Toxicity of Aluminum to Ceriodaphnia dubia in Low-Hardness Waters as Affected by Natural Dissolved Organic Matter
Abstract
We conducted a series of 7-d toxicity tests with Ceriodaphnia dubia in dilutions of low-hardness natural waters, which contained dissolved organic carbon (DOC) concentrations up to 10 mg/L. Stream waters were mixed with well water to achieve 2 target hardness levels (20 and 35 mg/L) and 4 DOC concentrations. Tests with aluminum (Al)-spiked waters were conducted in a controlled CO2 atmosphere to maintain the pH at a range of 6.0 to 6.5. The results were used to estimate effect concentrations for survival and reproduction, expressed as total (unfiltered) Al concentrations. There were small differences in total-Al thresholds between waters with 20 and 35 mg/L hardness, but effect concentrations for C. dubia survival (median lethal concentrations) and reproduction (effect concentrations, 20%) increased log-linearly with increasing DOC concentrations in the range, 0.3 to 6 mg/L. Slopes of these regressions were similar to slopes from data used to revise the US Environmental Protection Agency water quality criterion for Al, but toxic effects in the present study occurred at total-Al concentrations 8- to 10-fold greater than toxicity values used for criteria development. This difference probably reflects the long equilibration (aging) times of Al test waters used in the present study (up to 192 h) compared with short (3-h) equilibration times in other studies used for criteria development. These results confirm the importance of DOC as a control on Al toxicity in low-hardness waters, but they also demonstrate that total-Al concentrations are not predictive of Al toxicity, except under defined water quality (pH, hardness, DOC) and exposure conditions (e.g., aging of test waters). Environ Toxicol Chem 2019;38:2121–2127. Published 2019 Wiley Periodicals, Inc. on behalf of SETAC. This article is a US government work, and as such, is in the public domain in the United States of America.
INTRODUCTION
Previous US ambient water quality criteria for aluminum (Al; US Environmental Protection Agency 1988) consisted of an acute criterion (750 μg/L) and a chronic criterion (87 μg/L), expressed as total Al, that were intended to apply across a pH range of 6.5 to 9.0. However, research has long since demonstrated that the bioavailability of metals is also influenced by water hardness and other water quality factors (Ankley et al. 1996), notably pH and dissolved organic carbon (DOC; Wang et al. 2011). There is broad scientific support for metal criteria based on bioavailability models, such as the criteria for copper based on biotic ligand models (US Environmental Protection Agency 2007). Aluminum is an amphoteric metal with complex in-solution behaviors and toxicities. The toxicity of Al does not correspond closely to concentrations of dissolved Al species, due to the occurrence of toxicologically relevant solid phases of Al (Santore et al. 2018). Aluminum forms multiple pH-dependent hydrolytic monomer and polymer species (e.g., Al(OH)2+, Al(OH)2+) with distinct toxicity, solubility, stability, and binding capabilities. Aluminum toxicity varies due to pH-dependent equilibria among Al species, competitive binding of calcium and magnesium at biotic ligand sites on body surfaces of aquatic animals, and formation of stable sols and settleable precipitates that may redissolve or reprecipitate as solutions age.
A recent revision of the water quality criteria for Al is based on multiple linear regression (MLR) models that characterize the influences of pH, hardness, and DOC on Al toxicity (US Environmental Protection Agency 2018; DeForest et al. 2018). Modeling the toxicity of total-Al toxicity based on water chemistry characteristics is necessary because the available analytical methods for Al do not identify specific bioavailable Al species. Unlike most metals, the toxicity of Al does not correspond closely to concentrations of dissolved Al species. Instead, variation in Al toxicity with pH corresponds to pH-dependent equilibria among Al species with differing solubility and toxicity, and variation of Al bioavailability with hardness reflects competitive binding of calcium and magnesium at biotic ligand sites on body surfaces of aquatic animals. Dissolved organic matter, including both humic and fulvic acids, makes up the characteristic brown color of many streams and lakes, and binding to these compounds has been shown to decrease Al toxicity (Gundersen et al. 1994). Dissolved organic matter is typically quantified as DOC. Humic compounds and other dissolved organic compounds generally reduce toxicity by binding metals directly, thus reducing the pool of labile metals available to cause toxicity. In low-pH and low-hardness waters, high concentrations of DOC may have a greater capacity for binding metals, due to less competition from hardness cations and also to the predominance of more reactive humic compounds (Breault et al. 1996). The objective of the present study was to evaluate the effect of natural DOC concentrations on Al bioavailability in low-hardness test waters.
MATERIALS AND METHODS
We collected high-DOC stream water from western Massachusetts (USA) and manipulated hardness and DOC levels to produce test waters for toxicity tests. Two separate samples of stream water were collected from Beaver Brook, near Royalston (MA, USA; 42°37′10′′; 72°08′12′′). Sample 1 (DOC ~6–7 mg/L) was collected in December 2016, and sample 2 (DOC ~10 mg/L) was collected in February 2017. Samples were held in Teflon-lined carboys (20 L each; Entegris) and shipped on ice to the US Geological Survey's Columbia Environmental Research Center (Columbia, MO, USA) for testing and analysis. Before amendments, stream waters were acidic (pH 3.5–6.2) and had low hardness (~12 mg/L as CaCO3). None of the field-collected waters showed visible evidence of suspended particulates, so we did not filter the samples before use. Field-collected waters were amended with small amounts of well water (~5 and 10% by volume, respectively) to produce 2 hardness levels: 20 and 35 mg/L (20-hardness and 35-hardness). These hardness levels were selected to approach the lowest hardness range tolerated by Ceriodaphnia dubia, based on previous studies (Deleebeeck et al. 2007). After these initial hardness adjustments, each water was mixed with a low-DOC (<0.5 mg/L) laboratory water of the same hardness (prepared by diluting well water with deionized water) to produce 4 test waters, which were tested concurrently in a test cycle. Each complete test cycle consisted of a single hardness level and 4 DOC treatments: (A) high DOC (100% site water); (B) intermediate DOC (50% site water); (C) low DOC (25% site water); and (D) control (100% laboratory water). The percentage of site water in these designations is approximate: the waters designated 100% site water contained 90 to 95% site water, due to amendment with diluted well water (for hardness adjustment), and the true proportion of site water in other waters produced by dilutions would be reduced proportionately.
Cycle 1 consisted of 4 20-hardness waters (tests A1–D1), and cycle 2 consisted of 4 35-hardness waters (tests A2–D2). Tests that did not produce satisfactory concentration–response results in the first 2 test cycles (tests A1 and D2) were retested (tests A3, A4, and D3; Table 1). The pH of all test waters was initially adjusted with HCl or NaOH to achieve the target pH range of 6.0 to 6.5, and all toxicity tests were conducted in temperature-controlled plant growth chambers with controlled-CO2 atmosphere to maintain the pH (typical CO2 concentrations 1.0–1.2%; Mount and Mount 1992). The pH range for these tests supports at least 3 major hydrolytic Al species (Newman and Jagoe 1994) and is consistent with the stated pH for Al MLR models (US Environmental Protection Agency 2018).
| Sample characteristics | Survival (and immobilization) | Reproduction (young/female) | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Site water | Hardness | DOC | EC50 | lcl | ucl | EC20 | lcl | |||||||||
| Test water | Test | (%) | (mg/L as CaCO3) | pH | (mg/L) | (mg/L as total Al) | Method | (mg/L as total Al) | ucl | EC10 | lcl | ucl | Comments | |||
| 20-hardness | ||||||||||||||||
| A1 | 100 | 20 | 5.98 | 3.57 | 20 | 12 | 34 | TSK | No model | No model | No baseline | |||||
| A3 | 100 | 22 | 5.60 | 6.78 | 2.7 | 1.6 | 4.4 | TSK | No model | No model | No baseline | |||||
| A4 | 100 | 21 | 6.19 | 6.91 | 18 | 11 | 31 | TSK | 4.7 | 1.6 | 14 | 3.6 | 0.91 | 14 | OK | |
| B1 | 50 | 20 | 6.20 | 1.90 | 43 | 8.2 | 227 | Probit | 1.4 | 0.29 | 6.9 | 0.87 | 0.09 | 8.1 | OK | |
| C1 | 25 | 23 | 6.20 | 1.12 | >6.2 | No model | None | 3.4 | 0.21 | 57 | 2.8 | 0.07 | 110 | OK | ||
| D1 | 0 | 28 | 6.23 | 0.33 | 1.1 | 0.54 | 2.2 | TSK | 0.73 | 0.24 | 2.3 | 0.58 | 0.10 | 3.2 | Weak baseline | |
| 35-hardness | ||||||||||||||||
| A2 | 100 | 36 | 6.46 | 6.35 | 76 | 7.4 | 781 | Probit | 16 | 5.24 | 47.80 | 14 | 3.3 | 59 | 1 partial | |
| B2 | 50 | 35 | 6.40 | 3.54 | >12 | No model | None | 5.7 | 2.86 | 11.40 | 4.6 | 1.8 | 12 | OK | ||
| C2 | 25 | 36 | 6.48 | 1.94 | >5.6 | No model | None | 4.6 | 2.43 | 8.76 | 3.8 | 1.2 | 11 | 1 partial | ||
| D2 | 0 | 37 | 6.53 | 0.40 | >2.5 | No model | None | No model | No model | No baseline | ||||||
| D3 | 0 | 35 | 6.44 | 0.40 | >2.8 | No model | None | No model | No model | No baseline | ||||||
- DOC = dissolved organic carbon; EC50 = median effect concentration (for mortality/immobilization; lcl and ucl = lower and upper 95 % confidence limits; EC20 and EC10 = 20 % and 10 % effect concentration (for reproduction); TSK = trimmed Spearman–Karber.
Seven-day chronic toxicity tests were conducted with the cladoceran C, dubia, one of the taxa that provided the basis for the MLR model for Al toxicity (Deforest et al. 2018). Tests followed ASTM International (2015) and US Environmental Protection Agency (2002) standard methods unless otherwise noted. Tests were started with <24-h-old neonates (parthenogenic females), which were cultured at a hardness of approximately 20 mg/L for approximately 8 wk before the start of the tests. One neonate was stocked in each of 10 replicate 30-mL exposure cups/treatment, with 20 mL of test water/cup, and the organisms in each cup were fed daily with 0.1-mL portions of 2 diet suspensions: 1) yeast–cerophyll–trout chow (1800 mg/L stock solution), and 2) algae (Pseudokirchneriella subcapitata, 3.0 × 107 cell/mL; Aquatic Bio Systems). On each day of the test, stocked C. dubia were recorded as live or dead, live animals were transferred to fresh test solutions, and young were counted and discarded. Tests were ended after 7 or 8 d, with the end date determined by when at least 60% of stocked adults in the control group had produced at least 3 broods averaging 15 or more young/female.
Each test consisted of 5 Al concentrations in a 0.5× dilution series of one of the test waters, plus a control without added Al. Test solutions prepared 1 d before the start of the test were used for water replacements for the entire test, based on stability studies demonstrating that measured total-Al concentrations remained stable over a 7-d period. Test solutions were prepared in glass jars and stored in a walk-in cooler at 4 °C between uses. The highest Al concentration for each test was prepared by dissolving Al chloride hexahydrate salt (reagent grade; Sigma-Aldrich) directly into each test water, adjusting the pH as needed, and preparing 3 additional Al concentrations by 0.5× serial dilutions of the high-Al test water with unspiked, pH-adjusted test water. The volumes of test solutions prepared on day 0 were enough to conduct an entire 7-d test. Exposure cups with test water were equilibrated in a temperature-controlled (25 °C) incubator for 24 h before animals were stocked and fed to start the test. On each subsequent day of the test, the unused portions of Al-spiked test waters were used to fill additional sets of 10 cups for each treatment for equilibration, and each test organism was transferred into a cup with freshly equilibrated test water.
Test waters were analyzed for total Al (unfiltered samples), syringe-filtered Al (<0.45-μm polyethersulfone membrane; Whatman Puradisk; GE Healthcare Bio-Sciences), DOC, major ions, and other water quality parameters. Total Al was used as the primary measure of Al exposure for these tests, as it was for other toxicity tests (Gensemer et al. 2018; Oregon State University 2018) used to derive the US Environmental Protection Agency (2018) water quality criteria. This approach applies only acidification to the samples prior to analysis; more rigorous digestion methods are not used. The US Environmental Protection Agency (2018) considers the total-Al method equivalent to “total-recoverable” Al. Samples for analyses of total Al, filtered Al, and major cations (sodium, magnesium, potassium, calcium, strontium, iron, and manganese) were preserved by addition of nitric acid (in-house sub-boiling distilled from BDH Aristar ACS; VWR International) to a final acid content of 2% (v/v). Aluminum and major cation analyses were performed using inductively coupled plasma–mass spectrometry (ICP–MS; model NexION 2000; PerkinElmer). The ICP–MS was calibrated using a minimum of 3 National Institute of Standards and Technology (NIST)-traceable standards, and performance was evaluated throughout the analyses using second-source continuing calibration standards and blanks; laboratory control standards; analytical spikes, duplicates, and dilutions; and certified reference materials.
Samples for DOC analysis were collected in precleaned amber glass bottles, vacuum-filtered through 0.45-μm polyethersulfone membranes (Supor 450; Pall), and acidified with 9 N high-purity sulfuric acid (BDH Aristar Ultra; VWR) to pH ≤ 2 within 48 h of collection. The DOC was measured as nonpurgeable organic carbon by high-temperature combustion catalytic oxidation–nondispersive infrared spectroscopy using a total organic carbon (TOC) analyzer (model TOC-L CSH; Shimadzu Scientific Instruments). Samples were analyzed for DOC within 28 d after collection. The TOC analyzer was calibrated with a minimum of 3 NIST-traceable standards, and performance was verified throughout the analyses using second-source continuing calibration standards and blanks, and analytical duplicates and spikes. Samples for analyses of chloride, nitrate, and sulfate were syringe-filtered (0.45-μm polyethersulfone), collected in precleaned plastic bottles, and preserved at 4 °C in a laboratory refrigerator for up to 30 d before analysis using an ion chromatography system (ICS; model ICS-1100; Dionex) equipped with an Ionpac AS22 anion exchange column and a suppressed conductivity detector. The ICS was calibrated with a minimum of 3 NIST-traceable standards, and performance was verified throughout the analyses using second-source continuing calibration standards and blanks, laboratory control standards, and analytical duplicates and spikes.
Acceptance of data from chemical analyses was consistent with standard quality assurance/quality control guidelines, and acceptance of toxicity data was based on test acceptability criteria described by the US Environmental Protection Agency (2002). Chronic effect concentrations for effects of total Al on reproduction (e.g., 20% effect concentration [EC20]) were determined using the Toxicity Relationship Analysis Program (TRAP), Ver 1.30a (Erickson 2010). Median effect concentrations (EC50s) for lethality or immobilization of test organisms were estimated using trimmed Spearman–Karber or probit procedures (Sebaugh 1998).
RESULTS AND DISCUSSION
Chemical characterization of test waters
Routine water quality analyses indicated that tests were generally conducted within target ranges for hardness, pH, and DOC. Hardness levels were close to the nominal treatment levels of 20 and 35 mg/L as CaCO3 (Table 1 and Supplemental Data, Table S-1). Control of pH in test waters by manipulating atmospheric CO2 was generally successful, but we encountered difficulties maintaining pH in tests with 20-hardness/100% site water. The first test with this water (test A1) had an average pH of 5.98—at the low end of our target range—and had poor toxicity results. The second attempt to test this water (test A3) had an even lower average pH, of 5.72. This apparent overshoot of the pH target likely reflects the very low buffering capacity (alkalinity 3–4 mg/L as CaCO3) in the second sample of site water. In our third attempt to test this water (test A4), we reduced the CO2 level, which produced an average pH of 6.01 and a successful toxicity test.
The 2 samples of site water had somewhat different profiles of major cations and anions (Supplemental Data, Table S-1). Sample 1 (as tested in test A1) had greater concentrations of sodium, potassium, and chloride than the second sample of site water that was used for tests A3 and A4. The laboratory water was relatively enriched with calcium and magnesium compared with either site water. These differences in ionic constituents were less prominent in test waters consisting of 50 or 25% site water. Site water sample 1 (tested in cycle 1) had a starting DOC concentration of approximately 6 mg/L, compared with approximately 10 mg/L in sample 2 (tested in cycles 2–4). Average DOC concentrations during tests ranged from approximately 65% of the starting concentration in 100% site water to >80% of the starting concentration in the 25% site water treatments. Decreases in measured DOC concentrations in Al-spiked test waters (Supplemental Data, Figure S-1) may reflect formation of settleable (or otherwise unfilterable) Al CaCO3 DOC complexes, especially at higher Al concentrations.
Total-Al concentrations during tests were generally close to nominal values across different test waters and across Al concentrations, but there was a general trend for total-Al concentrations to fall below nominal values in the low-DOC laboratory waters and to exceed nominal concentrations in 100% site water (Supplemental Data, Figure S-2). Average total-Al concentrations ranged from 154% of nominal values in test A1 (20-hardness site water) to 72% of nominal values in test D1 (20-hardness laboratory water). The high recovery of total Al in test A1 reflected the high total-Al values in several high-Al treatments. The occurrence of individual samples with high total-Al concentrations in 100% site water may reflect the presence in these waters of nonuniformly dispersed Al–OH–DOC agglomerates and precipitates, which are difficult to sample uniformly. Floc or precipitates were visible in most Al-spiked test waters, with greater quantities in high-Al treatments. This is not surprising, because most Al toxicity is known to occur at concentrations greater than solubility limits (US Environmental Protection Agency 2018). Filterable Al concentrations sampled in selected waters demonstrated that almost all exposure concentrations exceeded Al solubility (Supplemental Data, Table S-2). In most samples with total Al >100 μg/L, filtered Al made up <50% of total Al, with this percentage dropping to <0.5% at higher total-Al concentrations (Supplemental Data, Figure S-3). Two of 8 test waters (A2 and B2) showed a different trend, with an initial decrease in filtered Al, followed by a reversal to >40% filtered Al at the highest concentration. The mechanism for this reversal is uncertain—perhaps dissolution of Al particulates during solution aging. However, both samples had total-Al concentrations greater than the EC20s from most C. dubia tests, suggesting that these relatively high concentrations of filterable Al did not dramatically affect test results.
Al toxicity
All 11 tests with C. dubia met minimum test acceptability criteria for control performance: 1) survival of at least 8 (of 10) stocked animals on day 7, and 2) at least 15 young/female in 3 or more broods from at least 6 females by day 8 (US Environmental Protection Agency 2002; Supplemental Data, Table S-3). Nominal Al concentrations were selected based on range-finding tests to prioritize collection of data on sublethal effects (reproduction) and avoiding treatments that would cause excessive mortality. Only one test (A3) had more than one treatment with survival <50%, and several tests had >50% survival at the highest Al concentration tested (Supplemental Data, Table S-3). Median effect concentrations for lethality (plus immobilization) were estimated for 6 of the 11 tests, with the other 5 tests having unbounded lower estimates.
The fit of concentration–response models for the reproduction data varied among tests (Table 1 and Supplemental Data, Figure S-4). Three of 4 tests in both cycle 1 (tests B1, C1, and D1) and cycle 2 (tests A2, B2, and C2) produced acceptable estimates of EC20s for reproduction (Table 1). Two tests (A2 and C2) were flagged by the TRAP program because they had insufficient partial effect concentrations for reproduction (i.e., <2 concentrations with effects between 10 and 90% of the model maximum), but we concluded that data from these tests adequately defined the concentration–response curve in the range that defined EC20s for reproduction. Concentration–response models for reproduction data from tests A1 and A3, the first 2 tests conducted with 20-hardness site water, had strongly negative slopes, indicating increasing toxicity with increasing total Al, but lacked a stable baseline response at low Al concentrations. We suspect that these atypical concentration–response models reflect the low pH of these test waters (means, 5.98 and 5.60, respectively) in these tests. The third test with this water (test A4) had a higher pH (6.19) that was consistent with other successful tests, and the concentration–response plot showed an adequate baseline and a well-defined sigmoid shape. Two attempts to model reproductive effects in the 35-hardness laboratory water (tests D2 and D3) also produced models without defined baselines. Neither model allowed estimation of a toxicity threshold, and we did not conduct further tests with this water.
Effect concentrations for both survival and reproduction showed strong trends for decreasing toxicity with increasing DOC concentrations and a weaker trend for differences between hardness levels. In both 20-hardness and 35-hardness waters, the median lethal concentrations (LC50s) differed by a factor of approximately 13 between 100% laboratory waters (low DOC) and 100% site waters (high DOC), with weaker trends for differences in LC50s between hardness levels (Table 1). The EC20s fluctuated across the series of 20-hardness test waters, and no reliable EC20 could be determined for the 20-hardness laboratory water (Table 1). However, toxicity values for both survival and reproduction had strong log-linear regressions with DOC (Figure 1). The slopes of these regressions (0.72 for survival and 0.93 for reproduction) were steeper than the slope (of 0.66) that is part of the MLR model (US Environmental Protection Agency 2018), but the most obvious differences between these regressions were the higher thresholds determined from tests in the present study. Across a range of DOC values from 0.5 to 5.0 mg/L, the MLR model predicts chronic values ranging from 0.08 to 0.40 mg/L, but the EC20s from our study were 10- to 18-fold higher (0.73–16 mg/L; Table 1).
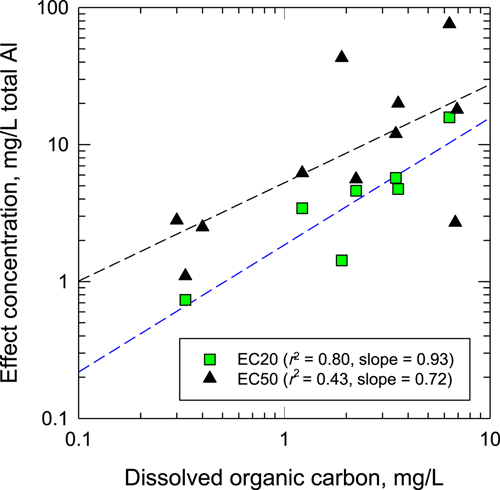
Relationships of aluminum (Al) effect concentrations for Ceriodaphnia dubia (survival median effect concentration [EC50]; reproduction effect concentration, 20% [EC20]) versus dissolved organic carbon (DOC) in low-hardness test waters. Some EC50 values are unbounded lower limits (see Table 1).
The present study overlapped in time with a broader research effort to revise the US water quality criteria for Al. As part of this effort, Cardwell et al. (2018), Oregon State University (2108), and Gensemer et al. (2018) conducted extensive testing with fish and invertebrates in a wide variety of test waters, including 7-d chronic tests with C. dubia. Deforest et al. (2018) used these data to develop MLR models to predict Al toxicity based on pH, hardness, and DOC, and these models were used to generate new Al criteria (US Environmental Protection Agency 2018). The results of 7-d C. dubia tests in the present study show both consistencies and differences relative to the results of tests conducted by Gensemer et al. (2018) and by Oregon State University (2018). The results of all 3 studies produced EC20s that had positive log-linear trends for increases in reproduction EC20s with increasing DOC (Figure 2), but there was substantial variation in EC20s both among and within studies. The general trend was for highest EC20s in the present study, intermediate EC20s in the Oregon State University (2018) study, and lowest EC20s in the Gensemer et al. (2018) study. Because the measured EC20s were influenced by the different water chemistries tested in each study, we used the MLR model to convert each measured EC20 to a “normalized” EC20 predicted for standard water quality conditions (pH 7.0, hardness 100 mg/L, DOC 1.0 mg/L; Figure 2). Normalizing EC20s from all 3 studies eliminated associations between EC20s and DOC, but normalized EC20s from the present study remained approximately 10-fold greater than normalized EC20s for other 2 studies.
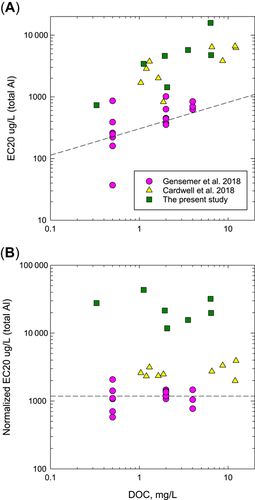
Relationships of chronic aluminum (Al) effect concentration, 20 % (EC20) values for reproduction of Ceriodaphnia dubia with dissolved organic carbon (DOC) concentrations in recent tests. (A)<measured EC20 values. (B) EC20 values normalized to standard water (pH 7.0, 100 mg/L hardness, 1.0 mg/L DOC) using multiple linear regression models (US Environmental Protection Agency 2018). Dashed lines in (A) indicate values for chronic water quality criteria and in (B) indicate predicted genus mean chronic value for C. dubia in standard water (US Environmental Protection Agency 2018).
The differences in sensitivity of C. dubia to Al toxicity in the 3 studies could reflect multiple factors that modify Al bioavailability. Some of the differences among the 3 studies may reflect differences in condition or genetic strain among cultured clones of C. dubia. After normalization, the EC20s from Oregon State University (2018) remained slightly greater than those from Gensemer et al. (2018), suggesting that some factor other than water quality resulted in lesser sensitivity of animals in the Oregon State University (2018) study. There may be other subtle differences in test conditions among the 3 studies that could affect sensitivity, such as the different methods used for pH control (CO2 atmosphere in the present study, proton buffers in the Oregon State University [2018] and Gensemer et al. [2018] studies) or differences in the form of Al tested (chloride in the present study, nitrate in previous studies), but these factors seem unlikely to result in order-of-magnitude differences in sensitivity. A more substantive difference in the design of these studies was our use of natural, field-collected waters to manipulate DOC in the present study, compared with laboratory-refined extracts used in the previous studies. However, we feel that none of these factors adequately explain the large differences in toxicity across test waters from the 3 studies. The most likely explanation is the different ways that Al-spiked test waters were prepared and stored before testing. In the present study, test solutions were prepared 24 h before the start of each test, and the same batches of test solutions were used for daily water replacements throughout each 7-d test, resulting in aging times for test waters in each test that ranged from 24 to 192 h. In contrast, Gensemer et al. (2018) and Oregon State University (2018) started tests after a short (3-h) temperature equilibration of freshly prepared test solutions.
The hypothesis that differences in reproduction EC20s between our study and the 2 previous studies can be attributed to the longer aging period for our test waters is supported by several other studies. The tendency of freshly mixed Al solutions at neutral pH to decrease in toxicity over time has been widely reported (e.g., Witters et al. 1996; Kroglund et al. 2001), and these changes are usually attributed to physical changes occurring as dissolved Al species precipitate and become more stable solids over time (e.g., Teien 2004; Angel et al. 2016). Gensemer at al. (2018) directly tested the issue of equilibration/aging time and reported 2- to 3-fold decreases in acute toxicity values for Al test waters aged for up to 99 h at pH 6.0 and increases in chronic toxicity values of approximately 50% after aging for 24 h at pH 6.3. It is widely accepted that the decrease in toxicity of aged Al solutions reflects precipitation and chemical transformation of toxic dissolved Al species that are above solubility limits. Teien et al. (2004) documented transformation of Al species in acidic waters after increased pH due to liming and estimated short half-times (seconds to minutes) for transformations from low-molecular-weight cationic species (presumed to be most toxic), to high-molecular-weight cationic species, to neutral or anionic high-molecular-weight species, with corresponding decreases in toxicity. The rates of these changes were greater at pH 6.4 than at pH 6.0, and rates also increased in waters with greater DOC concentrations. If changes in speciation and toxicity of Al species in water are as rapid as reported by Teien et al. (2004), most of these changes would have occurred within the 3-h equilibration period used for previous Al toxicity studies. However, Angel et al. (2016), in studies of Al-spiked marine waters, reported that precipitation of dissolved Al species may take several days, and they recommended aging for 1 wk before toxicity testing to minimize the influence of dissolved Al species. They also reported that further changes in Al bioavailability may occur for several weeks after precipitation is complete, including gradual formation of mineral species that may differ in bioavailability (e.g., by coprecipitation of Al with magnesium). These results suggest that aging of Al test solutions can result in a wide range in Al bioavailability, even for tests conducted at similar ranges of pH, hardness, and DOC.
Implications for revised Al water quality criteria
Overall, our findings, particularly the slope of our regression of total-Al EC20s versus DOC, support the approaches used for modeling water quality criteria based on total-Al concentrations. Regressions of EC50s and EC20s with DOC concentrations from the present study had slopes similar to the toxicity data used to generate the water quality criteria. The MLR models used to derive the Al water quality criteria are based on toxicity tests in waters that were subjected to a minimal equilibration period (typically 3 h) before testing, a condition that probably best represents Al toxicity in many waters with elevated Al concentrations, such as streams receiving Al inputs from industrial effluents or acid mine drainage. However, the results of our tests with aged test solutions suggest that the MLR-based criteria may be overprotective of natural waters away from point sources or tributary mixing zones. The results of the present study suggest that chronic EC20s for Al toxicity to C. dubia in aged waters could be approximately 10-fold greater than those predicted by the MLR model at similar water chemistries.
The 10-fold range of toxicity values from 3 different chronic toxicity datasets based on total Al—as well as the more limited variation in toxicity values in response to aging reported by Gensemer et al. (2018)—provide evidence that total-Al concentrations alone are not a definitive measure of Al bioavailability, even when adjusted for effects of DOC on Al bioavailability. The results of the present study suggest that Al toxicity values differ as much with differences in aging times as they do across a wide range of DOC concentrations. If this is true, it follows that bioavailability models based on total-Al measurements may only accurately predict toxic effects under defined exposure conditions (i.e., short equilibration times). The differences between data from the present study and the data used to develop the MLR model suggest that a long equilibration allowed a greater proportion of the total-Al concentration to be transformed to unreactive and less toxic species, a change that was not evident from differences in total-Al concentrations.
The inability of the bioavailability model based on total Al to predict differences in Al bioavailability across different aging times suggests that there is a need for better methods of sampling or analysis to reliably characterize bioavailable Al fraction(s) in freshwaters (US Environmental Protection Agency 2018). One promising approach would use a version of the “acid-soluble” Al extraction, a method that calls for acidification of unfiltered water samples in a pH-4.0 buffer for 3 h, followed by filtration at 0.45 μm. This method has been shown to differentiate between freshly precipitated Al and Al bound to suspended sediment particles in natural waters (Rodriguez et al. 2019). It would be helpful to determine whether Al concentrations in the “acid-soluble” Al fraction can explain differences in toxicity better than total-Al analyses. A better analytical approach to measure bioavailable Al or determine Al speciation (e.g., X-ray diffraction, light scattering photometry) during toxicity testing could further improve the reliability of predictions of Al toxicity.
Supplemental Data
The Supplemental Data are available on the Wiley Online Library at DOI:10.1002/etc.4523.
Acknowledgment
The present study was funded by Joint Funding Agreement 16ENMA000000021 between the State of Massachusetts Department of Environmental Protection and the US Geological Survey (USGS) New England Water Science Center. We thank K. Groff (State of Massachusetts) and J. Colman (USGS, retired) for their contributions. Research at the Columbia Environmental Research Center Toxicology Branch is also supported by the Center's USGS Environmental Health Mission Area/Contaminant Biology Program. We thank the USGS personnel who assisted with experimental apparatus (E. Brunson), invertebrate culture (R. Dorman), water quality (D. Hardesty and R. Gettler), and aluminum analyses (M. Walther). This manuscript was reviewed and approved in accordance with USGS policy. Peer reviews were provided by A. Cardwell, A. Allert, and anonymous reviewers. Laura Blake's current address is HydroAnalysis, Acton, MA, USA.
Disclaimer
The present study was conducted in accordance with the US Geological Survey-Columbia Environmental Research Center Animal Welfare Plan, but it did not undergo formal review by the Institutional Animal Care and Use Committee, because the research exclusively involved invertebrate animals. Any use of trade, firm, or product, or firm names is for descriptive purposes only and does not imply endorsement by the US Government.
Open Research
Data Accessibility
Data and metadata for this manuscript are available online in Ivey and Besser (2019; see the Reference list).




