Different organophosphate flame retardant and metabolite concentrations in urine from male and female university students in Beijing and an assessment of exposure via indoor dust
Abstract
Organophosphate flame retardants (OPFRs) have been found in human samples and associated with adverse health effects. In the present study, OPFR and dialkyl and diaryl phosphate (DAP) concentrations in human urine were determined and differences in the concentrations in urine from males and females were investigated. Urine samples from 22 male and 26 female university students, paired dust samples from the dormitories (13 each for males and females), and 10 dust samples from university teaching buildings were analyzed. The tri-o-cresyl phosphate (TOCP), tri-p-cresyl phosphate (TPCP), and tris(2-chloroisopropyl)phosphate (TCIPP) concentrations were significantly higher (p = 0.049, 0.023, and 0.027, respectively) in urine from the female students than in urine from the male students. Similar differences were found between males and females in terms of OPFR exposure and OPFR concentrations in urine for three-fourths of the OPFRs. Questionnaire answers and calculations indicated that disparities in OPFR concentrations in urine were mainly caused by females spending much more time than males in dormitories. Organophosphate flame retardants may pose degrees of health risk similar to those of polybrominated diphenyl ethers (PBDEs), and this must be considered when making decisions about controlling flame retardants. We are not aware of any previous studies that simultaneously monitor OPFRs and DAPs in human urine in China. Environ Toxicol Chem 2019;38:760–768. © 2019 SETAC
INTRODUCTION
Restrictions on polybrominated diphenyl ethers (PBDEs) and the excellent flame retardant properties and plasticities of organophosphate esters have led to organophosphate flame retardants (OPFRs) being used increasingly as additive flame retardants in polyurethane foam, textiles, upholstery, and varnish (Li et al. 2015; Ma et al. 2016). The wide use of OPFRs in consumer products has caused concern about the toxicities of OPFRs. Some OPFRs have been found to be carcinogenic, mutagenic, and neurotoxic; other OPFRs may be developmental and reproductive toxins (Behl et al. 2015; Hendriks and Westerink 2015; Vykoukalová et al. 2017).
Research has shown that OPFRs have different concentrations in men and women. Qiao et al. (2016) found significantly higher total OPFR, triphenyl phosphate (TPHP), 2-ethylhexyl diphenyl phosphate, and tri(2-ethylhexyl)phosphate concentrations in female hair than in male hair. Diverse OPFR and dialkyl and diaryl phosphate (DAP) concentrations have been observed in males and females. Fromme et al. (2014) discovered significantly higher (p = 0.016) di-(2-butoxyethyl)phosphate concentrations in urine samples from males than in urine samples from females. It is imperative to investigate what causes these differences and the possible effect on women and men.
Numerous studies of OPFRs in human samples such as serum and milk have been performed (Sundkvist et al. 2010; Kim et al. 2014; Ma et al. 2016). Recently, quantified DAPs in urine have been identified as biomarkers to assess human exposure to OPFRs. However, DAP data are limited in helping our understanding of the quantities and possible ways in which OPFR degrades in the human body. Some OPFRs are poorly metabolized and form diesters and oxidative dehalogenation products such as tris(2-chloroethyl)phosphate (TCEP; Van den Eede et al. 2013a). Certain metabolites of organophosphorus pesticides are the same as those of OPFRs. For example, diethyl phosphate (DEP) is not only the metabolite of chlorpyrifos and diazinon but also of triethyl phosphate (TEP; Takigami et al. 2009; Naeher et al. 2010). Simultaneously monitoring OPFRs and DAPs provides valuable information for understanding the accumulation of OPFRs and also delivers more comprehensive insight into the metabolism of OPFRs in the human body. We are not aware of any previous studies that concurrently monitor OPFRs and DAPs in human urine in China. Systematic review of OPFRs and DAPs in urine could allow for more all-inclusive studies of human exposure to OPFRs to be performed than have previously been possible.
Relationships between chemical concentrations in indoor environments and human samples have been studied, and indoor environments have been shown to be important sources of OPFRs to human beings (Takigami et al. 2009; Fromme et al. 2014; Cequier et al. 2015). No distinction has previously been made between males and females when calculating the risks posed by exposure to OPFRs. It is critical to establish whether differences among the patterns of exposure to indoor dust for males and females cause males and females to be exposed to dissimilar amounts of OPFRs. Total daily OPFR intakes have been estimated from DAP concentrations in urine (Fromme et al. 2014; Chen et al. 2018); however, the factors affecting human intakes of OPFRs are still poorly understood.
A correlation has previously been detected between human exposure to PBDEs and exposure to indoor dust (Zhu et al. 2015; Li et al. 2018). Restrictions on PBDE utilization have led to increasing use of OPFR products (Ma et al. 2016). It is vital that the risks posed by exposure to PBDEs and OPFRs are compared.
The aims of the present study were to: 1) investigate and estimate exposure of male and female students at Minzu University of China, in Beijing, to OPFRs by analyzing urine samples, 2) explore the role played by exposure to indoor dust in differences between OPFR concentrations in the urine samples provided by the male and female students, and 3) compare the risks posed by exposure to PBDEs and OPFRs.
MATERIALS AND METHODS
Sampling
Urine and dust samples were gathered from Minzu University of China. Urine samples were provided by 48 healthy students (22 males and 26 females). Each student supplied a 50.0-mL morning spot urine sample from the midstream flow of urine collected when the student first urinated after waking up in the morning. A 1.0-g dust sample was gathered from each of 26 dormitories (13 used by males and 13 used by females). Each dormitory dust sample was paired with the urine sample(s) provided by the 1 or 2 students using that dormitory. Each volunteer completed a lifestyle questionnaire. If 2 people sharing a dormitory gave different answers about their dormitory, the average of the answers was used. The questionnaire and the results are shown in Supplemental Data, Tables S1 and S2, respectively.
The dust samples were gathered on the day before urine sample collection, in consideration of the short half-lives of OPFRs. Dust samples from female dormitory 1 and male dormitory 1 were labeled FD1 and MD1, respectively, and the other dust samples were labeled in the same way. The urine samples supplied by females and paired to dust sample FD1 were labeled FU1-1 and FU1-2; the urine samples provided by males and paired to dust sample MD1 were labeled MU1-1 and MU1-2; and the other urine samples were labeled similarly. Dust samples were also collected from 3 classrooms on each of the 10 floors of the main teaching buildings (buildings no. 1 and no. 2) that are used for classes and private study. The samples collected from a floor were combined, giving 10 pooled samples (1 for each floor). The classroom dust samples were labeled TB1-1 (teaching building no. 1, first floor), TB2-1 (teaching building no. 2, first floor), and so forth. Air purifiers were not present in any of the classrooms. Each dust sample was gathered from the floor and furniture using a vacuum cleaner containing a dust-collection unit that was easily removed and emptied. A new filter was used for each sample. Each dust sample was wrapped in aluminum foil and sealed in a plastic bag. Each urine sample was stored in a brown, glass bottle to prevent the compounds of interest from being photodegraded. The samples, collected in December 2016 and January 2017, were stored at −5 °C during transport to the laboratory and then at −20 °C in the laboratory.
Chemicals
Pesticide-grade acetone, n-hexane, ethyl acetate, methanol, toluene, and acetonitrile were obtained from J.T. Baker. Hydrochloric acid (37%) and potassium carbonate were purchased from Sinopharm Chemical Reagent. 2,3,4,5,6-Pentafluorobenzyl bromide was acquired from Aldrich Chemical. High-purity nitrogen was bought from Cheng Wei Xin. Ultra-pure water was produced using a Milli-Q system (EMD Millipore).
The derivatization reagent was 5 g pentafluorobenzyl bromide dissolved in 6 mL acetonitrile. Sep-Pak Vac 6 cc C18 cartridges (500 mg, 3 mL) were purchased from Waters. Bond Elut PSA (an anion exchanger) cartridges (500 mg, 3 mL) and Bond Elut FL (Florisil) cartridges (500 mg, 3 mL) were obtained from Varian. ISOLUTE ENV+ cartridges (100 mg, 3 mL) were acquired from Biotage. Standards of the individual OPFRs (unlabeled) shown in Table 1 were bought from AccuStandard, and TCEP-d12 and TPHP-d15 were purchased from Cambridge Isotope Laboratories. Standards of the individual DAPs (unlabeled) di-(2-chloroethyl)phosphate (DCEP)-d8 and diphenyl phosphate (DPHP)-d10 in Table 1 were obtained from TRC.
| No. | Name | Acronym | CAS | log Kow | BCF | RfD (ng/(kg bw d)) | Urinary metabolites analyzed | |
|---|---|---|---|---|---|---|---|---|
| 1 | Tris(2-chloroethyl)phosphate | TCEP | 114-96-8 | 1.44 | 1.37 | 2200 | Di-(2-chloroethyl)phosphate (DCEP) | |
| 2 | Tris(2-chloroisopropyl)phosphate | TCIPP | 13674-84-5 | 2.71 | 42.4 | 8000 | Di-(1-chloroisopropyl)phosphate (DCIPP) | |
| 3 | Tris(1,3-dichloro-2-propyl)phosphate | TDCIPP | 13674-87-8 | 2.35 | 13.5 | 1500 | Di-(1,3-dichloro-2-propyl)phosphate (BDCIPP) | |
| 4 | Tri-n-butyl phosphate | TNBP | 126-73-8 | 4.00 | 1.03 × 103 | 2400 | Dibutyl phosphate (DNBP) | |
| 5 | Triphenyl phosphate | TPHP | 115-86-6 | 4.59 | 113 | 7000 | Diphenyl phosphate (DPHP) | |
| 6 | Triethyl phosphate | TEP | 78-40-0 | 0.80 | 3.88 | Diethyl phosphate (DEP) | ||
| 7 | Tri-o-cresyl phosphate | TOCP | TCP | 78-30-8 | 5.11 | 8.56 × 103 | 1300 | Di-o-cresyl phosphate (DOCP) |
| 8 | Tri-m-cresyl phosphate | TMCP | 563-04-2 | 5.11 | 8.56 × 103 | Di-m-cresyl phosphate (DMCP) | ||
| 9 | Tri-p-cresyl phosphate | TPCP | 1330-78-5 | 5.11 | 8.56 × 103 | Di-p-cresyl phosphate (DPCP) | ||
- CAS = Chemical Abstracts Service; BCF = bioconcentration factor; RfD = reference dose; bw = body weight.
Sample preparation
Preparation of dust samples
A 25-mg aliquot of a dust sample was spiked with 50 ng TCEP-d12 and 50 ng TPHP-d15; then 2.5 mL of a 1:1, v/v mixture of n-hexane:acetone were added. The mixture was vortexed twice for 1 min and ultrasonicated for 20 min in a water bath at 38 °C. The solvent was removed and the dust sample was extracted 2 times more utilizing the same procedure. The 3 extracts were combined and evaporated to 500 μL under a gentle stream of nitrogen.
Preparation of urine samples. Preparation of urine for OPFR analysis
A urine sample was thawed and shaken, and a 10-mL aliquot was then transferred to a 15-mL tube. The sample was spiked with 10 ng TCEP-d12 and 10 ng TPHP-d15 and vortexed. A Sep-Pak Vac 6 cc C18 cartridge was conditioned by passing through 5 mL ethyl acetate, 5 mL methanol, and 5 mL ultra-pure water. The urine sample was loaded onto the cartridge at a flow rate of approximately 10 mL/min. The analytes were eluted with 10 mL ethyl acetate at a flow rate of approximately 10 mL/min and the eluate was collected in a 15-mL tube. The extract was evaporated almost to dryness and re-dissolved in 100 μL toluene. The extract was then transferred to a micro-insert for analysis. The cleanup procedure has been described previously (Ma 2017).
Preparation of urine for DAP analysis
A urine sample was thawed and shaken, and a 5-mL aliquot was then transferred to a 15-mL tube. The sample was spiked with 10 ng DCEP-d8 and 10 ng DPHP-d10, acidified by adding 125 μL 3 M HCl, and vortexed. An ENV+ cartridge was conditioned by passing through 4 mL acetonitrile, and equilibrated by passing through 4 mL 0.1 M HCl. The urine sample was loaded onto the cartridge at a flow rate of approximately 0.25 mL/min. The cartridge was vacuum dried for 5 min, washed with 1 mL 0.1 M HCl, and vacuum dried for 10 min to remove any remaining water. The analytes were eluted with 7 mL acetonitrile at a flow rate of approximately 0.25 mL/min, and the eluate was collected in a 10-mL tube containing 10 mg potassium carbonate to neutralize any remaining acid. The eluate was evaporated to dryness at 50 °C under a gentle stream of nitrogen.
The residue was dissolved in 1 mL acetonitrile; then 10 mg potassium carbonate and 100 μL pentafluorobenzyl bromide derivatization reagent were added. The vial was capped, vortexed, and incubated at 60 °C for 16 h to allow derivatization to proceed to obtain pentafluorobenzyl derivates of the analytes. The sample was cooled to room temperature and vortexed; it was then evaporated to dryness under a gentle stream of nitrogen and the residue was dissolved in 5 mL hexane. The sample was vortexed and cleaned up by using 2 more solid-phase extraction cartridges.
A Bond Elut PSA cartridge and a Bond Elut FL cartridge were connected using an adapter, with the Bond Elut PSA cartridge at the top and the Bond Elut FL cartridge at the bottom. The cartridge was conditioned by passing through 2.5 mL hexane, and the sample containing the DAP derivatives and potassium carbonate residue was added. A gentle vacuum was applied if necessary. The cartridge was washed with 5 mL hexane, and the analytes were eluted with 7 mL of a 30:70, v/v mixture of acetone:hexane. The eluate was concentrated to 1 mL and 200 μL toluene were added to act as a keeper solvent. The sample was evaporated to 150 μL and transferred to a micro-insert. The cleanup procedure was described earlier (Schindler et al., 2009a; Van den Eede et al. 2013b). The samples were analyzed by gas chromatography–tandem mass spectrometry.
Instrumental analysis
The OPFR and DAP concentrations in the extracts were determined using a Trace 1310 gas chromatograph coupled to a TSQ 8000 Evo tandem mass spectrometer (Thermo Fisher Scientific). The gas chromatograph was fitted with a TG-5HT column (15-m length, 0.25-mm inner diameter, and 0.10-mm film thickness; Thermo Fisher Scientific). The oven temperature for OPFR analysis started at 100 °C held for 2 min and increased at 20 °C/min to 300 °C held for 5 min. The oven temperature for DAP analysis started at 80 °C held for 0.5 min, increased at 25 °C/min to 110 °C held for 1 min, then increased at 25 °C/min to 270 °C and at 20 °C/min to 290 °C both held for 2 min. The final increase at 20 °C/min to 300 °C was held for 8 min. The injector temperatures for the OPFR and DAP analyses were 280 and 260 °C, respectively. The carrier gas was helium, and the flow rates for the OPFR and DAP analyses were 1.0 and 1.5 mL/min, respectively. A 1-μL aliquot of a sample was injected in splitless mode for both OPFR and DAP analyses. The splitless injection time was 1.2 min for DAP analysis. The tandem mass spectrometer was operated in electron impact ionization mode and selective reaction monitoring mode. The ion source and the transfer line were both at 280 °C for OPFR analysis and 260 °C for DAP analysis. All the DAP analytes eluted in < 19.5 min. The instrumental analysis procedure has been described previously (Schindler et al. 2009a, 2009b; Ma et al. 2016). Van den Eede et al. (2013b) has done the research to compare DAP analysis employed by liquid chromatography−electrospray ionization(−)tandem mass spectrometry and gas chromatography–tandem mass spectrometry. Use of the latter method was recommended for analysis of chlorinated DAPs, and was also acceptable for dibutyl phosphate (DNBP) and DPHP analyses. The selective reaction monitoring parameters (masses reacted, product masses, and collision energies) are discussed in detail in Supplemental Data, Table S3.
Quality assurance and quality control
The detection and quantification limits have been defined as the concentrations giving signal-to-noise ratios of 3 and 10, respectively. The OPFR and DAP detection limits were 0.02 to 0.5 pg and 0.05 to 0.5 pg, respectively. The OPFR and DAP quantification limits were 0.1 to 1.5 pg and 0.1 to 2 pg, respectively. The TCEP-d12, TPHP-d15, DCEP-d8, and DPHP-d10 recoveries were 68 to 102%, 84 to 110%, 57 to 97%, and 78 to 123%, respectively. Every sample batch included a method blank. The analyte concentrations in the blank samples were very low (<10% of the concentrations in the samples) and the signal-to-noise ratios were < 10; thus the concentrations in the samples were not blank-corrected. Wang et al. (2014b), Ma and colleagues (2016), Yuan and associates (2016), and Ma et al. (2017) have previously documented the method.
Statistical analyses
In the present study, the concentrations of urinary OPFRs and DAPs were not normalized to specific gravity, which was usually taken into consideration in earlier research. The urine that we used was collected from healthy subjects, with specific gravity ranging from 1.005 to 1.030 (Qin 1984). This did not affect the aims of the present study to any significant extent. For the purposes of statistical analyses, in calculating the disparities between men and women, we normalized the concentrations of all the women (higher concentrations) to a specific gravity of 1.030 and of all the men (lower concentrations) to a specific gravity of 1.005.
Statistical analyses were performed using SPSS software, Ver 22.0 (IBM). Disparities among concentrations in different groups of samples were identified utilizing independent sample t tests. Bivariate Spearman correlation coefficients were employed to pinpoint statistically significant correlations among the concentrations of diverse analytes.
RESULTS AND DISCUSSION
OPFR and DAP concentrations
Concentrations in indoor dust
In Supplemental Data, Table S4, all the OPFRs except tri-o-cresyl phosphate (TOCP) were detected in all of the dust samples. Some OPFR concentrations of indoor dust samples were higher than the BDE-209 in our earlier article (Li et al. 2018). This suggests that OPFRs are widely found in dormitories and classrooms at Minzu University and might pose exposure risks to the people using these rooms. The total OPFR concentrations in the classroom and dormitory dust samples were 4250 to 19 800 ng/g and 1080 to 12 600 ng/g, respectively. The mean total OPFR concentration in the classroom dust samples was 7810 ng/g, and the mean total OPFR concentration in the dormitory dust samples was significantly lower (p = 0.019), at 4720 ng/g.
The predominant OPFRs in the dust samples were TPHP, TCEP, tris(2-chloroisopropyl)phosphate (TCIPP), and tris(1,3-dichloro-2-propyl)phosphate (TDCIPP), as revealed in a previous study of samples collected in Canada, the Czech Republic, and the United States (Vykoukalová et al. 2017). Similar to the present study, TCIPP, TCEP, and TPHP were the 3 most predominant OPFRs in the dust samples gathered from dormitories in Beijing (Cao et al. 2019). Tris(2-chloroisopropyl)phosphate and TCEP, along with tris (2-butoxyethyl) phosphate, were the 3 most abundant OPFRs in dust samples collected from homes, offices, and daycare centers in Beijing (Wu et al. 2016). The total OPFR concentrations in the dust samples were comparable with the concentrations found in dust in houses in Canada (206–9530 ng/g) and the United States (not detected–8940 ng/g) and higher than the concentrations found in dust in houses in the Czech Republic (56.0–1220 ng/g; Vykoukalová et al. 2017). However, the concentrations were much lower than those detected in dust in apartments (14 400–134 000 ng/g), houses (11 100–363 000 ng/g), primary schools (10 100–4 690 000 ng/g), and offices (34 500–1 980 000 ng/g) in Araraquara City, São Paulo State, Brazil (Cristale et al. 2018). The OPFR concentrations in our dust samples were lower than those in dust from a college dormitory in Guangzhou, southern China (He et al. 2015) and much lower than those in dust from 3 offices in another part of Beijing (11 100–212 000 ng/g; Cao et al. 2014).
Concentrations in urine samples―OPFRs in urine
All the OPFRs were found in the urine samples. Triethyl phosphate, TCIPP, and TDCIPP were present in all the samples but the TOCP, tri-m-cresyl phosphate, and TCEP detection frequencies were relatively low (6.3–14.6%). The total OPFR concentrations in the urine samples were 0.07 to 5.66 μg/L. The total chlorinated OPFR concentrations (0.04–0.56 μg/L) were significantly higher (p < 0.01) than the total unchlorinated OPFR concentrations (0.03–5.25 μg/L). The OPFR concentrations in the urine samples are shown in detail in Supplemental Data, Table S5.
Organophosphate flame retardant metabolites have been frequently found in human urine samples; however, parent OPFR concentrations in urine have been identified in only a few studies. He et al. (2018) observed OPFR concentrations in urine samples from young children similar to those in the present study. In the research of He and colleagues from 2018, TDCIPP and TCIPP were the 2 most frequently pinpointed OPFRs, with detection frequencies of 63 and 35%, respectively. The TDCIPP and TCIPP were the OPFRs most often observed in that study and in the present study. One hypothesis proposed was that both TDCIPP and TCIPP are used widely and have relatively low log KOW values (see Table 1). Thus they would be relatively soluble in water. However, additional evidence would be beneficial. The properties of TCEP (chlorination and relatively low log KOW values; Table 1), as well as its popular use, are almost identical with those of TDCIPP and TCIPP, whereas the detection frequency of TCEP was 14.6%—much lower than that of TDCIPP and TCIPP. A low detection frequency of TCEP (4–13%) was also determined by Van den Eede et al. (2015), whereas the detection frequency of DCEP, the metabolite of TCEP, was 100% in the present study. Different metabolic efficiency and speed of OPFRs may also be the possible explanation for recurring observations of TDCIPP and TCIPP. On the other hand, the high detection rates for TDCIPP and TCIPP could also be caused by their relatively long half-lives in human beings. Despite the scarce in vivo research on these half-lives, in vitro studies suggest low clearance of TDCIPP (46%) and TCIPP (33%) incubating with human liver microsomes for 24 h (Van den Eede et al. 2013a).
DAPs in urine
Dialkyl and diaryl phosphate concentrations—except for di-(1-chloroisopropyl)phosphate (DCIPP) and di-(1,3-dichloro-2-propyl)phosphate (BDCIPP)—were generally higher (Supplemental Data, Table S6) than the OPFR concentrations. This suggests that most of the OPFRs were metabolized within the body. The DAP detection rates (excluding BDCIPP) were high (41.3–100%), which indicates that OPFRs are ubiquitous in the tissues of people living in the study area. As mentioned, the frequency of detection for both parent substances, TDCIPP and TCIPP, respectively, was 100%; however, BDCIPP and DCIPP rates of detection were relatively low, at 2.17 and 66.7%, respectively. In a study of primary school children in Guangzhou and Shenzhen, China, DCIPP and BDCIPP were also identified less often than the other DAPs (Chen et al. 2018). This may be because diester metabolites are possibly not excreted effectively in urine (Ding 2016).
The total DAP concentrations in the urine samples were 5.80 to 104 μg/L (mean 26.5 μg/L; median 17.5 μg/L). Similar concentrations (0.23–74.3 μg/L) were found in urine from children living in Guangzhou (Chen et al. 2018); slightly lower concentrations (<0.15–24.9 μg/L) were observed in urine from people in Bavaria, Berlin, and North Rhine–Westphalia in Germany (Fromme et al. 2014); and higher concentrations (0.29–414 μg/L) were shown in urine from individuals in Shenzhen (Chen et al. 2018). Diphenyl phosphate was the dominant DAP in the present study urine samples, and was found at concentrations between not detected and 94.5 μg/L (mean 17.0 μg/L). Comparable results were observed in Australia by Van den Eede et al. (2015), who found DPHP concentrations of 10.2 to 225 μg/L (geometric mean 63.4 μg/L) that were markedly higher than the concentrations of bis(1-chloro-2-propyl) 1-hydroxy-2-propyl phosphate, a hydroxylated metabolite of TCIPP, which was the DAP with the second highest concentrations (mean 1.86 μg/L). This was probably because urinary DPHP can be formed within the body from many OPFRs (e.g., resorcinol bis diphenyl phosphate, bisphenol A bis diphenyl phosphate, 2-ethylhexyl diphenyl phosphate, and TPHP; Van den Eede et al. (2015); He et al. 2017). The various parent compounds of DPHP may cause its concentration to be higher than the concentrations of other DAPs. As an alternative, there may be some direct intake of DPHP from the environment. Nevertheless, further confirmation would be helpful.
Assessments of the exposure of male and female students to OPFRs
Diverse OPFR concentrations were found in urine provided by the volunteers. The total OPFR concentrations in the urine samples from males and females were 0.06 to 0.56 μg/L (mean 0.65 μg/L) and 0.07 to 2.86 μg/L (mean 1.02 μg/L), respectively. Independent sample t tests were performed, and significant differences were shown between the OPFR concentrations in the urine samples from males and females. It can be seen from Table 2 that the TOCP, tri-p-cresyl phosphate (TPCP), and TCIPP concentrations were significantly higher (p < 0.05) in the female urine than in the male urine and that the concentrations of the other OPFRs were higher (but not statistically significantly higher) in the female urine than in the male urine.
| TEP | TNBP | TPHP | TOCP | TMCP | TPCP | TCEP | TCIPP | TDCIPP | |
|---|---|---|---|---|---|---|---|---|---|
| Comparison | male < female | male < female | male < female | male < female | male < female | male < female | male < female | male < female | male < female |
| p | 0.183 | 0.534 | 0.908 | 0.049 | 0.085 | 0.023 | 0.248 | 0.027 | 0.586 |
- TEP = triethyl phosphate; TNBP = tri-n-butyl phosphate; TPHP = triphenyl phosphate; TOCP = tri-o-cresyl phosphate; TMCP = tri-m-cresyl phosphate; TPCP = tri-p-cresyl phosphate; TCEP = tris(2-chloroethyl)phosphate; TCIPP = tris(2-chloroisopropyl)phosphate; TDCIPP = tris(1,3-dichloro-2-propyl)phosphate.
- The TOCP, TPCP, and TCIPP concentrations were significantly higher (p < 0.05) in the urine from the women than in the urine from the men
We attempted to investigate the relationships between the total daily OPFR intakes and the OPFR concentrations in the urine samples. The total daily OPFR intakes were estimated using the equation 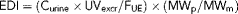 , which had been used in previous studies (Fromme et al. 2014; Chen et al. 2018). In this equation, EDI (ng/(kg body wt d)) is the estimated daily intake of an individual OPFR and Curine (mg/L) is the concentration of the metabolite in urine. The volume of urine excreted each day is UVexcr. Urine excretion volumes of 23.7 and 25.9 mL/(kg body wt d) for males and females, respectively, were utilized in an earlier study, giving 1570 mL in 24 h for males and 1485 mL in 24 h for females (Tang et al. 2015) employing body weights of 66.2 and 57.3 kg for males and females, respectively, as suggested by the National Health Commission of the People's Republic of China (2015). The molar fraction of the metabolite excreted in urine with respect to the parent compound is FUE. Data on OPFR metabolism kinetics are limited; hence the FUE of 0.18 for DNBP was used to calculate EDIs for the other OPFRs because the different OPFR metabolites have similar half-lives (Suzuki et al. 1984; Chen et al. 2018). It should be noted that the real EDIs for the other OPFR metabolites may differ from the EDIs estimated using the DNBP FUE. The molecular weights (g/mol) of the parent OPFR and the metabolite, respectively, are MWp and MWm.
, which had been used in previous studies (Fromme et al. 2014; Chen et al. 2018). In this equation, EDI (ng/(kg body wt d)) is the estimated daily intake of an individual OPFR and Curine (mg/L) is the concentration of the metabolite in urine. The volume of urine excreted each day is UVexcr. Urine excretion volumes of 23.7 and 25.9 mL/(kg body wt d) for males and females, respectively, were utilized in an earlier study, giving 1570 mL in 24 h for males and 1485 mL in 24 h for females (Tang et al. 2015) employing body weights of 66.2 and 57.3 kg for males and females, respectively, as suggested by the National Health Commission of the People's Republic of China (2015). The molar fraction of the metabolite excreted in urine with respect to the parent compound is FUE. Data on OPFR metabolism kinetics are limited; hence the FUE of 0.18 for DNBP was used to calculate EDIs for the other OPFRs because the different OPFR metabolites have similar half-lives (Suzuki et al. 1984; Chen et al. 2018). It should be noted that the real EDIs for the other OPFR metabolites may differ from the EDIs estimated using the DNBP FUE. The molecular weights (g/mol) of the parent OPFR and the metabolite, respectively, are MWp and MWm.
The reference dose for each compound was calculated. The reference doses indicated the maximum safe body burdens of the chemicals of interest, and were determined by dividing published chronic no-observable-adverse-effect-level and no-observable-effect-level values by a safety factor of 10 000, as described by Van den Eede et al. (2011). The total daily OPFR intakes for a person, measured by using the equation in the preceding paragraph, were much lower than the OPFR reference doses (Supplemental Data, Table S7).
Independent sample t tests of the total daily OPFR intakes for males and females were performed, and the outcomes are shown in Table 3. The TEP and tri-n-butyl phosphate intakes were significantly higher for females than for males. However, these results were not consistent with the differences between the OPFR concentrations found in the urine samples.
| TEP exp | TNBP exp | TPHP exp | TOCP exp | TMCP exp | TPCP exp | TCEP exp | TCIPP exp | TDCIPP exp | |
|---|---|---|---|---|---|---|---|---|---|
| Comparison | male < female | male > female | male < female | male > female | male < female | male < female | male < female | male < female | male < female |
| p | 0.001 | 0.025 | 0.993 | 0.709 | 0.215 | 0.094 | 0.757 | 0.917 | 0.363 |
- a See Table 2 abbreviations footnote.
- exp = exposure.
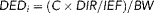 (1)
(1)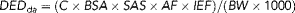 (2)
(2)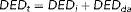 (3)
(3)In Equations 1, 2, and 3, DEDi is the daily exposure dose for an OPFR in dust (ng/kg · d); DEDda is dermal absorption of the OPFR from dust (ng/kg · d); DEDt is total exposure dose of the OPFR from dust; C is the OPFR concentration (ng/g) in indoor dust; DIR is the daily indoor dust ingestion rate (0.05 g/d); BW is body weight (60 kg); BSA is body surface area (5700 cm2); SAS is the amount of dust adhering to skin (0.07 mg/(cm2 · d)); AF is the fraction of the OPFR absorbed through the skin (0.03); and IEF is the indoor exposure fraction (0.88). The values used for the parameters were taken from a PBDE exposure assessment and handbooks published by the US Environmental Protection Agency in 2011.
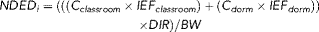 (4)
(4)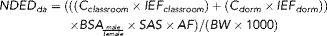 (5)
(5) (6)
(6)In Equations 4, 5, and 6, N of NDEDi and NDEDda stand for “new equation of”; Cclassroom is the mean OPFR concentration in classroom dust (ng/g); Cdorm is the OPFR concentration in dormitory dust (ng/g); IEFclassroom is the classroom exposure fraction; IEFdorm is the dormitory exposure fraction; BSAmale and BSAfemale are the body surface areas for male and female students, 5500 and 4704 cm2, respectively (Wang et al. 2008); SAS is the amount of dust adhering to skin; AF is the fraction of the OPFR absorbed through the skin; and BW is body weight.
Independent sample t tests were conducted on the daily intakes of OPFRs in dust by male and female students. Tables 4 and 5 display differences in OPFR intakes by male and female students. The disparities were more consistent when only dormitory dust data rather than both classroom and dormitory dust data were used. This contrasted with the results of past research on human exposure to PBDEs (Li et al. 2018). These outcomes may have been caused by the fact that the OPFR concentrations in the classroom and dormitory dust were not significantly different (Figure 1) and because students spend significantly more time (p < 0.001) in dormitories (10.96 h/d) than in classrooms (6.92 h/d). This would mean that the students would be exposed to a smaller amount of OPFRs in classroom dust than in dormitory dust. The female students spent a longer time in their dormitories than the male students, and this would explain the dissimilarities between the OPFR concentrations in the urine samples provided by male and female students.
| TEP exp | TNBP exp | TPHP exp | TOCP exp | TMCP exp | TPCP exp | TCEP exp | TCIPP exp | TDCIPP exp | |
|---|---|---|---|---|---|---|---|---|---|
| Comparison | male < female | male < female | male < female | male < female | male < female | male < female | male < female | male < female | male < female |
| p | 0.908 | 0.055 | 0.213 | 0.019 | 0.093 | 0.019 | 0.042 | 0.034 | 0.187 |
- a See Table 2 abbreviations footnote.
| TEP exp | TNBP exp | TPHP exp | TOCP exp | TMCP exp | TPCP exp | TCEP exp | TCIPP exp | TDCIPP exp | |
|---|---|---|---|---|---|---|---|---|---|
| Comparison | male < female | male < female | male < female | male < female | male < female | male < female | male < female | male < female | male < female |
| p | 0.696 | 0.031 | 0.115 | 0.016 | 0.015 | 0.008 | 0.010 | 0.007 | 0.166 |
- a See Table 2 abbreviations footnote.
The DNBP and DEP concentrations in the urine samples provided by the male and female students were significantly different (DNBP 1.49 and 0.38 μg/L for males and females, respectively, p = 0.017; DEP 0.30 and 0.85 μg/L for males and females, respectively, p = 0.001). Fromme et al. (2014) found a significant difference (p = 0.016) between the di-(2-butoxyethyl)phosphate concentrations in urine supplied by females and males. Van den Eede et al. (2013a) observed that P450 enzymes affect the concentrations of OPFR oxidative metabolites in human urine. There are disparities in P450 enzyme expression in males and females (Waxman and Holloway 2009). This may have been one reason for the difference in DPHP-OH concentrations in the urine samples from male and female students. Several enzymes may be involved in transforming OPFRs into DAPs and these enzymes may be expressed to varying degrees in males and females.
Hazard Quotients for Exposure to OPFRs and BDEs in Dust
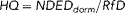 (7)
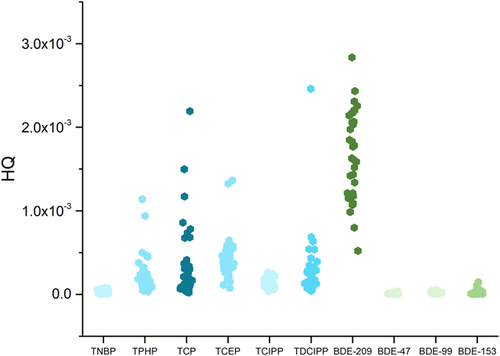
(7)In Equation 7, HQ is the hazard quotient. An HQ > 1 indicates that the compound of interest poses risks to humans and is likely to cause adverse health effects (Wang et al. 2014a). The new equation of daily exposure dose in the dormitory is NDEDdorm; and the reference dose is RfD.
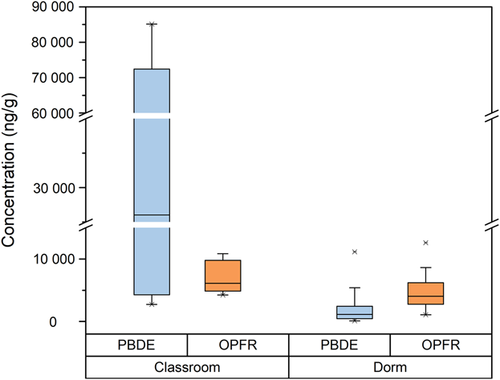
The hazard quotients for all the OPFRs and PBDEs (Figure 2) are much lower than 1. This indicates that the risks of OPFRs and PBDEs causing adverse health effects in the students are very low. The BDE-209 has the highest hazard quotient but the OPFRs have relatively high hazard quotients compared with the other PBDEs. In particular, the tricresyl phosphate hazard quotient is almost as high as the BDE-209 hazard quotient. The OPFR and PBDE concentrations found in the dust samples are shown in Figure 1. The PBDE concentrations are far higher than the OPFR concentrations in the classroom dust; however, the various compounds have different reference doses. This means that the OPFRs may pose levels of health risks to students similar to those of the PBDEs. It is imperative that this factor be taken into consideration when selecting flame retardants and developing legislation to control flame retardant use.
CONCLUSIONS
Male and female students were estimated to be exposed to different amounts of OPFRs in dormitory dust, and the OPFR concentrations in the male and female urine samples support this premise. These results indicate that differences in male and female student exposure to dormitory dust probably caused the variations in the OPFR concentrations in the male and female urine samples. We conclude that the OPFR concentrations were much higher in the female urine samples than in the male urine samples mainly because the female students spent much more time than the male students in their dormitories. The OPFRs were detected at lower concentrations than the PBDEs had been found previously in indoor dust; however, the OPFRs may pose levels of health risks similar to those of the PBDEs because both have different reference doses. These factors need to be taken into consideration when decisions are made about controlling flame retardants.
Supplemental Data
The Supplemental Data are available on the Wiley Online Library at DOI: 10.1002/etc.4365.
Acknowledgment
The present study was supported by the Collaborative Innovation Center for Ethnic Minority Development, Minzu University of China; the Chinese Fundamental Research Funds for the Central Universities (0910KYQN50 and 2015MDTD23C); and the Institution of Higher Education Innovation Talent Recruitment Program (111 Program; B08044).
Data Accessibility
Data, associated metadata, and calculation tools are accessible from the corresponding author ([email protected]).




