An analysis of lethal and sublethal interactions among type I and type II pyrethroid pesticide mixtures using standard Hyalella azteca water column toxicity tests
Abstract
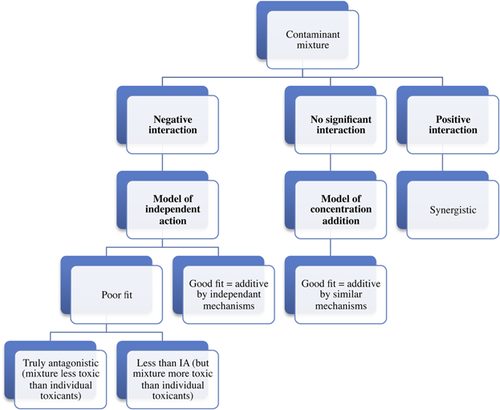
A novel 2-tiered analytical approach was used to characterize and quantify interactions between type I and type II pyrethroids in Hyalella azteca using standardized water column toxicity tests. Bifenthrin, permethrin, cyfluthrin, and lambda-cyhalothrin were tested in all possible binary combinations across 6 experiments. All mixtures were analyzed for 4-d lethality, and 2 of the 6 mixtures (permethrin–bifenthrin and permethrin–cyfluthrin) were tested for subchronic 10-d lethality and sublethal effects on swimming motility and growth. Mixtures were initially analyzed for interactions using regression analyses, and subsequently compared with the additive models of concentration addition and independent action to further characterize mixture responses. Negative interactions (antagonistic) were significant in 2 of the 6 mixtures tested, including cyfluthrin–bifenthrin and cyfluthrin–permethrin, but only on the acute 4-d lethality endpoint. In both cases mixture responses fell between the additive models of concentration addition and independent action. All other mixtures were additive across 4-d lethality, and bifenthrin–permethrin and cyfluthrin–permethrin were also additive in terms of subchronic 10-d lethality and sublethal responses. Environ Toxicol Chem 2016;35:2542–2549. © 2016 SETAC
INTRODUCTION
Pyrethroid insecticides are commonly detected at toxic concentrations in the surface water and sediment of water bodies worldwide 1-3. These pesticides are applied to urban and agricultural landscapes, and their use has been progressively increasing over the past 20 yr 4. Many pyrethroids are classified as contaminants of emerging concern because of their broad-spectrum toxicities, and their persistence and prevalence in the environment 5. These chemicals are lethal to multiple nontarget aquatic invertebrates at low parts per billion concentrations, and cause chronic and sublethal effects at fractions of those levels 6. Multiple field studies have implicated pyrethroids in environmental toxicity and ecological degradation 7, 8, although sediment concentrations of pyrethroids are low or moderate in some areas, and not always toxic to Hyalella 9, 10, possibly because of different regional use patterns. Where toxicity does occur, bifenthrin is often implicated as the major contributor in urban areas 11-14, and in other areas multiple pyrethroids (i.e., mixtures) are often the culprit 7, 11, 12. Regulations on maximum allowable concentrations in the environment are important for the ecological health of water bodies that receive urban or agricultural discharge. Fish populations worldwide are in decline 15-18, and pesticides are one of the many stressors to which fish are exposed. Although few studies have implicated direct effects of pesticides on fish mortality in nature, effects on prey abundance may have a significant impact on fish populations.
The present study investigates the interactive or additive effects of 4 type I and type II pyrethroid insecticides on an ecologically important epibenthic amphipod, H. azteca. Resident to streams and estuaries throughout the United States, the genus Hyalella includes many species that are important prey to native fish, birds, and amphibians. Hyalella azteca is certified by the US Environmental Protection Agency (USEPA) for sediment (10-d) and water reference (4-d) toxicity testing 19. Although it is particularly sensitive to pyrethroid pesticides, H. azteca is a species complex that appears to develop resistance to pyrethroids in the field 20. Using H. azteca, the present study investigated the lethal and sublethal interactions between chemicals commonly found in the aquatic environment. Concentrations tested were within environmentally detected ranges 1, 21, 22.
The primary targets of pyrethroid insecticides are voltage-gated sodium channels in the nervous system. Pyrethroids disrupt sodium channel activity by binding to channel receptors in the activated state and inhibiting their inactivation, causing nerve hyperactivity, neuromuscular dysfunction, and paralysis. The 2 types of pyrethroids are divided based on their chemical structures and consequent effects on sodium channels. Type II pyrethroids (i.e., cyfluthrin, lambda-cyhalothrin) contain a cyano group at the α-position of the 3-phenoxybenzyl alcohol moiety and cause longer lasting delays in channel inactivation, whereas type I pyrethroids (i.e., bifenthrin, permethrin) do not contain a cyano group and produce shorter acting delays 23. These classes are not absolute, and some pyrethroids exhibit intermediate properties 24.
In vitro studies have found both concentration-additive and less-than-additive interactions between pyrethroid pesticides 25-27, whereas in vivo studies have shown less than concentration-additive interactions 28, 29. The present study examines the interactions of all possible mixtures of 2 type I and 2 type II pyrethroids on mortality, swimming motility, and growth, and thus provides the most comprehensive in vivo analysis of pyrethroid mixture toxicity available to date.
MATERIALS AND METHODS
Toxicity testing
Water column toxicity testing methods (4 d and 10 d) for H. azteca were adapted from USEPA protocols for sediment toxicity testing 30, 31. Pesticides were purchased from Sigma-Aldrich and were used within 3 mo of purchase. Pesticides were prepared in methanol, and all treatments were spiked with a combination of stock and methanol as needed to attain 0.01% methanol, including a solvent control. Amphipods were purchased from Aquatic Research Organisms. All batches were acclimated to test conditions for a period of 72 to 96 h, and exposures were initiated with 10-d-old to 14-d-old H. azteca.
Each month, reference tests were conducted to evaluate the sensitivity of test organisms over time using sodium chloride (NaCl) as the reference toxicant. All control survival fell within the USEPA test acceptability criteria for water column toxicity testing (90% survival). Effect concentrations also fell within the 95% confidence interval of results across the present study period, indicating that organism sensitivity was consistent.
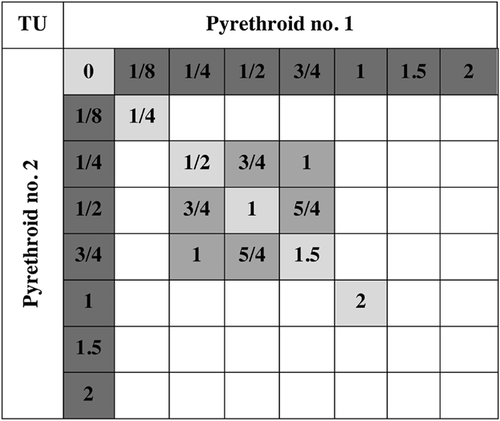
Five replicates of 10 amphipods were exposed in 100 mL of treatment water contained in 250-mL beakers. Individual pesticide toxicity tests were conducted within each of the mixture tests. Experimental treatments included 7 concentrations of each individual chemical, 6 equipotent concentrations of their mixture, and a 3 × 3 factorial design of treatments encompassing nonequipotent mixtures (Figure 1). Mortality was scored daily, and all mixtures were analyzed for acute 4-d lethality. Organisms were considered dead if all appendages, including pleopods, were immobile after repeated stimulation using a water stream. Two mixtures, permethrin–bifenthrin and permethrin–cyfluthrin, were also tested for 10-d (subchronic) lethality, as well as the sublethal responses swimming motility and growth after test termination.
Motility
Where motility was measured, individual amphipods were videotaped at the end of the 10-d exposure. Surviving organisms were transferred (along with their own treatment water) via pipette into a custom high-density polyethylene 5-welled plate, placed on a light-board, and recorded using a Panasonic black and white CCTV camera (12 V DC) in MPEG-2 format at 30 frames/s. Individual animal movement was tracked by the method of dynamic subtraction using Ethovision XT (Ver 6.1) software (Noldus Information Technology). Organisms were analyzed for the total distance traveled over a 90-s recording period (calculated as mean velocity in millimeters per second).
Weight
In experiments in which weight was measured, surviving amphipods were weighed at the end of the 10-d exposure. A subsample of amphipods was weighed on day 0 (test initiation) to confirm growth across treatments. At test termination, amphipods were pooled per replicate beaker, transferred to an aluminum weigh vessel (∼1.0 g), and desiccated at 80 °C overnight. Mean dry weight per amphipod was calculated using the tare-subtracted total weight divided by the number of H. azteca per vessel. Because of the limited scale sensitivity, only vessels containing 5 or more amphipods were included in the analyses.
Water chemistry
Water quality was measured on test initiation, at each test renewal, and at test termination. Both initial and final treatment water chemistry was recorded, including pH, specific conductance, dissolved oxygen (DO), and temperature. Dissolved oxygen was measured using a YSI model 58 meter, specific conductance was measured using a YSI model 30/10 FT, and pH was measured with a Beckman Coulter IP67 pH meter. Meters were calibrated according to the manufacturer's specifications prior to any measurements and checked for instrument drift after every 20 samples. Ammonia–nitrogen, alkalinity, and hardness were measured in control water for each experiment. Ammonia–nitrogen was tested using a Hach DR-890 portable colorimeter and a low-range Hach AmVer Ammonia Test'N Tube Reagent Set. Alkalinity and hardness were analyzed using titrimetric methods.
Water quality parameters in the present study were within the physiologically optimal ranges of H. azteca. Exposure temperatures were continuously monitored throughout the experimental period and remained within 2 °C of 23 °C. Total and un-ionized ammonia concentrations were below the detection limit in all treatments, at 0.00 mg/L to 0.05 mg/L. Control water chemistry included a hardness range of 84 mg/L to 110 mg/L as CaCO3 and an alkalinity range of 52 mg/L to 58 mg/L as CaCO3. Initial control water specific conductance was 245.1 μS/cm to 339.8 µS/cm, pH was 7.84 to 8.26, and DO was 7.8 mg/L to 9.7 mg/L. Treatment water chemistry between initial and final water measurements included an specific conductance range of 293.1 μS/cm to 378.4 μS/cm, a pH range of 7.1 to 8.27, and a DO range of 4.0 mg/L to 9.5 mg/L.
Analytical chemistry
Individual chemical dose–response curves generated within each experiment are used to calculate the expected response in the mixtures associated with treatment levels, and results from statistical analyses and model comparisons are the same irrespective of the number placed on pesticide concentration. Thus the analyses of mixture interactions based on regression statistics and model comparisons conducted in the present study are entirely based on the potency (effects) of individual chemicals and do not rely on measured concentrations. Therefore, only 1 concentration per chemical, and a control sample (blank), were tested per experiment, to confirm toxicant concentrations across experiments and ensure that spiking had occurred accurately. Analyses were run on calculated measured concentrations based on the measured-to-nominal fraction of the sample analyzed. One-liter samples for organic chemical analysis were preserved by the addition of 10 ml/L dichloromethylene immediately after exposure solutions were prepared (day 0). Samples were stored at 3 °C (±3 °C) until they were extracted within the standard holding time of 7 d. Treatment samples and the matrix control (blank) were submitted to the California Department of Fish and Game Water Pollution Control Laboratory (Rancho Cordova, CA, USA). Pyrethroids were measured by gas chromatography (Agilent 6890 Plus) with dual columns (DB5 and DB7) and dual microelectron capture detectors. Concentrations were confirmed using gas chromatography–mass spectrometry (GC–MS) or GC–tandem MS (GC–MS/MS). Detected concentrations of bifenthrin averaged 35% of nominal concentrations (standard error [SE] = 2%, n = 3), whereas concentrations of permethrin averaged 61% (SE = 9%, n = 3), and concentrations of lambda-cyhalothrin averaged 58% (SE = 8%, n = 3). In 1 of the 3 experiments containing cyfluthrin, the detected concentration was 3.75 times the expected nominal concentration, likely because of an error in spiking calculations, but otherwise concentrations of cyfluthrin averaged 58% of nominal concentrations (SE = 8%, n = 2). The fraction of recovery in the present study is consistent with tests across previous years of analysis at the Aquatic Health Program Laboratory at the University of California, Davis. 31. The results of the present study are based on concentrations calculated from the measured-to-nominal fraction of the analyzed samples. For example, if the measured concentration of permethrin was 61% of the nominal concentration in the sample analyzed for a given experiment, then nominal concentrations of permethrin were multiplied by 0.61. Thus effect concentrations (e.g., median lethal concentration [LC50] values) alone should be interpreted as estimates.
Statistical analysis
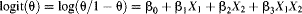 (1)
(1)
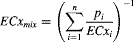 (2)
(2) (3)
(3)| Experiment/chemical | 4-d LC50 (ng/L) | 10-d LC50 (ng/L) |
|---|---|---|
| Bifenthrin–permethrin | ||
| Bifenthrin | 1.89 (1.74–2.05) | 1.48 (1.37–1.59) |
| Permethrin | 49.06 (43.98–54.14) | 35.84 (33.59–38.09) |
| Permethrin–cyfluthrin | ||
| Permethrin | 18.2 (15.33–21.08) | 15.69 (13.83–17.54) |
| Cyfluthrin | 2.80 (2.38–3.22) | 2.16 (1.93–2.38) |
| Bifenthrin–cyfluthrin | ||
| Bifenthrin | 2.70 (2.52–2.87) | – |
| Cyfluthrin | 1.99 (1.72–2.27) | – |
| Permethrin–cyhalothrin, lambda | ||
| Permethrin | 40.76 (37.20–44.32) | – |
| Cyhalothrin, lambda | 2.53 (2.37–2.69) | – |
| Bifenthrin–cyhalothrin, lambda | ||
| Bifenthrin | 2.23 (2.09–2.36) | – |
| Cyhalothrin, lambda | 1.00 (0.86–1.14) | – |
| Cyfluthrin–cyhalothrin, lambda | ||
| Cyfluthrin | 3.22 (2.92–3.52) | – |
| Cyhalothrin, lambda | 2.03 (1.86–2.22) | – |
Because of the high level of mortality by the end of each subchronic exposure, few treatments and replicates were available for sublethal testing, and were not sufficient for model comparisons. As a result, sublethal data were analyzed only using logistic regression statistics.
RESULTS
Most mixtures of pyrethroids were concentration-additive, the only exception being the acute 4-d mixtures with cyfluthrin, a type II pyrethroid, with either of the 2 type I pyrethroids bifenthrin and permethrin. All pyrethroids significantly affected motility and growth individually at sublethal concentrations, and were concentration-additive in mixtures.
Dose–response data: mortality
Average control survival in experimental tests conducted throughout the present study period was 98%. Vehicle control survival averaged 98.3% whereas nonvehicle control averaged 97.5%. Table 1 shows the LC50 values for each toxicant within each mixture experiment.
Mixture effects: mortality
Two of the 6 acute 4-d mixture exposures (permethrin-cyfluthrin and bifenthrin-cyfluthrin) negatively interacted and were additive by the independent action model, whereas neither of the subchronic 10-d mixtures interacted (Table 2 and Figures 3-6). All other pyrethroid mixtures, including combinations of the type I and type II pyrethroid pesticides, did not interact and were concentration-additive.
| Mixture | Exposure time (d) | p value | Parameter estimate | Standard error |
|---|---|---|---|---|
| Bifenthrin–permethrin | 4 | 0.108 | –0.008545 | 0.005288 |
| Bifenthrin–permethrin | 10 | 0.402 | –0.01031 | 0.01225 |
| Permethrin–cyfluthrin | 4 | 0.00594* | –0.02019 | 0.007225 |
| Permethrin–cyfluthrin | 10 | 0.118 | –0.01910 | 0.01216 |
| Bifenthrin–cyfluthrin | 4 | <0.001* | –0.2623 | 0.06025 |
| Cyfluthrin–cyhalothrin, lambda | 4 | 0.174 | 0.1897 | 0.1389 |
| Permethrin–cyhalothrin, lambda | 4 | 0.156 | –0.008694 | 0.006097 |
| Bifenthrin–cyhalothrin, lambda | 4 | 0.219 | –0.3589 | 0.2908 |
- * Statistically significant at p < 0.05.
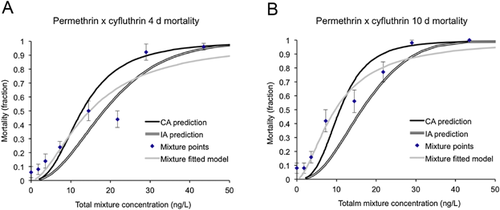
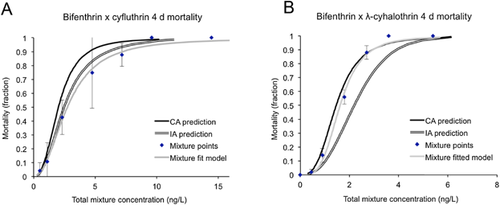
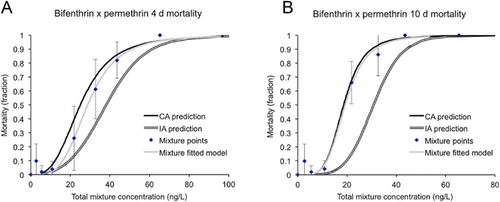
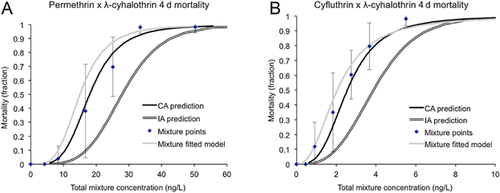
Mixture effects: motility
Swimming motility was affected in a concentration-dependent manner by all pyrethroids, and all mixtures were additive for this endpoint (Table 3). Regression statistics resulted in no significant interactions among any of the mixtures, indicating that these mixtures are concentration-additive on amphipod motility.
| Effect | p value | Parameter estimate | Standard error |
|---|---|---|---|
| Bifenthrin–permethrin | |||
| Bifenthrin | 0.00198* | −0.071454 | 0.02216 |
| Permethrin | <0.001* | −0.017029 | 0.002911 |
| Interaction | Not significant | NAa | NAa |
| Permethrin–cyfluthrin | |||
| Permethrin | <0.001* | −0.01247 | 0.00214 |
| Cyfluthrin | <0.001* | −0.19873 | 0.03416 |
| Interaction | Not significant | NAa | NAa |
- a Not applicable; interactions are omitted from the regression models where they are not significant.
- * Significant at p = 0.001 to 0.05.
Mixture effects: growth
Growth was affected in a concentration-dependent manner by all pyrethroids, and all mixtures were additive for this endpoint (Table 4). Data were analyzed using linear regression statistics, and no significant interactions were found among any of the mixtures, indicating that these mixtures are concentration-additive in their effect on growth.
| Effect | p value | Parameter estimate | Standard error |
|---|---|---|---|
| Bifenthrin–permethrin | |||
| Bifenthrin | <0.001* | −0.4120 | 0.0828 |
| Permethrin | <0.001* | −0.0234 | 0.0038 |
| Interaction | Not significant | NAa | NAa |
| Permethrin–cyfluthrin | |||
| Permethrin | <0.001* | −0.0298 | 0.0097 |
| Cyfluthrin | 0.003736* | −0.1985 | 0.0717 |
| Interaction | Not significant | NAa | NAa |
- a Not applicable; interactions are omitted from the regression models where they are not significant.
- * Significant at p < 0.05.
DISCUSSION
The toxicity of pyrethroid mixtures to H. azteca was predominantly concentration-additive across lethal and sublethal endpoints. Significant interactions were detected in just 2 of the 8 mixtures tested (4-d cyfluthrin–bifenthrin and 4-d cyfluthrin–permethrin). Both mixtures interacted antagonistically on 4-d lethality and followed the independent action model; however, the mixture of cyfluthrin–permethrin was also tested for effects on 10-d lethality, swimming motility, and growth, and was additive by concentration addition for all 3 of these endpoints. Although antagonistic interactions were the exception in the present study, they have been the norm for all studies testing type I and type II pyrethroid mixtures on lethal endpoints to date 28, 29. Brander et al. 28 calculated an antagonistic interaction between cyfluthrin and permethrin after a 10-d exposure to H. azteca. Although the authors did not compare mixture data with the independent action model, the antagonism is described as “slight,” and appears to concur with the 4-d results from the present study. Schleier and Peterson 29 found an interaction between permethrin and cypermethrin in Drosophila melanogaster survival, a mixture not tested in the present study; therefore a direct comparison cannot be made.
Mechanistically, site-specific competition could explain the interactions and additive responses of pyrethroid mixtures found in the present and previous studies. Toxicants with similar mechanisms of action can be competitive agonists if they either compete for the same binding site in a cell or tissue, or bind at different sites and inhibit toxicant affinity or efficacy allosterically 32. An antagonistic effect could occur if 2 toxicants targeted the same site, but had different efficacies, particularly if the toxicant with the lower efficacy possessed a higher binding affinity. In this case, at concentrations at which binding sites in a cell or tissue are limiting, the toxicant with the higher binding affinity (and lower efficacy) would displace the other, thereby reducing the toxicological effect, and an antagonistic interaction would result. Allosterically, toxicants can compete by causing a conformational change at the binding site, thereby reducing the binding affinity or hindering the target effect. Also in this case, one would expect greater competition at higher concentrations, where binding is more likely to overlap.
It is especially noticeable that the permethrin–cyfluthrin mixture interaction was concentration-dependent. At the low concentrations, the mixture followed the concentration addition model most closely, but was less than additive as the concentrations increased. To a small degree this trend is also noticeable in the bifenthrin–cyfluthrin mixture, the only other mixture in which an antagonistic effect was significant. Given a limited number of sodium channel binding sites for pyrethroids to interact with, one could postulate that at low concentrations, at which binding sites are not a limiting factor, pyrethroids would be additive, whereas at higher concentrations competition for binding sites would be greater and an antagonistic effect could result. This effect was not found in the other type I and type II pyrethroid mixtures containing lambda-cyhalothrin, possibly because of a lower relative binding affinity than cyfluthrin.
Studies conducted on isolated mammalian neurons indicate that competitive interactions at specific target sites do occur between type I and type II pyrethroids. Song et al. 27 tested the interactions of tetramethrin and fenvalerate on rat dorsal root ganglion neurons, and found that tetramethrin competitively inhibited the effects of fenvalerate. The effect was reversible after tetramethrin washout, suggesting either competitive binding at the same site or allosteric activity at different sites. In a similar study, Motomura and Narahashi 26 tested the interactions of tetramethrin (type I) and deltamethrin (type II) on voltage-gated sodium channels in rat hippocampal neurons. Cells were treated subsequently with each chemical, and neuron pulses exhibited effects of tetramethrin only in the mixture, again indicating that tetramethrin competitively displaced or allosterically inhibited the effects of deltamethrin. Additional research in cell cultures from aquatic species is warranted. Testing the competitive or allosteric interactions of type I and type II pyrethroids, particularly mixtures between cyfluthrin and bifenthrin or permethrin, would help to confirm the molecular mechanism of the interactions detected.
Two of the pyrethroid mixtures, bifenthrin–permethrin and permethrin–cyfluthirn, were tested beyond 4-d lethality for subchronic and sublethal effects: 10-d lethality, swimming motility, and growth. Both mixtures were additive across all subchronic and sublethal effects, including the permethrin–cyfluthrin mixture in which an interaction was significant in the 4-d exposure. Although the present study is, to our knowledge, the first to investigate the sublethal interactions of pyrethroid mixtures in H. azteca, previous studies in other species have found similar results. Hasenbein et al. 33 measured the effects of ternary mixtures of permethrin, lambda-cyhalothrin, and the organophosphate pesticide chlorpyrifos on chironomid (Chironomus dilutus) motility, and found significant effects at sublethal concentrations, but did not specifically test whether the pesticides were additive or interactive. Barata et al. tested the combined effects of pyrethroid–metal binary mixtures on Daphnia magna feeding 34 and reproduction 35, and both studies found the mixtures to be additive by concentration addition. In mammalian species models, Wolansky et al. 36 found dose additivity in equipotent mixtures of 11 pyrethroids (including type I and type II) in terms of rat motor activity after oral gavage administration, whereas Yang 37 found that individual pyrethroids and their mixtures significantly affected embryogenesis and morphology, although data were not statistically analyzed for interactions, nor were they compared with the additive concentration addition or independent action models.
CONCLUSIONS
Multiple studies have confirmed that mixtures of pyrethroids occur at toxic concentrations in the environment and cause ecosystem effects 7, 8, 21. This is not surprising because of the potent effects of pyrethroids on nontarget organisms and their increasing use for urban and agricultural pest control. The present study confirms that pyrethroids are highly toxic to nontarget species and are additive or close to additive regardless of type. In addition, pyrethroids inhibit growth and swimming motility in H. azteca at sublethal concentrations and are concentration-additive for these endpoints. As a result, to protect native species, environmental water quality regulations should assume dose additivity among pyrethroid pesticides, and safety factors incorporated into risk assessment should account for mixture effects.
Acknowledgment
The present study was supported by the California State Water Resources Control Board, contracts 09-093-150 and 10-067-150. We thank M. Junghans (Risk Assessment, Oekotoxzentrum Ecotox Center) and T. Famula (University of California, Davis) for providing invaluable statistical advice and examples, the California Department of Fish and Game for their excellent work in conducting chemical analyses, and the Aquatic Health Program staff for their contribution to the present study, including laboratory work, data organization, video analysis and more, specifically D. Markiewicz, A. Javidmehr, G. Merrill, J. Tse, L. Liang, P. Kumar, Y. Jaramillo, E. Siemion, and R. Porter-Blackwell, as well as S. Hasenbein and M. Hasenbein.
Data availability
Data are available on request. Please contact [email protected]




