at levels toxic to C. dubia. In the current study, diazinon concentrations as high as 14,000 ng/L were observed in the ditch, and tailwater flow rates were as high as 16 L/sec. Combined with restrictions on acreage available for treatment systems, these factors limit the viability of traditional treatment systems to reduce this pesticide to nontoxic concentrations. The management goals for diazinon and chlorpyrifos in tailwater runoff currently being considered by the Central Coast Regional Water Quality Control Board require that their concentrations not exceed 150 ng/L and 25 ng/L, respectively. In addition, no toxicity to C. dubia will be allowed (A. Jones, Central Coast Regional Water Quality Control Board, personal communication). Although stakeholders are working with growers to reduce the volumes of irrigation water runoff, the combination of management practices likely will be insufficient to eliminate diazinon toxicity without the use of additional measures, such as treatment with Landguard.
Reduction of particle runoff is an important characteristic of VTS because pyrethroid and organophosphate pesticides associated with sediments have been demonstrated to cause toxicity in downstream receiving waters in Central California coastal watersheds 2, 3, 21. In addition, elevated tissue concentrations of DDT and other organochlorine pesticides in sediment infaunal species are one source of ecosystem degradation in harbors and estuaries in California. Several water bodies along California's central coast are listed as impaired for this reason (http://www.swrcb.ca.gov/rwqcb3/water_issues/programs/tmdl/303d_list.shtml). Sediment concentrations of DDT were below the LC50 for the amphipod Hyalella azteca in the current study (LC50 = 11,000 ng/g; 22). Chlorpyrifos and diazinon were detected in sediment from both stations, and higher concentrations of both of these organophosphates were present at station C. The chlorpyrifos concentration was 160 ng/g (Table 3), which is considerably higher than the diazinon concentration. When normalized to the sediment total organic carbon, the chlorpyrifos concentration was 6.99 µg/g organic carbon (OC), which exceeds the OC-normalized chlorpyrifos LC50 of 2.96 µg/g OC 23. The OC-normalized LC50 for cypermethrin toxicity to Hyalella azteca is 0.40 µg/g OC 24, and the OC-normalized cypermethrin concentration in the station C sediment was 0.348 µg/g OC. The OC-normalized lambda-cyhalothrin concentration in the station C sediment was 0.147 µg/g OC (LC50 = 0.45 µg/g OC;25). Of the pyrethroid pesticides detected, cypermethrin and lambda-cyhalothrin concentrations were within the range toxic to amphipods, especially if additivity is assumed 26.
Our analyses of the water and sediment pesticide concentrations suggest that the VTS was effective at removing most of the organochlorine and pyrethroid pesticides from water. When the sediment concentrations are considered in the context of turbidity measurements at the four ditch stations, the results show that although most of the particles were removed in the VTS, those particles that accumulated as sediments in the Pyrex dishes at station C contained sufficient concentrations of pyrethroids and chlorpyrifos to be toxic to amphipods. We did not measure these pesticides in sediments downstream of the Landguard treatment area; however, based on the water chemistry and toxicity data, would expect little to no chlorpyrifos in the station D sediment. Landguard is effective at hydrolyzing particle-associated op-pesticides (Craig Clark, Orica Watercare, Australia, personal communication). Because pyrethroid pesticides and chlorpyrifos have been shown to be responsible for sediment toxicity in central California coastal watersheds 4, 27, one goal of future research will be to further reduce runoff of suspended particles.
Our research demonstrates that the combination of sedimentation, vegetation, and Landguard OP-A treatment sections in this ditch system were effective at reducing water column pesticide concentrations and toxicity associated with agricultural tailwater runoff. In addition, the ditch system was effective at significantly reducing turbidity of tailwater. These results corroborate results of previous research on vegetated treatment systems showing that these systems reduce runoff of hydrophobic pesticides such as organochlorine and pyrethroid pesticides. Residual concentrations of the more soluble organophosphate pesticides were effectively removed by the Landguard enzyme. In addition to evaluating methods to maximize removal of suspended particles and pesticides by increasing the hydraulic retention time in ditch treatment systems, future experiments are planned to determine the minimum concentrations of Landguard necessary to effectively treat the range of organophosphate pesticide concentrations observed in Salinas Valley tailwater runoff.
Acknowledgements
An early draft of this manuscript was greatly improved through review comments provided by Robert Kroger, Mississippi State University. Funding for this project was provided by the Office of the Monterey County, California, Agriculture Commissioner. Implementation of the Landguard OP-A treatment system could not have been accomplished without the assistance of Rachelle Antinetti, Craig Clarke (Orica Watercare), and Terri Pritchard (University of California at Davis, Cooperative Extension), and we are very grateful for their cooperation and expertise.
SUPPLEMENTAL DATA
Table S1. Summary of vegetated treatment system (VTS) ditch hydrology.
Table S2. Summary of vegetated treatment system (VTS) ditch water sampling times for 5 trials (51KB DOC).
Figure S1. Hourly diazinon concentrations at the four sampling stations during Trial 1.
Figure S2. Hourly diazinon concentrations at the four sampling stations during Trial 2.
Figure S3. Hourly diazinon concentrations at the four sampling stations during Trial 3.
Figure S4. Hourly diazinon concentrations at the four sampling stations during Trial 4.
Figure S5. Hourly diazinon concentrations at the four sampling stations during Trial 5 (26KB PDF).