Chronic ammonia toxicity to juveniles of 2 tropical Australian freshwater mussels (Velesunio spp.): Toxicity test optimization and implications for water quality guideline values
Abstract
Freshwater mussels play key roles in aquatic ecosystems, but are experiencing a global decline. Although studies have reported high acute sensitivity of mussels to some contaminants, chronic toxicity data are lacking for deriving high-reliability water quality guideline values. Ammonia is a contaminant of potential concern in some catchments of tropical northern Australia, where freshwater mussels are important ecological and cultural components. The extremely soft waters (hardness < 5 mg/L) of these environments can result in increased toxicity of many contaminants including ammonia, and regionally relevant tropical guideline values are needed to adequately protect these unique ecosystems. An optimized 14-d toxicity test protocol was used to assess the chronic toxicity of ammonia for 2 species, the lotic Velesunio sp. and the lentic Velesunio angasi. Ammonia exposures were conducted at pH 6.0 and 27 ± 0.5 °C to represent local environmental conditions, using shell length growth rate as the endpoint. Chronic toxicity estimates indicated high sensitivity to ammonia, with mean median effect concentrations (in total ammonia nitrogen) being 7.0 mg/L for V. angasi from the semi-urbanized Lake Bennett, 9.2 mg/L for V. angasi from Sandy Billabong, and 11.3 mg/L for Velesunio sp. from Gulungul Creek. When the 10% effect concentration values were compared with other chronic ammonia data (normalized to pH 7.0 and 20 °C), Velesunio spp. were found to be more sensitive than 8 of 16 other temperate and 7 of 9 tropical invertebrate and fish species. These chronic toxicity estimates will be used to further inform regionally relevant and site-specific guideline values. Environ Toxicol Chem 2019;38:841–851. © 2019 Commonwealth of Australia. Published by Wiley Periodicals Inc. on behalf of SETAC.
INTRODUCTION
Freshwater mussels play important roles in aquatic ecosystems, including biofiltration of the water column, nutrient and energy cycling, and sources of food for other animals and humans (Vaughn 2017). The global decline of freshwater mussel species is well documented, and is generally attributed to habitat loss, overexploitation, invasive species, and their high sensitivity to some contaminants (Lydeard et al. 2004; Augspurger et al. 2007; Lopes-Lima et al. 2017). Tropical waters with low ionic strength (hardness < 5 mg/L) have been shown to increase the toxicity to tropical freshwater taxa of certain contaminants, such as metals (van Dam et al. 2010, 2016; Harford et al. 2015) and ammonia (Mooney et al. 2019). Mussels inhabiting these waters could also be at greater risk, but knowledge of their response to such contaminants is limited. Therefore, further research is needed to better understand the impact of contaminants on tropical mussel species, for the protection of these important taxa (Augspurger et al. 2007).
Although chronic toxicity data are preferred for generating high-reliability water quality guideline values (Warne et al. 2018), the extrapolation of such values from short-term (acute) toxicity data is commonly practiced due to a lack of chronic data for many contaminants (Australian and New Zealand Environment and Conservation Council/Agriculture and Resource Management Council of Australia and New Zealand 2000; Raimondo et al. 2007; May et al. 2016). A lack of chronic toxicity data for freshwater mussels has been attributed to the challenges researchers face when the mussels are used for longer term toxicity testing. These challenges include difficulties culturing stocks in the laboratory, uncertainty regarding appropriate test durations and sublethal endpoints, and the need for including sediment in some juvenile mussel tests to sustain growth and survival (Augspurger et al. 2003; Newton and Bartsch 2007). Progress has been made in recent years through the development of specialized culturing and toxicity testing methods for freshwater mussel early life stages (Neves 2004; American Society for Testing and Materials 2006; Barnhart 2006; Ingersoll et al. 2007). However, these methods were developed using temperate North American species (Unionidae), which may vary in culture requirements and toxicological sensitivity compared with species from other regions of the world.
Ammonia contamination in aquatic ecosystems is an important global issue, and reliable water quality guideline values or criteria are needed to protect local receiving waters (Camargo and Alonso 2006; Miao et al. 2013; US Environmental Protection Agency 2013). Because freshwater mussels have a higher sensitivity to ammonia, it has been recommended that they be included when water quality guideline values or criteria are derived (Augspurger et al. 2003; Mummert et al. 2003; Wang et al. 2007a; US Environmental Protection Agency 2013). However, most of the available ammonia toxicity data for freshwater mussels are generated from temperate species (Augspurger et al. 2003; US Environmental Protection Agency 2013; Clearwater et al. 2014). In the US Environmental Protection Agency (USEPA) ammonia water quality criteria revision (2013), temperate freshwater mussels were ranked the 4 most sensitive among 69 genera in all the acute data and the 2 most sensitive among 16 genera in all the chronic data. Studies have reported that although tropical species were more tolerant of some organic chemicals, they were generally more sensitive to ammonia than their temperate counterparts (Kwok et al. 2007; Wang and Leung 2015).
Ammonia is used for ore-processing at the Ranger Uranium Mine in tropical northern Australia, and has been identified as one of several contaminants of potential concern. Hence, site-specific toxicity studies that include the use of freshwater mussels are needed to determine the risk that ammonia presents to aquatic biota. Only limited acute ammonia data exist for some Australian tropical freshwater species, for example, barramundi, Lates calcarifer, the eastern rainbowfish, Melanotaenia splendida splendida, a freshwater shrimp, Caridina nilotica (Økelsrud 2004; Økelsrud and Pearson 2007), and 2 freshwater mussel species, Velesunio angasi and Velesunio sp. (Kleinhenz et al. 2018). Both Velesunio species were more sensitive to ammonia than most temperate species, and were thus considered valuable additions to the data set for chronic ammonia toxicity. Chronic data were recently reported for the tropical green hydra, Hydra viridissima (Mooney et al. 2018), and for 6 tropical freshwater species (Mooney et al. 2019). At the time of the present study, no chronic ammonia toxicity estimates were available for tropical freshwater mussel species.
The aims of the present study were to: 1) optimize a standardized test protocol for the chronic exposure of tropical freshwater mussels to water-borne contaminants, and 2) use the test protocol to assess ammonia toxicity to the juvenile life stage of 2 tropical Velesunio species, thereby contributing to the global chronic toxicity data set for ammonia.
MATERIALS AND METHODS
The starting points for the toxicity test protocol used in the present study were the American Society for Testing and Materials International Standard (2006; ASTM E2455-06, Standard guide for conducting laboratory toxicity tests with freshwater mussels), and early work by Humphrey (1987a, 1987b, 1991), who successfully reared juvenile mussels of the same species (V. angasi and Velesunio sp.) in laboratory and field settings after exposing fish to the mussels’ parasitic glochidial larvae.
Mussel species and collection
Several species of the genus Velesunio (Hyriidae family) are found throughout tropical northern Australia, the most common being V. angasi (Supplemental Data, Figure S1). Recent genetic analysis (J.M. Hughes, D.J. Schmidt, Griffith University, QLD, Australia, unpublished data) has confirmed the presence of V. angasi, a lentic species predominantly found in permanent billabongs, lakes, and impoundments, and has identified a lotic species of Velesunio (Velesunio sp.), inhabiting creeks and streams that commonly cease to flow during the dry season (April–November). Adult Velesunio sp. and V. angasi were collected from 3 locations within the Alligator Rivers Region of the Northern Territory, Australia, Magela Creek (latitude 12°40′28″S, longitude 132°55′52″E), Gulungul Creek (latitude 12°39′21″S, longitude 132°52′42″E), and Sandy Billabong (latitude 12°54′4″S, longitude 132°46′38″E), and one location outside of the Alligator Rivers Region, the semi-urbanized Lake Bennett located 80 km south of Darwin (latitude 12°57′39″, longitude 131°09′59″). Velesunio sp. was sourced from the Magela Creek and Gulungul Creek sites, and V. angasi from Sandy Billabong and Lake Bennett. Mussels were transported back to the laboratory within 4 h of collection, in aerated plastic 20-L drums containing approximately 15 L of water collected from the sampling site.
Glochidia isolation
Glochidia were isolated from female mussels according to the method described in Kleinhenz et al. (2018). Viability testing was undertaken on a subsample of mature glochidia by exposure to a weak salt solution according to the method detailed in ASTM International (2006). Only glochidia that had achieved >80% viability (preferably >90%) were used for host fish exposure, as recommended in ASTM International (2006). The subsamples were then discarded, and the remaining glochidia were pooled into a 400-mL watch-glass and gradually acclimated to filtered Darwin tap water at a temperature of approximately 26 to 27 °C for at least 3 h, to prepare for the host fish exposure in filtered Darwin tap water.
Host fish exposure and selection of juveniles
The northern trout gudgeon, Mogurnda mogurnda, was used as a host fish for all toxicity tests. This species is common in the Alligator Rivers Region, and has previously been identified as a host fish for Velesunio spp. (Humphrey and Simpson 1985). For the host-fish exposure, 2 M. mogurnda of approximately 12 cm length (∼6 mo old) were exposed to glochidia from the pooled sample for 35 min, in 4 L of vigorously aerated filtered Darwin tap water at a final density of approximately 19 000 glochidia/L. Exposed fish were transferred to a plastic tub containing approximately 60 L of filtered Darwin tap water, and held for up to 10 d until the required number of juvenile mussels had been collected. Fish were fed Hikari® sinking carnivore pellets (Kyorin) and one-third of the water was exchanged daily.
Excysted juveniles were collected by siphoning the material in the bottom of the tub through a 63-μm sieve. Collected juveniles were acclimated to the test diluent (Magela Creek water) for a minimum of 3 h, and used in toxicity tests within 24 h of being isolated from fish. The development of the host-fish exposure method is described in further detail in the Supplemental Data, Section S1.
Juveniles were selected under a stereomicroscope (Leica MZ8), and transferred into test vessels using a 1-mm-diameter Pasteur pipette. Criteria for selection of juveniles included opaque appearance, the presence of internal organs observed through the part-translucent shell and pedal gape, and a moving foot. During all tests, vessels were arranged randomly on Perspex trays and placed into constant-temperature incubators (27.5 ± 1 °C; Labec).
General laboratory procedures
All plastic and glassware used throughout experiments were cleaned by soaking in a 5% (v/v) nitric acid (HNO3) bath for 24 h, triple rinsing in deionized water (Elix; Millipore), and then washing in a laboratory dishwasher (Miele) using deionized water and a phosphate-free detergent (Neodisher® Laboclean; Dr. Weigert).
Test diluent
The test diluent, Magela Creek water, is representative of the extremely soft waters of northern Australia. These waters are slightly acidic (pH 5.5–6.5), have low ionic strength (hardness 3–6 mg/L as CaCO3), low alkalinity (5–10 mg/L as CaCO3), and low electrical conductivity (5–20 μS/cm; van Dam et al. 2010). Values for key physicochemical variables associated with the Magela Creek water used for definitive ammonia tests during the present study are shown in the Supplemental Data, Table S2. The Magela Creek water was collected monthly from a permanent water body (Bowerbird Billabong, latitude 12°46′15″S, longitude 133°02′20″E). Water was pumped into 20-L acid-washed plastic containers and transported at ambient temperature to the laboratory within 4 h of collection, and was then filtered (3 μm Sartopure PP2 MidiCap filter; Sartorius Stedim) and stored at 4 °C until required for use.
Toxicity test optimization
The 14-d toxicity test optimization experiments involved trialling different variables, including test species, test volumes, test vessels, the addition of fine sediment, food and feeding frequency, pH control, and biological endpoints. For these experiments, control performance and endpoint responses were assumed to be comparable among the mussel species and populations used. One-way and 2-way analysis of variance (ANOVA) tests or Student's t tests were used (α = 0.05; SigmaPlot 13.0) to compare test optimization results for selected treatments. The optimization experimental methods are described in detail in the Supplemental Data, Section S2.
Toxicity testing
The final toxicity testing protocol (Table 1) was based on the results of the test optimization experiments. Using the optimized protocol, 6 definitive chronic ammonia tests were undertaken (2 tests with Velesunio sp. from Gulungul Creek, 2 tests with V. angasi from Sandy Billabong, and 2 tests with V. angasi from Lake Bennett) to estimate and compare ammonia toxicity between juvenile mussels from different sites and species. Test duration was set at 14 d, the minimum test period required to classify the test as chronic, using the guidance of Warne et al. (2018). Other chronic toxicity studies with temperate mussel species have used a 28-d test duration (Wang et al. 2007b, 2011) as recommended in ASTM International (2006), but Warne et al. (2018) note that test durations may be reduced for tropical species whose life cycles are much shorter than those of temperate species.
| Test organism | Species | Velesunio spp. |
| Life stage | Juvenile <24-h-old post excystment | |
| Host-fish exposure method for acquiring juvenile mussels | Host fish | 2× Mogurnda mogurnda juveniles ∼15 cm |
| Exposure volume | 4 L tap water in 5-L beaker, aerated with air stone | |
| Exposure duration | 35 min, agitated every 10 min with air stone | |
| Glochidia quantity | Medium (100 mL of ∼750 glochidia/mL) | |
| Toxicity test description | Type | Static renewal |
| Test duration | 14 d | |
| Test vessels | 6-cm plastic jars (175 mL) with lids | |
| Test volume | 100 mL | |
| No. of organisms per test vessel | 10 | |
| No. of replicates per concentration | 2 | |
| Dilution water | Natural Magela Creek water | |
| Test solution renewal | 100% every 2 d | |
| Feeding | Chlorella algae (∼7.0–8.0 × 104 cells/mL) every 2 d | |
| Aeration | None | |
| Turbidity at start and at water changes | 100 NTU using fine sediment ≤63 μm | |
| Assessment endpoint | Growth rate, survival | |
| Quality control | Test acceptability | Survival >80% in each control treatment |
| Temperature | 27.5 ± 1 °C | |
| pH | 6.0 ± 0.3 | |
| pH control | 1 mM HEPES buffer adjusted to pH 6.0 | |
| Dissolved oxygen | 80–120% saturation | |
| Photoperiod | 12:12-h (light:dark) | |
- NTU = nephelometric turbidity unit; HEPES = N-2-hydroxyethylpiperazine-N′-2-ethane-sulfonic acid.
The growth rate endpoint was determined using image analysis software (Leica application suite, Ver 4.6.1) to measure the maximum shell length of each mussel photographed on days 0 and 14 (Leica MC170HD camera, Leica M205C microscope at 8.0× magnification).
Preparation of test solutions
Analytical grade ammonium sulfate (NH4)2SO4 (Sigma-Aldrich) was added to high-purity water (MilliQ; Millipore) to prepare a 1000-mg/L total ammonia nitrogen (TAN) stock solution. A bulk batch of Magela Creek water and sediment was prepared to achieve a diluent with a turbidity of 100 nephelometric turbidity units (NTUs; TPS 90-FLT meter, probe 125186). Each test concentration was prepared by mixing the diluent with the required volume of ammonia stock solution and 1 mM of N-2-hydroxyethylpiperazine-N′-2-ethane-sulfonic acid (HEPES) buffer. The initial pH was adjusted to 6.0 ± 0.1 by adding 5% H2SO4 or 5% NaOH dropwise.
Test vessels were prepared by adding the required volume of algae (Chlorella sp.), and then dispensing 100 mL of each test concentration into the vessels to ensure thorough mixing. Dispensed test vessels and water samples were warmed to the testing temperature, and the pH was further adjusted if necessary, by adding 1% H2SO4 or 1% NaOH dropwise to ensure the starting pH was 6.0 ± 0.1.
Quality control
Test waters from each treatment were measured for water quality using a WTW Multi 340i meter, with specific probes: Sentix 41 for pH, Orion 013005MD for electrical conductivity, and CellOx 325 for dissolved oxygen. New waters were measured at the start of each test and during each water change; pooled replicate subsamples from old waters were measured during each water change and at the end of the test. For each treatment, measurements taken over the test duration were averaged, and were reported and used in further analyses of ammonia toxicity. Incubator temperature was monitored at 5-min intervals using data loggers (Testo Saveris™) throughout each test.
Total organic carbon (TOC) and dissolved organic carbon (0.45-μm filtered) for each batch of Magela Creek water were measured in-house (Shimadzu TOC-V CSH device) within 48 h of collection.
Blank, procedural blank, and diluent water samples from each test were filtered (0.45-µm, Minisart RC25 filter; Sartorius Stedim), acidified using 1% HNO3, and sent to an external laboratory (Envirolab, Chatswood, NSW, Australia) for chemical analysis of a standard suite of metals and major ions (Al, Ca, Cd, Co, Cr, Cu, Fe, Mg, Mn, Na, Ni, Pb, Se, SO4, U, and Zn) using inductively coupled plasma–mass spectrometry and inductively coupled plasma–atomic emission spectroscopy. Ammonia concentrations (as TAN) of unfiltered subsamples were measured at the start and end of tests using an ammonia test kit (Palintest), followed by spectrophotometry (Shimadzu UV-2550, wavelength 640 nm; USEPA method 350.1; US Environmental Protection Agency 1993), and were averaged for concentration–response analysis.
Tests were considered valid if control growth was ≥12.8 μm/d (see the Toxicity test optimization section in the Results), control survival was ≥80% at the end of the test (ASTM International 2006), average changes in effect concentration (EC) remained within 10% of the values at test commencement, drift in pH did not exceed ±0.3 units from the starting pH in a 48-h period, dissolved oxygen levels remained >80%, and temperature of the incubator remained within ±1 °C of the target.
Data analysis
Measured TAN concentrations were used for concentration–response modeling and estimation of ECX values. Growth rate data for each definitive ammonia toxicity test were transformed to percentage of control, and the data from each of the 2 tests/site were pooled to generate toxicity estimates for the 3 sites and 2 mussel species (Velesunio sp.: Gulungul Creek; V. angasi: Sandy Billabong and Lake Bennett) using CETIS™ (Ver 1.9.0.9; Tidepool Scientific) for statistical analysis. The toxicity estimates were calculated from concentration–response curves generated using nonlinear (3-parameter logistic) regression. Ammonia toxicity estimates were calculated for a 10% (EC10), 20% (EC20), and 50% (EC50) reduction in growth relative to the control responses, along with their 95% confidence limits. For comparisons with other chronic ammonia data, ECX values were adjusted to pH 7 or 8 and 20 °C using the algorithms derived in Emerson et al. (1975) and described in the USEPA method (US Environmental Protection Agency 2013). See the Supplemental Data, Section S3, for further information about each method.
RESULTS
Toxicity test optimization
Test volume and test vessels
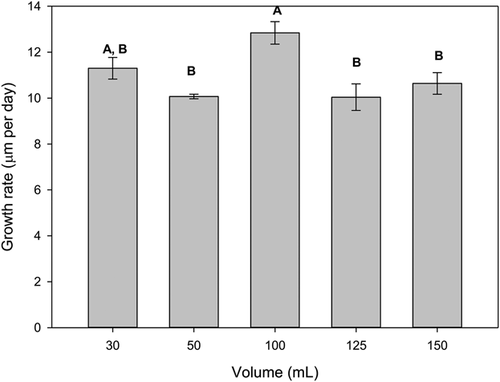
All 4 preliminary trials comparing growth and survival among different test volumes and test vessels showed consistently best performance in the 100-mL treatments in plastic jars with lids (Supplemental Data, Figure S4). In a test comparing 2 feeding regimes (Chlorella sp. and shellfish diet, both at ∼8 × 104 cells/mL), growth was almost doubled in the 100-mL treatments compared with the 30-mL treatments regardless of feeding regime (Supplemental Data, Figure S4a). In 2 tests comparing 100 with 50 mL (Supplemental Data, Figure S4b and c) and 1 test comparing 100 with 150 mL (Supplemental Data, Figure S4d), growth was higher in the 100-mL treatments, whereas survival was 100% in all treatments. In the final growth trial, the 100-mL treatments also achieved the greatest growth rate (12.8 μm/d), with significant differences found between treatments (p = 0.007; Figure 1). Survival rates remained within acceptable levels (≥80%), ranging from 83% (125-mL treatment) to 97% (150-mL treatment).
Sediment and feeding
In turbidity trials, growth rates were highest in treatments with the highest turbidity (100 and 200 NTU), regardless of feeding regime. Growth was significantly reduced in treatments with no sediment added (Supplemental Data, Table S3: tests 2 and 3). Results of the final combined feeding and turbidity trial are described below.
In feeding trials, shellfish diet or Chlorella sp. supplemented with FFV (Fermented Food with Vitamins, see supplemental Data, Section 2: Sediment and feeding), produced higher growth than algae alone, but survival was reduced (Supplemental Data, Table S4: tests 1 and 2), and fungal growth was increased on the mussel shell surface. The optimum cell density for the shellfish diet was 8 × 104 cells/mL (Supplemental Data, Figure S5). The Chlorella sp. diet resulted in higher growth rate than the shellfish diet (Supplemental Data, Table S4; tests 3 and 4), and mussels were healthier, with sediment and algae tending to adhere to the mussel shell surface in treatments using the shellfish diet (Supplemental Data, Figure S6).
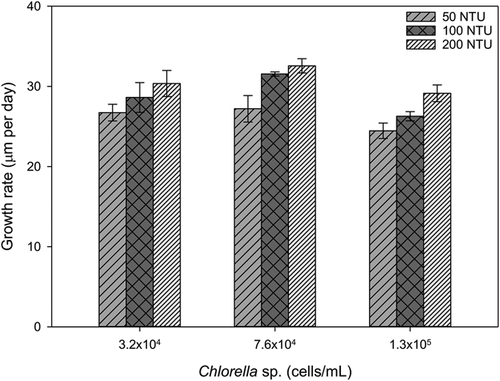
In the final combined feeding and turbidity trial (Figure 2), significant differences in growth rates were observed among turbidity treatments (p = 0.005), with 200 NTU producing the greatest growth. Significant differences were also observed among algal treatments (p = 0.01), and greatest growth was observed in treatments using 7.6 × 104 cells/mL Chlorella sp. in all turbidities. Two treatments with 100 and 200 NTU using this algal cell count produced the greatest growth (31.5 and 32.6 μm/d, respectively), with no significant difference (p = 0.23, one-way ANOVA) found between these 2 treatments. Therefore, the smallest possible amount of added sediment that produced no significant difference in growth compared with the next highest sediment concentration tested was chosen (100 NTU) to minimize factors that may modify ammonia toxicity and to simplify transfer of juveniles during water changes. On the basis of these results, approximately 7.0 to 8.0 × 104 cells/mL Chlorella sp. and 100 NTU were used in the final test protocol.
Water changes and pH control
Growth was reduced when water was changed less frequently than every 2 d, with growth and survival lowest when water was changed every seventh day (Supplemental Data, Table S5). In the absence of added toxicants and buffers, water quality remained acceptable in all treatments: (pH 6.2–6.4, electrical conductivity 13.0–13.5 μS/cm, dissolved oxygen 98.9–99.3%). Based on these results, water changes every 2 d were undertaken in subsequent toxicity testing.
Both the HEPES and the 2(N-morpholino)ethanesulfonic acid (MES) buffers controlled pH within acceptable levels (6.0–6.1; Supplemental Data, Table S6). When either buffer was used, no significant difference (p = 0.24) was seen in median growth (HEPES 31.9 and MES 33.5 μm/d), and survival remained at ≥95%. Both buffer treatments showed an increase in electrical conductivity compared with Magela Creek water (∼14 μS/cm), with the treatments using MES measuring a higher average electrical conductivity (59.4 μS/cm) than the HEPES treatments (25.9 μS/cm), and thus HEPES was chosen as the preferred buffer.
Endpoints
Dry weight measurements were more variable than shell growth rate measurements (Supplemental Data, Figures S7 and S8), and the 2 endpoints had a low correlation in both tests (r2 = 0.468 and 0.065, respectively; Supplemental Data, Figures S9 and S10). On this basis, dry weight was discontinued as a viable and useful endpoint. Survival data recorded during tests were used for quality control, and to optimize concentration ranges to minimize mortality. Control growth rates measured during optimization tests averaged 21.2 ± 5.8 μm/d (± standard deviation [SD]), ranging from 12.8 to 31.9 μm/d with various feeding and turbidity regimes. The minimum growth rate in these tests (12.8 μm/d) was used for the control growth test acceptability criterion for toxicity tests.
Toxicity tests
Quality control
For all ammonia toxicity tests, control survival met test acceptability criteria, averaging 98.3 ± 2.6% (± SD). Control growth rates averaged 25.0 ± 4.6 μm/d (± SD), also meeting the test acceptability criterion. Mean physicochemical variables (± SD) remained within acceptability criteria in all tests with pH 6.01 ± 0.02, dissolved oxygen 100.6% ± 1.2% saturation, and temperature 27.2 ± 0.2 °C (Table 2). Mean electrical conductivity of the control treatment using Magela Creek water was 14.9 μS/cm ± 1.9 (Supplemental Data, Table S2). The mean electrical conductivity among ammonia treatments (1.4–31.1 mg/L TAN) ranged from 19 to 345 μS/cm (Supplemental Data, Table S7).
| Test | Date | Species | Site | Control survival (%) | Control growth (μm/d) | pH (units)a | Temp. (°C) | EC (μS/cm)a | EC shift (%)a | Dissolved oxygen (%)a |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 12/03/16 | Velesunio sp. | GC | 95 | 32.7 ± 0.8 | 6.00 ± 0.05 | 26.9 | 139.1 | 1.2 ± 0.7 | 100.2 ± 2.7 |
| 2 | 29/04/17 | Velesunio sp. | GC | 100 | 19.9 ± 0.5 | 5.97 ± 0.04 | 27.4 | 68.7 | 1.1 ± 1.3 | 100.7 ± 2.5 |
| 3 | 05/06/17 | Velesunio angasi | SB | 100 | 22.9 ± 0.2 | 6.03 ± 0.06 | 27.2 | 77.5 | 2.5 ± 2.1 | 102.3 ± 3.1 |
| 4 | 25/06/17 | Velesunio angasi | SB | 95 | 21.6 ± 0.8 | 6.00 ± 0.05 | 27.2 | 104.7 | 2.2 ± 1.1 | 98.8 ± 3.4 |
| 5 | 23/03/17 | Velesunio angasi | LB | 100 | 30.7 ± 0.4 | 6.02 ± 0.04 | 26.9 | 117.5 | 1.1 ± 1.3 | 100.6 ± 2.8 |
| 6 | 13/05/17 | Velesunio angasi | LB | 100 | 21.0 ± 1.3 | 6.01 ± 0.05 | 27.4 | 71.7 | 1.5 ± 1.9 | 101.2 ± 2.5 |
| Mean ± SD | 98.3 ± 2.6 | 24.8 ± 5.5 | 6.01 ± 0.02 | 27.2 ± 0.2 | 96.5 ± 28.5 | 1.6 ± 0.6 | 100.6 ± 1.2 | |||
- a Values represent measurements of test waters at 0 h and 2 replicate samples at 2, 4, 6, 8, 10, 12, and 14 d from all treatments from each test (tests 2 and 6: n = 7; test 1: n = 8; tests 3–5: n = 9).
- EC = electrical conductivity; GC = Gulungul Creek; SB = Sandy Billabong; LB = Lake Bennett.
The analyses of blank and procedural blank samples from the start of each test showed that no confounding contamination was present. Measured chemical concentrations of the control treatments (Magela Creek water containing algae and sediment) at the start of each test reflected normal Magela Creek water composition (Supplemental Data, Table S8). Measured ammonia concentrations generally remained within 10% between the start and the end of tests, averaging a net decrease of 3.9% for the duration of the tests (Supplemental Data, Table S9).
Toxicity of ammonia
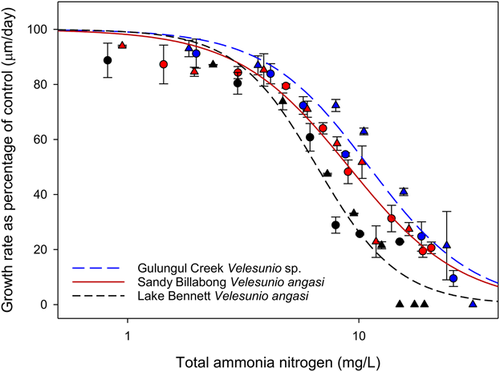
The toxicity of ammonia to mussels among the 3 different field sites was notably different (Figure 3 and Table 3). The toxicity estimates for Velesunio sp. from Gulungul Creek were the highest (EC50 = 11.3, EC20 = 5.6, EC10 = 3.7 mg/L TAN), whereas those of V. angasi from Lake Bennett were the lowest (EC50 = 7.0, EC20 = 4.1, EC10 = 2.9 mg/L TAN). The EC50 (9.2 mg/L) and EC20 (4.4 mg/L) values for V. angasi from Sandy Billabong were between the values of those found at the other 2 sites, whereas the EC10 value (2.8 mg/L) was comparable to that of V. angasi from Lake Bennett. Based on the EC50 and EC20 values from each site (at test pH and temperature), the order of sensitivity was Lake Bennett > Sandy Billabong > Gulungul Creek. Based on the EC10 values, the order of sensitivity was Sandy Billabong > Lake Bennett > Gulungul Creek. The mean EC50s among the sites varied by a factor of 1.6, the mean EC20s by 1.4, and the EC10s by 1.3.
| NH3 (mg/L) | Total ammonia nitrogen (mg/L) | ||||||
|---|---|---|---|---|---|---|---|
| Species | Site | pH (SD) | Temperature (°C) | EC50 | EC50 (95% CL) | EC20 (95% CL) | EC10 (95% CL) |
| Velesunio spp. | Gulungul Creek | 5.99 (0.02) | 27.2 | 0.007 | 11.27 (9.75–13.03) | 5.63 (4.24–6.93) | 3.68 (1.91–4.98) |
| V. angasi | Sandy Billabong | 6.02 (0.02) | 27.2 | 0.006 | 9.18 (8.21–10.28) | 4.38 (3.43–5.30) | 2.78 (1.62–3.71) |
| V. angasi | Lake Bennett | 6.02 (0.01) | 27.2 | 0.005 | 7.00 (6.18–7.93) | 4.06 (2.97–4.98) | 2.90 (1.09–3.87) |
- a Two pooled tests from each site; Gulungul Creek: n = 15; Sandy Billabong: n = 18; Lake Bennett n = 16.
- SD = standard deviation; EC = effect concentration; CL = confidence limit.
When the EC10 values for Velesunio spp. were adjusted to pH 7 and 20 °C using the USEPA method (US Environmental Protection Agency 2013), and compared with chronic data (EC10 or no-observed-effect concentration [NOEC] values) for 25 other species belonging to 12 taxa, Velesunio spp. were more sensitive than 8 of 16 temperate species, and more sensitive than 7 of 9 tropical species (Figure 4 and Supplemental Data, Table S10).
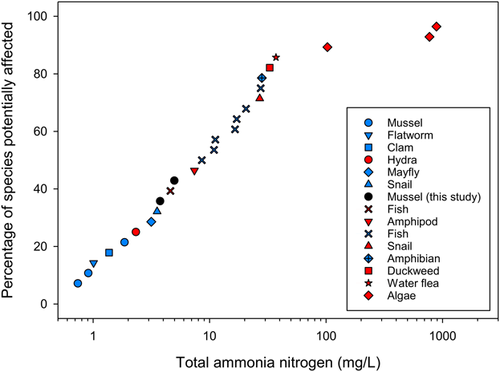
Using only the equations of Emerson et al. (1975) for adjusting data, Velesunio spp. were found to be more sensitive than all 16 temperate species, and all except 1 tropical species (H. viridissima; Supplemental Data, Figure S11 and Table S10).
DISCUSSION
Toxicity test optimization
Test volume and vessels
A wide range of test volumes have been used in juvenile mussel studies (ASTM International 2006), varying from 3.5 mL/replicate in static conditions to 1200 mL/replicate in flow-through conditions. Similarly, many different test vessels have been trialed, including Petri dishes, 12-well plates, crystallizing dishes, and beakers (ASTM International 2006). In the present study, the optimal test volume and vessel for the 14-d test protocol using 10 juveniles/vessel was 100 mL in a 175-mL plastic jar. No significant difference was observed between 100- and 150-mL volumes (p = 0.35), which further supported the use of 100-mL volumes. The water volume and depth appeared to be important factors influencing mussel growth, with the lowest growth rate observed in shallow, small-volume Petri dishes.
Addition of sediment
The addition of sediment proved necessary for achieving optimal mussel growth. Juvenile mussels feed on a mixture of fine particulate organic matter including detritus, bacteria, and algae (Cope et al. 2008). Sediment as a suspension or layer has been reported to support juvenile mussel growth and survival (Gatenby et al. 1996), by facilitating the collection of food using pedal-feeding (Gatenby et al. 1996; Hua et al. 2013), providing a nutritional resource, and facilitating digestion and feeding orientation (Jones et al. 2005). When testing with Velesunio spp., Humphrey (1987a, 1987b) applied the early North American culturing methods of Hudson and Isom (1984), who found that the addition of a silt suspension in test vessels increased growth and survival of postexcysted juveniles.
Different sediment particle sizes were not trialed in the present study, because the size of ≤63 μm proved to be appropriate for producing the required turbidity and subsequent high mussel survival and growth. Fine sediments of different size classes (45, 45–63, and 63–125 μm) and concentrations (0–10 g/L) have been shown to have no negative effects on mussel filtration (Lummer et al. 2016). Growth of Lampsilis siliquoidea (Unionidae) has been positively related to volatile solids, total phosphorus and particular fatty acids in sediment, and water particle size fractions <32 μm, and negatively related to fatty acids in the >63-μm fraction (Bartsch et al. 2017).
Studies have also shown that using sediment or detritus in combination with algal food produces higher growth and survival rates for juvenile mussels (Gatenby et al. 1996, 1997; Eybe et al. 2013). Gatenby et al. (1996) observed more algae in the juvenile gut of the rainbow mussel (Villosa iris) when they were reared on sediment and algae than those reared on algae alone. They concluded that fine sediment provided nutritional value and facilitated collection of food materials by pedal-feeding juvenile mussels, and suggested that fine sediment may aid in the digestion of algal cells by acting as an internal grinding substrate.
Although ammonia does not bind to sediment, using large amounts of sediment in ammonia toxicity tests can be challenging because of the tendency for partitioned ammonia to diffuse from sediment porewater into the overlying water. This may increase pH levels or cause a pH gradient in overlying water (Newton and Bartsch 2007). However, the small amount of sediment added to treatments during this study produced only a thin covering on the bottom of test vessels after settling, minimizing the potential for pH gradients. Wang et al. (2011) observed similar concentration–response relationships to ammonia in treatments with or without sediment, despite higher growth rates and survival in sediment treatments, indicating that sediment did not substantially influence mussel sensitivity to ammonia. Minimizing the amount of sediment used in the toxicity test would be more important if the protocol is used to assess metals, due to the potential of metals to bind to the sediment. Sediment-bound metals may become biologically unavailable to the mussels, reducing toxicity (Costello and Burton 2014; Cardoso-Silva et al. 2016).
Feeding
The most appropriate algal diet for juvenile Velesunio spp. during toxicity testing was Chlorella sp. The improved growth and survival of juveniles using the Chlorella sp. diet, compared with the mixture of algae present in the shellfish diet, may be due to the smaller cell size and nutritional content of the juveniles. The diet of V. angasi was previously investigated by Humphrey and Simpson (1985), who observed that V. angasi in Magela Creek were phytophagous and detritivorous filter feeders, with their main food comprising unicellular algae and organic detritus. Unicellular algae were considered to be the main usable energy source rather than organic detritus or diatoms. Laboratory-cultured green algae, and commercially available (nonviable) marine algae such as in the shellfish diet, have been used successfully by other researchers for temperate freshwater juvenile mussel propagation and toxicity testing (Gatenby et al. 1996; Ingersoll et al. 2007; Wang et al. 2007b, 2007c; Eybe et al. 2013).
The increased growth measured in treatments with FFV may have been due to its greater nutritional value but may also have been a result of the reduced survival and therefore less competition for food for remaining mussels. The reduced survival was likely to be a result of the FFV introducing unknown bacteria or fungi, which grew on mussel shells and agglomerated sediment particles (Supplemental Data, Figure S6). This growth around the valve opening may have affected their ability to pedal-feed. Jones et al. (2005) recommended careful preparation of sediment before use, including autoclaving to kill invertebrate predators that may induce mortality by consuming or encasing juvenile mussels. The sediment used in the present study was heated to 60 °C but was not autoclaved, which may have contributed to the fungal growth observed throughout test development. However, fungal growth was not observed during toxicity tests, which used the same sediment but did not use FFV or the shellfish diet mix. Sterilization of the sediment was therefore not investigated further, but could be a consideration for future studies to reduce the chances of fungal or bacterial infection.
Endpoints
Growth rate was the most sensitive and reliable endpoint we investigated. However, one complication we noted was that the smaller mussels were often killed following exposure to the higher ammonia concentrations, resulting in a higher average length of the larger surviving organisms. This effect was minimized by selecting more suitable sublethal concentration ranges. Dry weight was a problematic endpoint because it was difficult to weigh the very small size mussels accurately (∼280 μm starting length, ∼21 μg wt), and the sediment and algae adhered to mussel shells, confounding the weights. Wang et al. (2011) used dry weight as an additional endpoint to shell growth and survival during 28-d toxicity tests with older juveniles (2-mo-old). The same chronic effect concentration (geometric mean of lowest-observed-effect concentration and NOEC values) of 0.36 mg/L was estimated for growth and dry weight, indicating that either could be used as an endpoint (Wang et al. 2011). However, in that study, dry weight data were too variable in their responses to be used to calculate an EC10 or EC20 for use in the USEPA (US Environmental Protection Agency 2013) ammonia criteria update (Wang et al. 2011).
Measuring 14-d survival in the present study was useful as a comparison with other studies, and for determining the magnitude of ammonia concentrations that caused a lethal response. Survival as an endpoint has been compared with growth during 28-d chronic ammonia tests on juvenile mussels by Wang et al. (2007b). Chronic values for growth were equal to or lower than survival, indicating that growth rate was the more sensitive endpoint.
Toxicity of ammonia
Velesunio angasi and Velesunio sp. showed high chronic sensitivity to ammonia, in comparison with species used in other studies. When sensitivities between mussel species were compared, Velesunio sp. from Gulungul Creek were less sensitive than V. angasi based on EC50, EC20, and EC10 values. The V. angasi from Lake Bennett were slightly more sensitive than V. angasi from Sandy Billabong based on EC50 and EC20 values, but of similar sensitivity based on EC10 values. The higher sensitivity of Lake Bennett V. angasi was previously observed in acute ammonia toxicity tests with glochidia (Kleinhenz et al. 2018). Differences in water quality between Lake Bennett waters and the Magela Creek diluent water were suggested as a possible contributing factor.
The semi-urbanized environment of Lake Bennett may also have affected mussel sensitivity, through increased risk of pollution from domestic wastewater, livestock, or recreational boating activities. However, sensitivity differences between the 2 different species and 3 different sites did not vary to the degree found in other studies using multiple mussel species. In a recent multispecies study with temperate juvenile mussels, differences in sensitivity among 5 species tested with 10 chemicals (including ammonia) were assessed using acute 96-h tests (Wang et al. 2017). The EC50 values for all chemicals varied by factors of ≤2 to 12, with ammonia tests varying by a factor of ≤5 (1.5–8.0 mg/L TAN), which was considered low variability (Wang et al. 2017). The differences in chronic toxicity estimates from the present study were much lower by comparison, varying at the most by only a factor of 1.6 among all 3 EC50 values (7.0–11.3 mg/L). The small differences between EC50 values from the present study suggest that sensitivity between the 2 different local species was relatively comparable, and that the influence of genetic differences on sensitivity was negligible.
The physicochemistry of the diluent water (Magela Creek water) is likely to be a contributing factor in the high sensitivity of Velesunio spp. to ammonia. Velesunio spp. glochidia demonstrated high sensitivity to acute ammonia exposures in a previous study using Magela Creek water as the diluent water (Kleinhenz et al. 2018), and other studies have reported increased ammonia sensitivity for other species in similar waters of low ionic strength and low hardness (Ankley et al. 1995; Souza-Bastos et al. 2017). A recent study by Mooney et al. (2019) assessed chronic ammonia toxicity to 6 local species from the Alligator Rivers Region. As with the present study, all species were tested in waters of low ionic strength, and were consistently more sensitive to ammonia compared with international data for species from the same phylogenetic group, and other tropical species. The low ionic strength was hypothesized to increase species’ sensitivity to ammonia due to the lack of cations with the capacity to ameliorate toxicity, rather than to any physiological differences associated with tropical climatic zones. Further research with Velesunio spp. and ammonia under varying ionic conditions may demonstrate the influence of ionic strength on ammonia toxicity in comparison with the effect of pH and temperature.
The comparison of ammonia sensitivity with species used in other chronic studies was dependent on the method used to adjust pH and temperature. Using the USEPA adjustment method (US Environmental Protection Agency 2013), Velesunio spp. were less sensitive than temperate freshwater mussels and several other temperate taxa, and were highly sensitive compared with other tropical taxa. When the equations of Emerson et al. (1975) were used, Velesunio spp. were found to be highly sensitive compared with other temperate freshwater mussels and other (nonmussel) temperate and tropical taxa.
Similarly, EC20 data comparisons with chronic ammonia data for the 3 temperate freshwater mussel species (V. iris, L. siliquoidea, and Lampsilis fasciola; Wang et al. 2007b, 2011) used in the USEPA ammonia criteria update (US Environmental Protection Agency 2013), suggested that Velesunio spp. exhibit high sensitivity to ammonia. When EC20 data (Table 3) were adjusted to pH 7 and 20 °C using the USEPA adjustment method (US Environmental Protection Agency 2013), Velesunio spp. were found to be slightly less sensitive (EC20s of 5.5–7.6 mg/L TAN; Supplemental Data, Table S11) than the highly sensitive V. iris, L. siliquoidea, and L. fasciola (adjusted EC20s of 1.4–3.5 mg/L TAN; Wang et al. 2007b; 2011), but more sensitive than 16 of the other 18 invertebrate and fish species (EC20s of 3.3–73.7 mg TAN/L; US Environmental Protection Agency 2013) used in the update. When adjusted using the equations of Emerson et al. (1975), the EC20 values of the present study (Table 3) were lower (EC20s of 1.0–1.3 mg/L TAN) than the 3 temperate mussel species (EC20s of 6.4–15.9 mg/L TAN), also indicating high sensitivity. Although comparisons can be made, there were several notable differences in test conditions, for example, the data from Wang et al. (2007b, 2011) were derived from 28-d survival tests using 2-mo-old temperate juvenile mussels in flow-through conditions. No chronic data were available for other tropical freshwater mussel species to allow us to draw further comparisons with the data obtained from the present study.
Based on the findings of Mooney et al. (2018), the Emerson et al. (1975) equations may more accurately represent values derived in test waters of low ionic strength and low pH, but these authors acknowledged that more data was needed. The USEPA model did not consider ionic strength as a variable affecting ammonia toxicity, and the data used to derive the equations was obtained across a pH range of 6 to 9, with considerable error possible at the lower end of this range (US Environmental Protection Agency 1999). Additional chronic ammonia toxicity studies with Velesunio spp. using a range of different pH values may reveal the most appropriate data adjustment method for tests conducted in these waters.
The chronic EC10 values for ammonia from the present study (2.78, 2.90, and 3.68 mg/L TAN) can be used with acute toxicity data for Velesunio spp. (median lethal concentration = 9.02 mg/L TAN; Kleinhenz et al. 2018) to calculate an acute-to-chronic ratio (ACR) of 2.9 for Velesunio spp., which is much lower than the ACRs for freshwater mussels provided by the USEPA (US Environmental Protection Agency 2013). The USEPA's ACRs range from 9.03 to 49.5, and were based on acute and chronic data from 4 temperate species, highlighting the variation between chronic and acute responses that can occur between different mussel species (US Environmental Protection Agency 2013). This variation demonstrates the inaccuracies in applying a single ACR value across species and that there is no substitute for actual chronic data. Reliance on limited and varying ACR values when site-specific guideline values are derived may result in under- or overprotection of species within that environment.
CONCLUSIONS
The chronic toxicity test protocol refined in the present study was shown to be effective for assessing the toxicity of ammonia to tropical freshwater mussels, and showed that Velesunio spp. were highly sensitive to ammonia compared with available chronic data for other invertebrate and fish species. Velesunio spp. were also similarly sensitive to ammonia compared with limited chronic data for temperate mussel species. The test produced high-quality chronic toxicity estimates for ammonia, which are suitable for deriving water quality guideline values using current best-practice methods in ecotoxicology. The test protocol can be used to assess the toxicity of other contaminants to tropical freshwater mussels, and the data obtained may be incorporated in the revision of national and/or international water quality guideline values.
Supplemental Data
The Supplemental Data are available on the Wiley Online Library at DOI: 10.1002/etc.4370.
Acknowledgment
The authors thank the staff of Supervising Scientist Branch (Department of the Environment and Energy, Canberra, ACT, Australia) for their support and assistance with field collections. The primary author was in receipt of an Australian Government Research Training Program scholarship administered through Royal Melbourne Institute of Technology University for the duration of the present study. Ethical approval to use host fish was obtained from Charles Darwin University's Animal Ethics Committee (project A15018). Field collections on public land were conducted under special permit 2015-2016/S17/3380 issued by the Fisheries Division, Department of Primary Industry and Resources (Berrimah, NT, Australia) and permit 57834 issued by the Parks and Wildlife Commission of the Northern Territory (Darwin, NT, Australia). Field collections within the Alligator Rivers Region were permitted under project RES-2015-025 and PAN-eriss protocols 2015-18, and permission was granted by the landholder for field collections at Lake Bennett.
Data Accessibility
Data, associated metadata, and calculation tools are available from the corresponding author ([email protected]).




