Occurrence of Toxigenic Microalgal Species and Phycotoxin Accumulation in Mesozooplankton in Northern Patagonian Gulfs, Argentina
Abstract
In the Northern Patagonian gulfs of Argentina (Golfo Nuevo and Golfo San José), blooms of toxigenic microalgae and the detection of their associated phycotoxins are recurrent phenomena. The present study evaluated the transfer of phycotoxins from toxigenic microalgae to mesozooplankton in Golfo Nuevo and Golfo San José throughout an annual cycle (December 2014–2015 and January 2015–2016, respectively). In addition, solid-phase adsorption toxin tracking (SPATT) samplers were deployed for the first time in these gulfs, to estimate the occurrence of phycotoxins in the seawater between the phytoplankton samplings. Domoic acid was present throughout the annual cycle in SPATT samplers, whereas no paralytic shellfish poisoning toxins were detected. Ten toxigenic species were identified: Alexandrium catenella, Dinophysis acuminata, Dinophysis acuta, Dinophysis tripos, Dinophysis caudata, Prorocentrum lima, Pseudo-nitzschia australis, Pseudo-nitzschia calliantha, Pseudo-nitzschia fraudulenta, and Pseudo-nitzschia pungens. Lipophilic and hydrophilic toxins were detected in phytoplankton and mesozooplankton from both gulfs. Pseudo-nitzschia spp. were the toxigenic species most frequent in these gulfs. Consequently, domoic acid was the phycotoxin most abundantly detected and transferred to upper trophic levels. Spirolides were detected in phytoplankton and mesozooplankton for the first time in the study area. Likewise, dinophysistoxins were found in mesozooplankton from both gulfs, and this is the first report of the presence of these phycotoxins in zooplankton from the Argentine Sea. The dominance of calanoid copepods indicates that they were the primary vector of phycotoxins in the pelagic trophic web. Environ Toxicol Chem 2019;38:2209–2223. © 2019 SETAC.
INTRODUCTION
Certain marine microalgae produce potent toxins that negatively affect human and ecosystem health, as well as economic activities that depend on marine resources when the toxins are transported and accumulate throughout marine food webs. In humans, phycotoxins can produce different types of gastrointestinal and neurological symptoms and even death due to the ingestion of contaminated seafood (Shumway 1990; Esteves et al. 1992; Lincoln et al. 2001). Impacts on marine organisms are generally observed as acute intoxications that include mass mortality of cultivated and wild organisms (such as fish, birds, and marine mammals; Geraci et al. 1989; Anderson and White 1992; Gulland 1999; Núñez-Vásquez et al. 2004; Gayoso and Fulco 2006; Montoya and Carreto 2007; De La Riva et al. 2009; Fire et al. 2010). Although toxic microalgae are consumed by various marine organisms (Turner 2010), it has been reported that the main entry point for phycotoxins into the pelagic food web is copepods (Turner 2014). Passive sampling techniques for monitoring the occurrence of phycotoxins and shellfish contamination events have recently been developed worldwide (MacKenzie et al. 2004; Lane et al. 2010; MacKenzie 2010). The techniques include the passive accumulation of phycotoxins from the water column onto porous synthetic resin–filled sachets (solid-phase adsorption toxin tracking [SPATT]) and their subsequent extraction and analysis (MacKenzie et al. 2004). The SPATT methodology has several advantages, such as that the adsorbed toxins are not subject to biotransformation and depuration (unlike in shellfish), nor to time- and space-integrated sampling of toxins that simulates shellfish adsorption. Although the use of SPATT samplers to provide an early warning of lipophilic toxins has been questioned (Pizarro et al. 2013), several studies have demonstrated that SPATT samplers coupled to sensitive analytical technologies such as liquid chromatography–tandem mass spectrometry (LC–MS/MS) form a highly sensitive tool allowing for early information on the presence of this group of phycotoxins (Turrell et al. 2007; Reguera et al. 2012; McCarthy et al. 2014).
In the Argentine Sea, harmful algal blooms occur frequently and have repeated with varying intensities over the years (Gayoso and Fulco 2006; Almandoz et al. 2007; Montoya et al. 2010; D'Agostino et al. 2015, 2018; Carreto et al. 2016). Microalgal toxins associated with 5 different shellfish poisoning syndromes have been reported in the Argentine Sea (reviewed by Krock et al. 2018). The North Patagonian Gulfs of Argentina (Golfo Nuevo, Golfo San José, and Golfo San Matías (Figure 1) are some of the most productive areas of the Patagonian Shelf ecosystem (Carreto et al. 1989; Acha et al. 2004). These gulfs are characterized by an important biodiversity, with the presence of marine mammals (Harris and García 1990; Crespo and Pedraza 1991; Bastida and Rodríguez 2009), marine birds (Yorio et al. 1998), fish (Elías 1998), natural shellfish banks (Orensanz et al. 2007; Amoroso et al. 2011), and seaweed beds (Boraso and Kreibohm 1984). In these gulfs, blooms of toxigenic microalgae as well as the detection of their respective toxins are recurring phenomena. Thus a Harmful Algal Bloom and Shellfish Toxicity Monitoring Program has been carried out in coastal waters of the Northern Patagonian gulfs since 2000. Paralytic shellfish poisoning (PSP), diarrhetic shellfish poisoning (DSP), and amnesic shellfish poisoning (ASP) toxins are frequently monitored, and the shellfish fisheries in both Golfo Nuevo and Golfo San José have been subject to periodic closures annually, because levels of paralytic shellfish toxins produced by the dinoflagellate Alexandrium catenella (formerly termed A. tamarense) were above the regulatory limit of 80 µg saxitoxin (STX) eq 100 g–1 of mussel tissue (Harmful Algal Bloom and Shellfish Toxicity Monitoring Program; Wilson et al. 2015). On the other hand, in only a few years (2009, 2011, 2017, 2018, and 2019) was the harvest of mollusks closed either in Golfo Nuevo and/or in Golfo San José, because DSP toxin (produced by dinoflagellates of the genera Dinophysis and Prorocentrum) levels in shellfish field samples exceeded the regulatory limits (Harmful Algal Bloom and Shellfish Toxicity Monitoring Program; Gracia Villalobos et al. 2015). In contrast, harvests have not been closed due to domoic acid, a neurotoxin produced by species of the diatom genus Pseudo-nitzschia (the regulatory limit for domoic acid being 20 μg g–1 of mussel tissue). Although no cases of ASP events have been documented to date either in wildlife or in humans in the Northern Patagonian gulfs, previous research carried out in Golfo Nuevo and Golfo San José showed the presence of domoic acid in samples of phytoplankton and mesozooplankton (mostly calanoid copepods), as well as in fecal samples of whales (Eubalaena australis) collected at similar sites and on similar dates to those of the present study (D'Agostino et al. 2017). These findings indicate that southern right whales are exposed to domoic acid through the ingestion of contaminated zooplankton, mainly copepods, while they remain in their calving grounds in these gulfs. This could, in turn, become a risk for other species due to the transfer of phycotoxins through the food web. In contrast, to date, spirolides (spiroimine shellfish poisoning [SSP]) and azaspiracids (azaspiracid shellfish poisoning [AZP]) have not been observed in these gulfs. In view of these data, the aim of the present study was to investigate the transfer of phycotoxins from toxigenic microalgae to mesozooplankton in Golfo Nuevo and Golfo San José throughout an annual cycle. In the present study, levels of phycotoxins dissolved in the water column of Northern Patagonian gulfs were quantified and analyzed for the first time. The present study contributes baseline knowledge on the occurrence of phycotoxins and their accumulation in higher trophic levels in Golfo Nuevo and Golfo San José, and provides new information about the seasonal dynamics of phycotoxins in this area throughout an annual cycle.
Site locations of sampling in Golfo Nuevo and Golfo San José (S1, S2, and S3).  Solid-phase adsorption toxin tracking (SPATT) sampler location.
Solid-phase adsorption toxin tracking (SPATT) sampler location.
MATERIALS AND METHODS
Sample collection
Plankton and seawater samples were collected on an approximately monthly basis at 3 sites (S) in Golfo Nuevo (S1 = 42.61°S, 64.29°W; S2 = 42.63°S, 64.27°W; S3 = 42.56°S, 64.34°W) and Golfo San José (S1 = 42.40°S, 64.12°W; S2 = 42.37°S, 64.08°W; S3 = 42.33°S, 64.08°W; Figure 1). Sampling was carried out in Golfo Nuevo from December 2014 to December 2015 (nphyto S1 = 11, S2 = 10, S3 = 9; nmesozoo S1 = 11, S2 = 10, S3 = 9), and in Golfo San José from January 2015 to January 2016 (nphyto S1 = 10, S2 = 10, S3 = 10; nmesozoo S1 = 10, S2 = 10, S3 = 10). The PSP toxin analyses of plankton (phyto- and mesozooplankton) were performed from samples collected in Golfo Nuevo from July 2015 to December 2015 (nphyto S1 = 5, S2 = 4, S3 = 3; nmesozoo S1 = 5, S2 = 4, S3 = 3) and in Golfo San José from August 2015 to January 2016 (nphyto S1 = 5, S2 = 5, S3 = 5; nmesozoo S1 = 5, S2 = 5, S3 = 5). At each sampling site, surface seawater temperature was measured in situ with a portable thermometer (for details, see D'Agostino et al. 2018). Seawater was subsequently sampled from a boat at 3- and 10-m depths using a 2.5-L Van Dorn bottle. One liter from each depth was mixed, and 500 mL were taken for the analysis of chlorophyll-a and phaeopigments; then a 250-mL aliquot was fixed with Lugol's solution at a final concentration of 0.4 mL 100 mL–1 (Ferrario et al. 1995) for quantitative analysis of toxigenic species. Phytoplankton samples for both relative abundance and phycotoxin analysis were collected using oblique net tows from a 20-m depth to the surface using a 20-µm mesh net while the boat was traveling over a 7-min period at a speed of 2 knots. Samples were collected in 500-mL plastic bottles. Net tow samples for relative abundance analysis were fixed with 4% formaldehyde, whereas samples for phycotoxin analysis were placed in portable coolers and immediately processed after return to the laboratory (see Phycotoxin analysis section following). Mesozooplankton samples for quantitative taxonomic and phycotoxin analysis were collected using a 335-µm mesh net equipped with a flow meter (General Oceanics model 2030 R) on the mouth of the net. Net tows were performed obliquely from a depth of 30 m to the surface for a 7-min period at a boat speed of 2 knots, and the samples were put into 250-mL plastic flasks. Mesozooplankton samples for taxonomic analysis were fixed with 4% formaldehyde, whereas samples for phycotoxin extractions were placed in portable coolers and immediately processed after return to the laboratory (see Phycotoxin analysis section following). The mesozooplankton samples collected in October in Golfo Nuevo could not be used in the analyses of phycotoxins, because of an intense Pseudo-nitzchia australis bloom, and long chains of this species were found in the respective mesozooplankton samples, which made it impossible to attribute any phycotoxins to mesozooplankton.
Phycopigment analysis
For chlorophyll-a determination, the seawater samples were filtered through GF/F filters (25-mm and 0.7-μm pore size) and then stored frozen at –20 °C. The chlorophyll-a was extracted over 24 h at 4 °C with 5 mL 90% acetone in the dark. Extracts were centrifuged at 1680 g for 5 min. Then chlorophyll-a and phaeopigments were quantified using a spectrofluorophotometer (Shimatzu model RF-5301PC) at λEx/λEm of 430/671 nm calibrated with a standard of Anacystis nidulans, and concentrations were estimated according to Holm-Hansen et al. (1965) equations. Chlorophyll-a is a photosynthetic pigment common to all autotrophic phytoplankton organisms. Concentration data from high-performance liquid chromatography (HPLC) analysis were then used to estimate phytoplankton biomass (Almandoz et al. 2011; Gonçalves-Araujo et al. 2016). Values of chlorophyll-a were corrected for phaeopigments by acidification with HCl (0.1 N). The phaeopigments (mainly phaeofitin) value was used as a principal indicator of chlorophyll-a degradation as a result of herbivorous zooplankton grazing (Lorenzen 1967; Helling and Baars 1985; Head and Harris 1992).
Species identification and abundance of planktonic organisms
Microalgae species in bottle samples were counted with an inverted microscope (Leica DMIL) at ×200 magnification following Utermöhl (1958). For identification of toxigenic diatoms, net samples were cleaned (Hasle and Fryxell, 1970). Naphrax-mounted slides (Ferrario et al. 1995) were observed with an optical microscope equipped with phase contrast at ×400 and ×1000 magnification. Scanning electron microscopy observations of the samples were made with a Jeol model JSM-6360 LV microscope at the Facultad de Ciencias Naturales y Museo, Universidad Nacional de La Plata (La Plata, Argentina), and with a Zeiss Supra 40 microscope at the Advanced Microscopy Center of the Universidad de Buenos Aires (Buenos Aires, Argentina) to identify Pseudo-nitzschia species. Taxonomic phytoplankton identifications were based on the specific literature (Balech 1995, 2002; Boltovskoy 1995; Tomas 1997; Ferrario et al. 1999, 2002; Lundholm et al. 2003; Fryxell and Hasle 2004; Sar et al. 2006; Almandoz et al. 2007; Reguera et al. 2012; Guiry and Guiry 2017). Phytoplankton abundances were expressed as cells per liter (cells L–1). For qualitative estimation, net samples were standardized into an abundance scale. Abundance estimates were obtained by counting the number of cells for toxic species in 3 0.1-mL aliquots. The abundance classification was performed using a relative abundance scale between 0 and 6 (0 = absent and 6 = bloom [>1 000 000]; for details, see Gracia Villalobos et al. 2015) according to the abundance of a species in natural populations. For data presentation, the abundance values of toxigenic species identified in bottle samples were standardized to the same relative abundance scale. When the same species was identified in both the bottle and net samples at the same site, the highest abundance was used in subsequent analyses.
A Pearson correlation was used to investigate the relationships between phytoplankton biomass and phytoplankton abundances. In all tests, the threshold for significance was set at 0.05. Statistical analyses were conducted in InfoStat (free version) software packages.
Mesozooplankton samples for species identification and enumeration were examined under a stereomicroscope (Nikon model SMZ645) at ×30, ×40, and ×50 magnification. Potential consumers of toxic microalgae were identified to the lowest possible taxonomic level using the appropriate literature (Boltovskoy 1981, 1999; Kirkwood 1982; Cervellini 1988; Harris et al. 2000; Young 2002). According to the abundance of organisms observed a priori in the samples, total or aliquot counts were applied. In the latter case, samples were subsampled (1/10; Boltovskoy 1981), and all individuals were then identified and counted. Mesozooplankton abundances were expressed as number of individuals per cubic meter (ind m–3).
SPATT samplers
The SPATT samplers were deployed to complement sporadic phytoplankton and toxin sampling with integrated toxin sampling over an extended period. The purpose of the deployment of SPATT samplers was also to evaluate the presence/absence of phycotoxins in the study area and to confirm that no phycotoxin classes were missed by discrete phytoplankton sampling. The SPATT samplers were prepared from 95-µm polyester mesh that contained approximately 10 g (dry wt) of Diaion HP-20 (Sigma) resin. For activation, SPATT samplers were conditioned in 100% MeOH for approximately 24 h. Then they were rinsed several times with distilled water and kept wet and refrigerated between 4 and 6 °C until they were placed on the study site in Golfo Nuevo and Golfo San José (Figure 1). In each gulf, 2 SPATT samplers were deployed at a fixed sampling site (Golfo Nuevo = 42.61°S, 64.27°W; Golfo San José = 42.41°S, 64.11°W; Figure 1), shallow (≈15 m) and anchored approximately 1 m from the seabed. One of the SPATT samplers was used for analysis of hydrophilic PSP toxins, and the other was used for analysis of domoic acid and lipophilic toxins. The SPATT samplers were replaced monthly (at the same time that plankton and seawater samples were collected) and kept at 4 °C until extraction.
Phycotoxin analysis
Phytoplankton samples for phycotoxin extraction were filtered through GF/F filters (25-mm and 0.7-μm pore size) and frozen (–20 °C) until analysis. Filters were transferred into FastPrep tubes containing 0.9 g of lysing matrix D (Thermo Savant), and 0.5 mL of methanol was added to extract multiple lipophilic toxins (such as domoic acid, gymnodimine, spirolides [SPXs], dinophysistoxins [DTXs], okadaic acid, pectenotoxins [PTXs], yessotoxins, and azaspiracids); then 1 mL 0.03 M acetic acid was added to extract hydrophilic PSP toxins. The samples were homogenized by reciprocal shaking at maximum speed (6.5 m s–1) for 45 s in a Bio101 FastPrep instrument (Thermo Savant). After homogenization, samples were centrifuged (Eppendorf model 5415 R centrifuge) at 16 100 g at 4 °C for 15 min. Supernatants were transferred to centrifugation filters (0.45-μm pore size; Millipore Ultrafree), centrifuged at 800 g at 4 °C for 30 s, and then transferred to autosampler vials (Krock et al. 2008).
Mesozooplankton samples for phycotoxin extraction were filtered through GF/F filters (47-mm and 0.7-µm pore size) and frozen (–20 °C) until analysis. Filters for lipophilic toxins extraction were cut in half and transferred into FastPrep tubes containing 0.9 g lysing matrix D (Thermo Savant), and 1 mL methanol was added. Samples were homogenized as just described. Filtrates of the same samples were combined and dried in a gentle nitrogen stream and reconstituted with methanol to a final volume of 0.5 mL. Subsequently, the extracts were filtered through centrifugation filters (0.45-μm pore size; Millipore Ultrafree) at 16 100 g at 4 °C for 5 min. Samples were transferred into an autosampler vial for LC–MS/MS analyses (Tammilehto et al. 2012). For extraction of hydrophilic toxins, filters were cut into small fractions and transferred into centrifuge tubes, and phycotoxins were extracted in acetic acid 0.03 M (4 mL) with the use of ultrasonication (Sonopuls HD 2070; Bandelin; 2 min/7 cycles/96% power). Samples were vortexed (Heidolph Reax Top Vortex Mixer) for 2 min and centrifuged (Eppendorf 5810 R) at 3220 g at 4 °C for 10 min, and the supernatant was transferred into centrifuge tubes and stored at 4 °C until use (Leandro et al. 2010). The residues were re-extracted once as just described. Then extracts were combined and evaporated using a rotary evaporator (Heidolph Rotary Evaporator, Laborota 4002), and the volumes were adjusted to 1 mL with 0.03 M acetic acid. Finally, the concentrates were filtered through centrifugation filters (0.45-μm pore size; Millipore Ultrafree), centrifuged at 3000 g at 4 °C for 30 s, and then transferred to autosampler vials.
Each SPATT sampler for lipophilic toxin extractions was rinsed 3 times with 500 mL of Milli-Q water. The SPATT samplers were subsequently dried on filter paper for 3 h at 50 °C in a drying oven. Then dry SPATT samplers were opened, the resins were transferred to 50-mL centrifuge tubes, and 30 mL of 100% MeOH was added. The mixture was vortexed for 1 min, and the tubes were left overnight to extract the lipophilic toxins adsorbed by the resins. Subsequently, the mixture of resins with MeOH was transferred into chromatographic glass columns (27 cm length, 13 mm ID, packed with a 2-cm layer of quartz wool and 1 cm of quartz sand), and the centrifugation tubes were rinsed with an additional 15 mL methanol. Methanol was eluted dropwise from the column until the liquid reached the top column layer, and subsequently another 25 mL was added to each column for complete extraction of toxins. Finally, fractions were combined and methanol evaporated using a rotary evaporator (Heidolph Rotary Evaporator, Laborota 4002) to a final volume of approximately 0.5 mL. The concentrates were transferred to HPLC vials and adjusted with methanol to 1 mL. The extracts were filtered through centrifugation filters (0.45-mm pore size; Millipore Ultrafree) at 3000 g at 4 °C for 0.5 min. Samples were transferred into an autosampler vial for lipophilic toxin analysis (Fux et al. 2008).
The PSP toxins accumulated in SPATT samplers were extracted following the methodology described in Rodríguez et al. (2011) with little modification. Each SPATT sampler was opened and the resin was transferred to a chromatographic column. The resin was then rinsed with 7 mL of Milli-Q water, and the PSP toxins were eluted twice with a solution of 10% MeOH (3.5 mL) containing 2% of 100% acetic acid. Finally, the rinsed water fraction and the 2 methanolic fractions were combined and evaporated using a rotary evaporator (Heidolph Rotary Evaporator, Laborota 4002) until approximately 0.5 mL was reached, and then the final volume was adjusted to 1 mL with acetic acid (0.03 N). Subsequently, the extracts were filtered through centrifugation filters (0.45-mm pore size; Millipore Ultrafree) at 3000 g at 4 °C for 0.5 min at 4 °C. Samples were transferred into an autosampler vial for PSP toxin analysis.
Analysis of multiple lipophilic toxins was performed by LC–MS/MS as described in Krock et al. (2008). In brief, samples were analyzed in the selected reaction monitoring mode by single injection and quantified against external standard solutions of domoic acid, DTX-1, PTX-2, PTX-11, and SPX-1 purchased from the Certified Reference Material Program of the Institute for Marine Biosciences of the National Research Council of Canada (IMB-NRC; Halifax, NS, Canada). Because no analytical standard of 20-methyl spirolide G is commercially available, 20-methyl spirolide G values were expressed as SPX-1 equivalents assuming a similar molecular response factor. The filtrates of the acetic acid extraction were analyzed for PSP toxins after separation of target analytes in reverse-phase mode by HPLC with postcolumn derivatization and fluorescence detection, according to the method described by Krock et al. (2007). The PSP toxins were analyzed by single injection and quantified against an external 4-point calibration curve of PSP mix solutions containing (N-sulfocarbamoyl compounds C1 and 2 [C1/2], gonyautoxin [GTX]1/4, GTX2/3, decarbamoyl [dc]GTX2/3, N-sulfocarbamoyl compound B1, dcSTX, neosaxitoxin, and STX). All toxins were purchased from the Certified Reference Material Program of the IMB-NRC. Phycotoxin levels in SPATT samplers were expressed as nanograms per SPATT and in plankton samples as nanograms per net tow (ng NT–1). A list of monitored phycotoxins is given in Table 1.
| Chemical classification and polarity | Syndrome | Toxin group | Toxin | Mass transition (m/z) |
|---|---|---|---|---|
| Hydrophilic, amino acid | ASP | DA | Domoic acid | 312 > 266 |
| 312 > 161 | ||||
| Lipophilic, cyclic imine | SSP | GYM | Gymnodimine A | 508 > 490 |
| Gymnodimine B | 526 > 508 | |||
| Gymnodimine C | 526 > 508 | |||
| Gymnodimine D | 524 > 506 | |||
| 12-Methyl gymnodimine A | 522 > 504 | |||
| 16-Desmethyl gymnodimine D | 510 > 492 | |||
| SSP | SPX | Spirolide A | 692 > 150 | |
| Spirolide B | 694 > 150 | |||
| Spirolide C | 706 > 164 | |||
| Spirolide D | 708 > 164 | |||
| 13-Desmethyl spirolide C | 692 > 164 | |||
| 13-Desmethyl spirolide D | 694 > 164 | |||
| Spirolide G | 692 > 164 | |||
| 20-Methyl spirolide G | 706 > 164 | |||
| SSP | PnTx | Pinnatoxin E | 784 > 164 | |
| Pinnatoxin F | 766 > 164 | |||
| Pinnatoxin G | 694 > 164 | |||
| Lipophilic | GD | Goniodomin A | 786 > 607 | |
| DSP | OA | Okadic acid | 822 > 223 | |
| DTX | Dinophysistoxin-1 | 836 > 237 | ||
| Dinophysistoxin-1b | 836 > 237 | |||
| Di-hydrodinophysistoxin-1 | 638 > 237 | |||
| Dinophysistoxin-2 | 822 > 223 | |||
| AZP | AZA | Azaspiracid-1 | 842 > 824 | |
| Azaspiracid-2 | 856 > 838 | |||
| Azaspiracid-3 | 828 > 810 | |||
| PTX | Pectenotoxin-2 | 876 > 213 | ||
| Pectenotoxin-2 seco acid | 894 > 213 | |||
| Pectenotoxin-11 | 892 > 213 | |||
| Pectenotoxin-12 | 874 > 213 | |||
| YTX | Yessotoxin | 1160 > 965 | ||
| 45-Hydroxy yessotoxin | 1176 > 981 | |||
| Homo yessotoxin | 1174 > 979 | |||
| 45-Hydroxy homo yessotoxin | 1190 > 977 |
- ASP = amnesic shellfish poisoning; SSP = spiroimine shellfish poisoning; DSP = diarrhetic shellfish poisoning; AZP = azaspiracid shellfish poisoning.
RESULTS
Phytoplankton biomass and occurrence of toxigenic Pseudo-nitzschia species
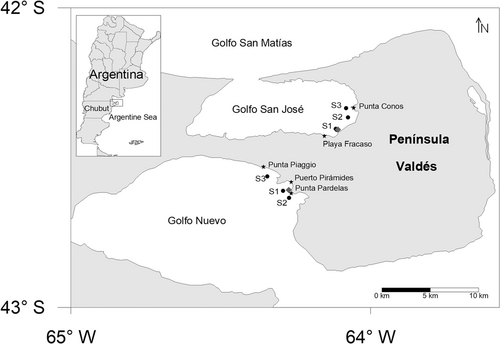
In Golfo Nuevo, phytoplankton biomass (chlorophyll-a) ranged between 0.23 and 4.45 µg L–1 throughout the sampling period, with minimum values found during late spring (0.23 µg L–1; December 2014) and early summer (0.39 µg L–1; January 2015; Figure 2A). A clear correlation between phytoplankton biomass and diatom abundances (Pearson's correlation: r = 0.97, p < 0.05, n = 11) was found. The maximum values were observed during late winter (1.04 µg L–1; September 2015) and spring (4.45 µg L–1; October 2015; Figure 2A). This peak was associated with an intense bloom of P. australis that occurred in October 2015 in this gulf (Figure 2C). Total diatom abundances (cells L–1) peaked in early summer (9.5 × 104 cells L–1, January 2015) and spring (6.72 × 105 cells L–1, October 2015) and attained their minimum in late spring and early winter (December 2014 and July 2015 = 4.4 × 102 cells L–1; Figure 2A). Pseudo-nitzschia spp. were found throughout the annual cycle except for July 2015, from low densities ranging from 1.1 × 102 cells L–1 in bottle samples and 2 in concentrated net samples to bloom densities (6.06 × 105 cells L–1 and 6 in relative scale abundance; Figure 2C). During October 2015 Pseudo-nitzschia spp. were dominant, representing >90% of the total diatoms (Figure 2A). The Pseudo-nitzschia species that were detected with the highest frequency and abundance during the study period in Golfo Nuevo were Pseudo-nitzschia calliantha and P. australis, which reached densities of 1.49 × 105 and 4.56 × 105 cell L–1, respectively, in October 2015 (Figure 2C).
Seasonal variability in Golfo Nuevo and Golfo San José (A and B) total diatoms; total cell densities of Pseudo-nitzchia species; chlorophyll-a (Chla-a) and phaeopigments (Phae); and (C and D) relative cell abundances of Pseudo-nitzchia species, and levels of domoic acid (DA). For data presentation the abundance of Pseudo-nitzchia spp. identified in bottle samples were standardized in the relative abundance scale (see Gracia Villalobos et al. 2015).
In Golfo San José, phytoplankton biomass ranged from 0.74 to 2.69 µg L–1, throughout the annual cycle (Figure 2B). The lowest values were found in late summer (0.84 µg L–1, March 2015) and during the winter (0.74 and 0.85 µg L–1, July 2015 and August 2015, respectively), and they were found to be highest in autumn (2.7 µg L–1, April 2015) and spring (2.56 µg L–1, October 2015; Figure 2B). No significant correlation between phytoplankton biomass and diatom abundances (Pearson's correlation: r = –0.173, p > 0.05, n = 10) was found. The highest diatom abundances were detected in late summer (1.93 × 105 cells L–1, March 2015) and spring (7.33 × 105 cells L–1, November 2015), which did not coincide with the highest biomass values found in this gulf, and were lowest during the winter (1.73 × 103 cells L–1, July 2015; Figure 2B). Pseudo-nitzschia spp. were present throughout the period studied (Figure 2D), ranging from 4.4 × 102 to 1.32 × 104 cells L–1 in bottle samples and from 2 to 4 in concentrated net samples (Figure 2D). The most abundant species were Pseudo-nitzschia pungens and P. calliantha, with maximum cell abundances of 1.32 × 104 cells L–1 observed in March 2015 and 1.23 × 104 cells L–1 in April 2015, respectively (Figure 2D).
In general, values of phaeopigments in Golfo Nuevo and Golfo San José showed a pattern similar to that of chlorophyll-a levels (Figure 2A and B). In Golfo Nuevo, the highest values of phaeopigments (1.22 µg L–1) were observed in October 2015 coincident with the bloom of P. australis and the highest chlorophyll-a levels registered in this gulf (Figure 2A). Likewise, in Golfo San José, the highest mean values of phaeopigments were detected in April and October 2015 (1.99 and 1.29 µg L–1, respectively) coincident with the peaks of chlorophyll-a (Figure 2B).
Toxigenic phytoplankton species and phycotoxin abundance
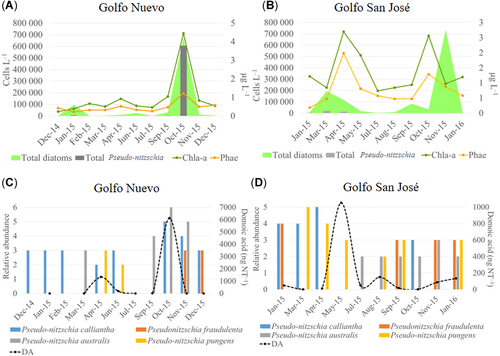
Taxonomic analyses showed the presence of 10 potentially toxic phytoplankton species, 6 of them dinoflagellates (Alexandrium catenella, Dinophysis acuminata, Dinophysis acuta [only in Golfo San José], Dinophysis tripos, Dinophysis caudata [only in Golfo Nuevo], Prorocentrum lima [only in Golfo Nuevo]; Figure 3A and B), and the other 4 diatoms (P. australis, P. calliantha, Pseudo-nitzschia fraudulenta, and P. pungens; Figure 2C and D). In Golfo Nuevo, A. catenella, the recognized source of PSP in the study area, was observed only during the summer (February 2015) and spring (October 2015) in the net samples, with abundances of 1 and 2, respectively, on the abundance scale (Figure 2A) and was not identified in the bottle samples from this gulf. Three of 5 phytoplankton samples were positive for PSP toxins at low levels. In October 2015 a correlation between A. catenella and PSP toxins in phytoplankton samples was found (Figure 3A). The genus Dinophysis, the source of PTX toxins in marine waters (Reguera et al. 2014), was observed to reach bloom densities of 103 cells L–1 (Maneiro et al. 2000). This genus was represented by D. acuminata, D. caudata, and D. tripos, which have all been associated with DSP events in the Argentine Sea (Fabro et al. 2015; Gracia Villalobos et al. 2015; Turner and Goya 2015; Figure 3A). The most abundant Dinophysis species in bottle samples was D. caudata, which reached a bloom density (1.32 × 103 cells L–1) in January 2015 and D. acuminata in net samples in February 2015, with an abundance of 2 on the abundance scale (Figure 3A). The toxin PTX2 was detected in 27.27% samples from this gulf, and only in February 2015 was there a correlation between Dinophysis spp. and PTX2 (Figure 3A). The genus Pseudo-nitzschia was found in 90.9% of the phytoplankton samples (Figure 2C). Domoic acid was the most abundant toxin, being present in 54.54% of samples, with levels between 1.47 and 6140 ng NT–1 (Figure 2C). The highest domoic acid abundances (6140 ng NT–1) coincided with the bloom of P. australis observed in bottle and net samples in October 2015 (Figure 2C). On the other hand, only traces of 20-methyl spirolide G (20-Me-SPX-G), 13-desmethyl spirolide C (SPX1), and pectenotoxin-11 (PTX11) were detected (<limit of detection [LOD]); data not shown).

Relative cell abundances of toxigenic dinoflagellate and phycotoxin levels detected in Golfo Nuevo (A) and Golfo San José (B). For data presentation the abundance of dinoflagellates identified in bottle samples was standardized in the relative abundance scale (see Gracia Villalobos et al. 2015). C = N-sulfocarbamoyl toxins; GTX = gonyautoxins; PTX = pectenotoxins; SPX = spirolides.
Toxigenic dinoflagellates were found most frequently in Golfo San José (Figure 3B). The most frequent species found in both bottle and net samples were A. catenella and D. tripos, which reached maximum cell densities of 4.4 × 102 and 1.76 × 103 cells L–1 in bottle samples and abundance levels of 2 and 3 in concentrated net samples, respectively (Figure 3B). Alexandrium catenella was identified in 60% of phytoplankton samples, from late autumn to spring 2015 with densities in bottle samples of 2.2 × 102 and 4.4 × 102 cells L–1 and of 1 and 3 on the abundance scale (Figure 3B). The PSP toxin pairs C1/2 and GTX2/3 were detected in all analyzed phytoplankton samples, except for the sample collected in January 2016 (Figure 3B). The C1/C2 abundances ranged between 36.8 and 266 ng NT–1 and GTX2/3 between 23.8 and 283 ng NT–1 (Figure 3B). Maximum levels of both toxins were detected in midwinter (August 2015; Figure 3B). In this gulf, the presence of A. catenella coincided with the detection of PSP toxins in the phytoplankton samples analyzed for PSP toxins (Figure 3B). The genus Dinophysis was observed in 50% of samples, with densities in bottle samples ranging between 4.4 × 102 and 1.76 × 103 cells L–1 and with levels between 1 and 3 in concentrated net samples (Figure 3B). The most frequently found Dinophysis species in this gulf was D. tripos (Figure 3B). Two PTXs were detected in this gulf, PTX2 and PTX11 (Figure 3B). The PTX2 was detected in 60% of samples and PTX11 in 20% of them (Figure 3B). A general concurrence between the presence of D. tripos and PTX2 was observed (Figure 3B). In fact, the maximum level of PTX2 (634 ng NT–1) was detected with the presence of a bloom of D. tripos (1.76 × 103 cells L–1) in April 2015 (Figure 3B). The genus Pseudo-nitzschia was observed in 100% of phytoplankton samples, with densities ranging between 4.4 × 102 and 1.32 × 104 cells L–1 and with abundances between 2 and 4 in concentrated net samples (Figure 2D). Phycotoxin analyses showed the presence of domoic acid in all phytoplankton samples, with abundances ranging between 1.3 and 1053 ng domoic acid NT–1 (Figure 2D). The 20-Me-SPX-G was detected in midspring (November 2015) and early summer (January 2016), whereas SPX1 was found during winter (July and August 2015); both spirolides were found in very low abundances (Figure 3B). On the other hand, trace levels of DTX1 were detected (<LOD; data not shown).
Potential zooplankton vectors and phycotoxin content
Analysis of mesozooplankton samples revealed the presence of primarily herbivorous organisms (appendicularians, ascidian larvae, cirripedian larvae, echinoderm larvae, and mollusc larvae), predominantly omnivorous (copepods [copepodites and adults] and euphausiids [larvae, juveniles, and adults]) and predominantly carnivorous (amphipods [only in Golfo Nuevo], cnidarians, chaetognaths, decapod larvae, polychaete larvae, and fish larvae) species as possible vectors of phycotoxins (Figure 4A and B).
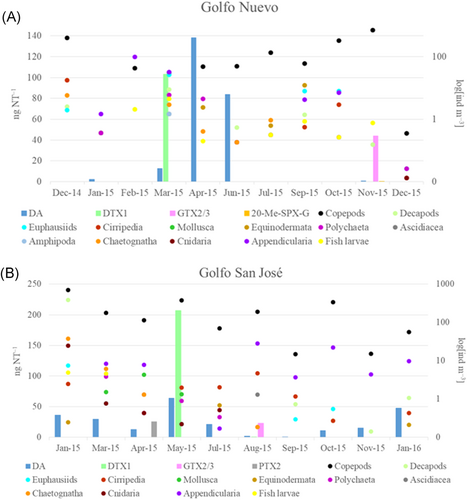
Abundance (ind m–3) of potential vectors of phycotoxin and phycotoxin levels present in mezooplankton samples from Golfo Nuevo (A) and Golfo San José (B). Domoic acid (DA) data from June to December 2015 in Golfo Nuevo and July to November 2015 in Golfo San José were taken from D'Agostino et al. (2017). C = N-sulfocarbamoyl toxins; GTX = gonyautoxins; PTX = pectenotoxins; SPX = spirolides; DTX = dinophysistoxins. Note that abundances (ind m–3) are displayed on a logarithmic scale.
In Golfo Nuevo, calanoid copepods dominated the mesozooplankton community throughout the annual cycle, except during summer 2015 when appendicularians (Oikopleura sp. and Fritillaria sp.) were the dominant group (Figure 4A). In general, the most abundant copepod species were the small copepods Ctenocalanus vanus and Paracalanus parvus and the large copepods Calanus australis and Calanoides carinatus (data not shown). Phycotoxin analysis of mesozooplankton samples showed the presence of low levels of domoic acid, DTX1, 20-Me-SPX-G, and GTX2/3 (Figure 4A). Domoic acid was the most frequent toxin, being present in almost 50% of the mesozooplankton samples (Figure 4A) ranging from 0.71 to 138 ng NT–1. The maximum level of domoic acid was detected in April (138 ng NT–1; Figure 4A) when the copepod C. australis was the most abundant species (35.12 ind m–3). In addition, PTX2 and dcGTX3 were detected at trace levels (<LOD; data not shown).
In Golfo San José, among the zooplankton that could act as potential phycotoxin vectors, calanoid copepods were the most abundant group throughout the annual cycle (Figure 4B). The most abundant species were C. vanus and P. parvus among the small copepods and the large abundant calanoids were C. carinatus and C. australis (data not shown). Analysis of phycotoxins revealed the presence of domoic acid, DTX1, PTX2, and GTX2/3 (Figure 4B) and trace levels of 20-Me-SPX-G and dcGTX3 (<LOD; data not shown). Domoic acid was the most frequent toxin, being present in 100% of the mesozooplankton samples and ranging from 0.69 to 64 ng NT–1 (Figure 4B). This latter value was detected in May 2015 when C. carinatus was the dominant species in the mesozooplankton community (271.97 ind m–3). The toxin DTX1 was only detected in May 2015 and was the most abundant phycotoxin in the mesozooplankton (Figure 4B). The other phycotoxins were only detected sporadically and at low levels on a few sampling dates (Figure 4B).
Adsorption of phycotoxins by SPATT samplers
Domoic acid was present in all SPATT samplers from Golfo Nuevo and in all but one (January 2015) from Golfo San José at low levels (Table 2). The maximum levels both in Golfo Nuevo and in Golfo San José were found during spring (December 2015 and September 2015, respectively; Table 2). In contrast, no PSP toxins or its analogs were detected in SPATT samplers from Golfo Nuevo and Golfo San José during the present study period.
| Golfo Nuevo | Golfo San José | ||
|---|---|---|---|
| Date | DA ng/SPATT | Date | DA ng/SPATT |
| Jan-15 | 3.20 | Jan-15 | ND |
| Feb-15 | 5.45 | Apr-15 | 6.47 |
| Apr-15 | 10.77 | May-15 | 3.61 |
| Jun-15 | 9.90 | Jul-15 | 5.34 |
| Jul-15 | 6.24 | Aug-15 | 12.16 |
| Sep-15 | 5.82 | Sep-15 | 17.89 |
| Oct-15 | 6.59 | Oct-15 | 9.29 |
| Nov-15 | 5.02 | Nov-15 | 17.46 |
| Dec-15 | 71.91 | Jan-16 | 16.33 |
- ND = not detected.
DISCUSSION AND CONCLUSIONS
The present study represents the most complete analysis carried out to date on the dynamics of phycotoxins produced by phytoplankton organisms and their accumulation in higher trophic levels in both Golfo Nuevo and Golfo San José. It is also important to highlight that this is the first study in which SPATT samplers were used to determine the phycotoxins dissolved in the water column in both Golfo Nuevo and Golfo San José in addition to sporadic phytoplankton sampling and toxin determination. The SPATT samplers are complementary to toxin determinations in plankton, because SPATT, despite not being quantitative in a strict analytical sense, provides integral data on toxin occurrence over an extended time period and therefore captures important toxin occurrences between individual plankton samplings. It is noteworthy that only domoic acid was detected on SPATT samplers. This clearly indicated that domoic acid–producing Pseudo-nitzschia species constitute a regular component of the phytoplankton communities in Golfo San José and Golfo Nuevo. In contrast, the absence of other phycotoxins on the exposed SPATT samplers indicates that the toxic species found in plankton samples did not dominate the plankton community over the study period. Nevertheless, this does not imply that these species might not form blooms under favorable conditions. To achieve a higher temporal resolution of toxic species and their associated toxins in the area, future investigations should consider the replacement of these samplers weekly. This would improve our knowledge of the phycotoxins present in Northern Patagonian gulfs, their persistence in the water column, and the frequency of the toxic episodes.
In both Golfo Nuevo and Golfo San José, only the PSP toxins C1/2 and GTX2/3 were found. Contrary to the results of previous studies carried out in these gulfs (Reyero et al. 1998; Andrinolo et al. 1999a), our findings showed higher levels of C1/2 than of GTX2/3 several times in both Golfo Nuevo and Golfo San José. Both previous studies (Reyero et al. 1998; Andrinolo et al. 1999a) found trace levels of C1/2 in field samples from Golfo Nuevo and Golfo San José or these toxins were not detected in phytoplankton samples. Interestingly, these same studies have documented that the profiles of PSP toxins were dominated almost exclusively by GTX1/4. This dominance of GTX1/4 in field samples from Golfo Nuevo and Golfo San José was also found in a study carried out during the winter and spring (Cadaillón 2012). Therefore, those results also differ from the findings of the present study in Golfo Nuevo and Golfo San José for the same seasons.
In addition, previous studies have reported the absence of C1/C2 or lower levels than those of the GTX2/3 in natural populations of A. catenella (formerly termed A. tamarense) from the Argentine Sea (Carreto et al. 2001; Montoya et al. 2010; Krock et al. 2015). According to these authors, the profiles of the PSP toxins obtained from the analysis of field samples of A. catenella were dominated by the GTX2/3 epimers, and even Krock et al. (2015) found only GTX2/3 toxins and trace amounts of STX. Notwithstanding, Montoya et al. (2010) and Krock et al. (2015) reported the presence of elevated levels of C1/2 toxins followed by GTX1/4, in culture strains of A. catenella (termed A. tamarense) that were isolated from the same area where the natural populations of this dinoflagellate were sampled during their studies. These differences in C1/2 levels between natural and cultured strains of A. catenella cells found by these and others authors (Oshima et al. 1992; Anderson et al. 1996) led to the hypothesis that the production of toxin by this dinoflagellate changes according to whether it is studied in natural or laboratory conditions (Andrinolo et al. 1999b; Montoya et al. 2010). However, the results obtained in our study show that this dinoflagellate is able to synthesize C1/2 in natural conditions. Another possible explanation for the absence of C1/2 in field samples could be related to the time that elapses between the collection of the samples at the study sites and the phycotoxin analyses in the laboratory, which would be sufficient for the chemical conversion of the C toxins to GTXs (Santinelli 2002; Krock et al. 2015). In line with this thinking, Krock et al. (2015) indicated that the detection of only GTX2/3 toxins in field samples could be associated with the transformation of C1/2 toxins, which could have occurred within the time (3 mo) between the collection of plankton samples in the study area and their analysis in the laboratory. However, in the present study this period of time was sometimes greater (1–7 mo). Therefore, it should not be ruled out that the differences between the profiles of PSP toxins observed in the present study and in previous studies carried out in the Argentine Sea could be due to an unexplored diversity of PSP-producing organisms in the Southwest Atlantic.
With respect to toxicity, it has been demonstrated that GTXs have an intermediate toxicity compared with STX, whereas the toxins of the sulfocarbamoyl group (B and C toxins) are the least toxic (Kwong et al. 2006). However, it must be taken into account that the N-sulfocarbamoyl group is chemically labile and the sulfonyl group is easily cleaved off at low pH and/or metabolic activity of the ingesting organisms, which converts the almost nontoxic N-sulfocarbamoyl B and C toxins into the more toxic carbamoyl toxins such as the GTXs (Krock et al. 2007). Via this mechanism a priori low toxic Alexandrium blooms can become more toxic through ingestion and biotransformation by vectors.
In both Golfo Nuevo and Golfo San José, only one sample of mesozooplankton was positive for PSP toxins in each gulf. In both cases, low levels of the epimers GTX2/3 were detected. The absence of C toxins in these samples is in line with the biotransformation of N-sulfocarbamoyl toxins as just discussed. In addition, dcGTX3 was detected in 5 samples (3 from Golfo Nuevo and 2 from Golfo San José) at trace levels (<LOD = 15 ng dcGTX3 NT–1). Because A. catenella has never been reported to produce decarbamoyl toxins (Montoya et al. 2010; Krock et al. 2015), the detection of dcGTX3 in mesozooplankton can been seen as an indication that copepods (the most abundant group among the potential toxin vectors in Golfo Nuevo and Golfo San José) also decarbamoylate carbamoyl toxins, as has been described for filter-feeding mollusks (Artigas et al. 2007; Turner et al. 2013). At this point the observation of decarbamoyl toxins in copepods is only a first indication, and the hypothesis of decarbamoylation of PSP toxins in zooplankton needs to be confirmed by feeding experiments.
Grazing experiments have documented that copepods select their prey based on levels of PSP toxins, feeding on dinoflagellates when the levels of toxins present in them are low, and when the concentrations of toxins in the dinoflagellates increased the copepods fed on non-toxic dinoflagellates (Turriff et al. 1995; Shaw et al. 1997; Teegarden 1999; Guisande et al. 2002). This selective behavior of copepods toward nontoxic dinoflagellates could explain the absence of PSP toxins recorded in the present study in the mesozooplankton samples dominated by copepod species. Evidence of this phenomenon could be the presence of PSP toxins in the phytoplankton samples analyzed, and the closure of the shellfish fisheries from the detection of PSP toxins in the study area during the period analyzed in the present study (Harmful Algal Bloom and Shellfish Toxicity Monitoring Program). By contrast, several investigations have reported that mesozooplankton, and especially copepods, are capable of feeding on toxic dinoflagellate species (Turrif et al. 1995; Turner et al. 2000; Teegarden et al. 2001; Durbin et al. 2002; Doucette et al. 2006) and accumulate PSP toxins produced by them (White 1981; Turriff et al. 1995; Lincoln et al. 2001; Bargu et al. 2002; Durbin et al. 2002; Hamasaki et al. 2003; Teegarden et al. 2003; Doucette et al. 2006). Therefore, future studies should analyze, through grazing experiments, whether the dominant copepod species in both Golfo Nuevo and Golfo San José are the key vector for the transfer of PSP to higher trophic level organisms.
In addition to the transfer of hydrophilic PSP toxins, the present study also addressed the transfer of lipophilic toxins in the marine food web. Only one study showed an association between Dinophysis spp. and DSP toxins in shellfish from Golfo Nuevo and Golfo San José by analysis of samples collected by the Harmful Algal Bloom and Shellfish Toxicity Monitoring Program as well as in phytoplankton samples collected in February 2005 in these gulfs (Gracia Villalobos et al. 2015). In agreement with our results, Gracia Villalobos et al. (2015) found that the DSP toxin profiles from phytoplankton samples consisted mostly of PTX2 and PTX11. These authors observed a clear association of D. tripos with PTX2 and PTX11, which is in agreement with our results from Golfo San José and to a lesser extent Golfo Nuevo. Our findings support the hypothesis that D. tripos could be the major PTX toxin producer species in Northern Patagonian gulfs (Gracia Villalobos et al. 2015). During the study period, PTX2 was detected more frequently and at higher levels in Golfo San José than in Golfo Nuevo. The highest levels of this phytoplankton toxin in Golfo San José were recorded in April (634 ng PTX2 NT–1), coincident with a bloom of D. tripos (1.76 × 103 cells L–1), and constituted the only detection of PTX2 in the mesozooplankton samples (26 ng PTX2 NT–1). Although the PTX2 level found in the mesozooplankton sample in April was low, this represents the first detection of PTX2 in the mesozooplankton, mostly copepods, in the study area reported to date. Likewise, trace levels of this toxin were detected in mesozooplankton from Golfo Nuevo. Therefore, these findings demonstrated that PTX toxins are transferred and accumulated by copepods in the study area. In agreement with this, several researchers have demonstrated that the copepods ingest toxic species of Dinophysis during natural blooms of these dinoflagellates (Jansen et al. 2006; Kozlowsky-Suzuki et al. 2006) and accumulate their toxins (Setälä et al. 2009).
Diatoms of the genus Pseudo-nitzschia were the potentially toxic species most frequently found in these gulfs during the annual cycle studied, with concentrations up to 4.56 × 105 cells L–1. Consequently, domoic acid was the phycotoxin that was most abundant and transferred to upper trophic levels in both gulfs. The highest domoic acid level recorded in phytoplankton samples was detected concurrently with the bloom of P. australis in Golfo Nuevo in October 2015. The attribution of toxicity to a species usually involves proof of the presence of toxins in cultures or isolated cells (Álvarez et al. 2009). However, in the present study the identification of a bloom of P. australis in net tow samples, and the high cell densities of this species observed in bottle samples in Golfo Nuevo in October, suggest that this species was the main producer of the highest levels of domoic acid. In the Argentine Sea, P. australis has been suggested to be the major producer of domoic acid (Almandoz et al. 2017). Furthermore, P. australis is considered to be a strong domoic acid producer (12–37 pg cell–1; Bates 2000; Kotaki et al. 2000), and for this reason it has been reported to be the most toxic species of the genus Pseudo-nitzschia (Trainer et al. 2000) and to be primarily responsible for ASP problems worldwide (Bates 2000; Fire et al. 2010). Therefore, it is worth highlighting the simultaneous detection of the highest levels of domoic acid and major densities of P. australis in the Argentine Sea (Negri and Inza 1998; Sastre et al. 2001; Negri et al. 2004; Almandoz et al. 2007, 2017) because proliferation of this species may pose a risk for fishery activities in the region that are focused on the Tehuelche scallop (Aequipecten tehuelchus) in Golfo San José (Orensanz et al. 2007) and on several species of marine animals that feed and reproduce in both gulfs.
The present findings indicate that calanoid copepods were the main potential vectors for the trophic transfer of domoic acid in both Golfo Nuevo and Golfo San José during the period studied. Evidence of this is the dominance of this group among the potential consumers of Pseudo-nitzschia spp. throughout the year in both gulfs, including those months in which the highest levels of domoic acid were recorded in phytoplankton and mesozooplankton samples. In the present study, C. vanus was the calanoid copepod that coincided with the highest domoic acid concentrations during April, June, and October 2015 in the plankton (phyto- and mesozooplankton) samples from Golfo Nuevo. In Golfo San José the highest levels of domoic acid were recorded in the plankton samples during January 2015, May 2015, and January 2016 when C. australis, C. carinatus, and Acartia tonsa were the most abundant species, respectively. These copepod species have been defined as herbivores or omnivores (Boltovskoy 1981, 1999; Lombard et al. 2010; D'Agostino 2013; Antacli et al. 2014 and references therein); therefore they could act as effective phycotoxin vectors, either by the direct consumption of domoic acid–producing species or by ingestion of organisms of lower trophic levels contaminated with toxins. Consequently, vectorial intoxication of pelagic food webs could occur either by the grazing of copepods on toxic microalgae and also by predation of organisms contaminated with domoic acid. In addition, taking into account that phaeopigments are related to degradation of chlorophyll-a due to grazing by zooplankton, the detection of the highest levels of phaeopigments together with the highest abundances of Pseudo-nitzschia spp. in both gulfs (including the bloom of P. australis and the highest levels of domoic acid observed in phytoplankton samples in Golfo Nuevo) shows other evidence that copepods were the main vector of domoic acid through the food web.
Although spirolides have been previously detected in the Argentine Sea (Almandoz et al. 2014; Turner and Goya 2015; Fabro et al. 2017; Krock et al. 2018), in the present study we report for the first time the presence of SPX1 and 20-Me-SPX-G in plankton (phyto- and mesozooplankton) samples from Golfo Nuevo and Golfo San José. However, Alexandrium ostenfeldii, the only known source of spirolides (Cembella et al. 2001; Franco et al. 2006), was not detected in phytoplankton samples from the present study. Until now, A. ostenfeldii was only found in the Beagle Channel of southern Argentina (Almandoz et al. 2014) and in Argentinean slope waters (Fabro et al. 2017; Guinder et al. 2018), and it has not been previously observed in the Northern Patagonian gulfs. However, the identification of these spirolides in phytoplankton samples suggests the presence of A. ostenfeldii in the study area. On the other hand, the detection of 20-Me-SPX-G in mesozooplankton is evidence that zooplankton, mainly copepods, accumulate these neurotoxins. It has been demonstrated that spirolides produce strong neurotoxic symptoms when they are administrated to laboratory rodents (Guéret and Brimble 2010). Therefore, although low levels of spirolides were detected in the present study, the presence of SPX1 and 20-Me-SPX-G in plankton indicates that it is important for monitoring programs to assess the transfer of these neurotoxins through food webs. This will generate knowledge about the impacts of spirolides exposure in marine fauna as well as on human health by consumption of seafood contaminated with these phycotoxins.
In the present study, the dinophysistoxin DTX1 was recorded for the first time in mesozooplankton samples from the Argentine Sea. To date, the presence of these phycotoxins had been demonstrated only in phytoplankton and shellfish from the Argentine Sea (Gracia Villalobos et al. 2015; Fabro et al. 2018; Krock et al. 2018). Although DTXs were absent in phytoplankton samples from Golfo Nuevo and Golfo San José, the presence of DTX1 in mesozooplankton samples indicates that toxigenic strains of P. lima were present in the phytoplankton and were consumed by the mesozooplanktonic organisms.
Our findings indicate that there are transfers of phycotoxins to higher trophic levels in Northern Patagonian gulfs. In line with this, the present study highlights the need for understanding the mechanisms of transfer of phycotoxins from phytoplankton producer–species to higher trophic levels in the study area. The important role that copepods play in pelagic food webs of Northern Patagonian gulfs (Hoffmeyer et al. 2010; D'Agostino et al. 2016, 2018) justifies the need to conduct studies under controlled conditions that evaluate the grazing of main copepod species on toxigenic microalgae species present in the area. This research will allow one to know whether copepods select among toxic and nontoxic phytoplankton species, as well the rate and the time of phycotoxin retention in their bodies. Likewise, future studies should focus on the phycotoxin content of copepod body tissues, as well as the possibility of biotransformations of the toxins by copepods following ingestion of toxic microalgae.
Acknowledgment
We thank all those who assisted with fieldwork, namely, M. Pollicelli, G. Voglino, J. Deías, G. Novacovsky, N. Sueyro, R. D'Agostino, and M. Roig. Special thanks are also due to M.S. Dutto, A.A. Berasategui, and M. García for their assistance with the taxonomic classification of some specimens of mesozooplankton and during chlorophyll-a extraction. We thank W. Drebing and A. Müller for expert technical assistance with phycotoxin extractions. Thanks are also due to the Dirección General de Conservación de Fauna y Flora and the Subsecretaría de Áreas Protegidas (Chubut Province) for their permission to carry out the present study in the Natural Reserve of Península Valdés in Chubut, Argentina. The study was supported by a grant to M.S. Hoffmeyer from the Argentinean Agencia Nacional de Promoción Científica y Tecnológica (grant PICT-2014-3091, entitled Dinámica planctónica y vectorización de ficotoxinas hacia consumidores de niveles superiores en tramas tróficas pelágicas de la región de Golfos Norpatagónicos y El Rincón), by the Helmholtz-Gemeinschaft Deutscher Forschungszentren through the research program PACES of the Alfred-Wegener-Institut, Helmholtz Zentrum für Polar- und Meeresforschung and the European Commission under the 7th Framework Programme through the Action–IMCONet (FP7 IRSES, Action 319718), and partially by Aluar, Buenos Aires, Argentina. V.C. D'Agostino was supported by a PhD fellowship from the Consejo Nacional de Investigaciones Científicas y Técnicas of Argentina. The manuscript benefited greatly from suggestions by 2 anonymous reviewers and the editor.
Open Research
Data Accessibility
Data pertaining to this manuscript are available from the corresponding author ([email protected]).




