Local and interannual variations in mercury and cadmium in eggs of eight seabird species of the Sinaloa coast, México
Abstract
Mercury (Hg) and cadmium (Cd) concentrations in eggs of 8 seabird species inhabiting 5 coastal ecosystems in Sinaloa, México were determined during 2 breeding seasons (2012 and 2013): blue-footed booby (Sula nebouxii), brown booby (Sula leucogaster), double-crested cormorant (Phalacrocorax auritus), magnificent frigatebird (Fregata magnificens), brown pelican (Pelecanus occidentalis), royal tern (Thalasseus maximus), laughing gull (Leucophaeus atricilla), and Heermann's gull (Larus heermanni). The interspecific differences found in the concentrations of both metals were attributed to the diet and foraging ecology of the species. The highest Hg concentrations were detected in piscivorous species (brown pelican, 0.42 µg/g; brown booby, 0.31 µg/g; blue-footed booby, 0.26 µg/g; and double-crested cormorant, 0.23 µg/g); whereas species with more varied diets presented the highest Cd concentrations (Heermann's gull, 0.31 µg/g; laughing gull, 0.27 µg/g; and magnificent frigatebird, 0.27 µg/g). Cadmium concentrations were significantly greater in 2013 than 2012 for most species, and brown pelican and laughing gull also had higher Hg concentrations in 2013 in Santa María Bay, suggesting a relationship as a result of the changes either in oceanographic conditions or in continental runoff. Mercury concentrations in brown pelican and Cd concentrations in Heermann's gull and laughing gull were above threshold levels for adverse effects on reproduction and survival. Environ Toxicol Chem 2016;35:2330–2338. © 2016 SETAC
INTRODUCTION
The coastline of Sinaloa State, located in northwest México, has great ecological, economic, and social importance 1. Highly productive ecosystems used for the reproduction and protection of many aquatic organisms 2, as well as well-developed fisheries, can be found along its 656 km of shoreline. In addition, some of the most productive agricultural valleys of México are located in the adjacent areas. Furthermore, more than 2 million people live in the Sinaloa coastal plain, and intense mining activity is undertaken in the highlands 3. As a consequence, studies have reported the presence of toxic metals in mollusks 4, crustaceans 5, and fish 2. These pollutants may pose a risk for the ecological integrity of the ecosystems and for the population dependent on their natural resources 6.
Assessment of the conditions affecting the presence and concentrations of metals in organisms is particularly important to understand so that risks associated with the presence of high levels of these metals to ecosystems and communities of the region might be ameliorated 7. Mercury (Hg) and cadmium (Cd) are perhaps the most relevant metals to the marine environment because of their toxicity and tendency to accumulate in organisms. These metals have been related to a variety of effects on health, reproduction, and survival of wild animals, including genetic damage, oxidative stress, neurotoxicity, kidney failure, abnormal behavior, and avian embryo mortality 8-10.
Coastal and marine ecosystems can be affected by significant metal inputs related to local hydrodynamics and other oceanographic processes 11. Sinaloa is located in the southern region of the Gulf of California and is characterized by highly variable oceanographic conditions attributable to the occurrence of intense upwelling events during the winter season and interannual global events, such as the El Niño Southern Oscillation 12, 13. Locally, the coastal lagoons of Sinaloa present internal circulation patterns strongly influenced by tidal currents that determine the spatial and temporal distributions of pollutants 14.
Seabirds have been used as indicators of metal pollution in several regions of the world 15, 16. The quantification of pollutants in eggs of seabirds has been proposed as a tool for assessing the levels of local contamination because the metal concentrations in eggs are directly related to the levels of metals in female blood during the egg formation process 16. At least 15 species of seabirds have their nesting grounds along the Sinaloa coast, some of which form colonies of several thousand pairs 17, 18. These seabirds obtain their food from the waters surrounding their nesting areas; therefore, they can be considered as indicators for assessing spatial and temporal changes in metal concentrations of local or regional food chains.
In the present study, Hg and Cd concentrations were determined in eggs of 8 seabird species: blue-footed booby (Sula nebouxii), brown booby (S. leucogaster), double-crested cormorant (Phalacrocorax auritus), magnificent frigatebird (Fregata magnificens), brown pelican (Pelecanus occidentalis), royal tern (Thalasseus maximus), laughing gull (Leucophaeus atricilla) and Heermann's gull (Larus heermanni). The breeding grounds of these species are located in 5 different coastal and marine ecosystems of Sinaloa: Farallón de San Ignacio (FSI site), Ohuira Bay (OHB site), the Navachiste–Macapule System (NMS site), Santa María Bay (SMB site), and Mazatlán Bay (MZB site; Figure 1).
These species feed at different trophic levels that can affect inputs and transfer of metal to their eggs 7, 19. In most cases, seabirds that include greater amounts of fish in their diets or consume larger fish have higher levels of metals than those who consume alternate prey items or smaller fish 7. High Hg concentrations may be related to feeding at higher trophic levels, whereas higher Cd concentrations are more common in seabirds that consume large quantities of marine invertebrates such as squid and crustaceans 15, 19. Therefore, greater Hg concentrations were predicted in eggs of 6 species (blue-footed booby, brown booby, double-crested cormorant, magnificent frigatebird, brown pelican, and royal tern) that are predominantly piscivorous. Larger Cd concentrations were predicted in 2 species of gulls, which are omnivores, with a diet including fish, marine and terrestrial invertebrates, and eggs and chicks of other seabirds nesting in the same areas 20, 21. It was hypothesized that we might find the same interspecific trends in 3 of the analyzed nesting sites (FSI, SMB, and NMS).
The Gulf of California shows an interannual variability in physical and ecological processes dependent on the El Niño Southern Oscillation, which may also affect the bioavailability of metals in the sea surface and their subsequent bioaccumulation in the food chain 22, 23. Hence, we predict an interannual variability in metal concentrations in the eggs as a response to possible changes in oceanographic processes. Because estuarine ecosystems are considered sinks of metals 24, we also hypothesized the presence of higher Hg and Cd concentrations in seabirds from coastal lagoons compared with seabirds from pelagic habitats. In addition, higher metal concentrations would be predicted for pelicans nesting in 2 coastal lagoons (NMS and SMB sites) than in the MZB site, a semi-enclosed bay with a lower influence from agricultural drains.
MATERIALS AND METHODS
Seabird species
In total, 416 eggs were collected from 8 seabird species in 5 coastal ecosystems located in Sinaloa State, México, (Table 1 and Figure 1). Egg collection was carried out on 1 d at each nesting site, so eggs with different developmental stages were collected. The nesting chronology was different among the 8 species; Pelecaniformes and Suliformes began nesting in the fall (November–December) and concluded in the spring or summer of the following year. The sampling period for these species included 2 breeding seasons, 2011 to 2012 and 2012 to 2013, and each season was identified by the last year of each period. The nesting period for Charadriiformes lasts for 3 to 4 mo, between spring and summer each year. Sampling was carried out in the 2 breeding seasons (2012 and 2013) for royal tern and Heermann's gull and in the 3 breeding seasons (2010, 2012, and 2013) for laughing gull. In 2010, only laughing gull eggs were collected from the SMB site. For the other 2 seasons, eggs were collected from 8 species at 3 different locations during 2012 and in 5 different locations during 2013. The species, species abbreviations, sampling locations, and sample numbers are shown in Table 1.
| Species | 2010 | 2012 | 2013 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Order | Abbreviation | Common name | Species | SMB | FSI | OHB | SMB | FSI | OHB | NMS | SMB | MZB |
| Suliformes | BFBO | Blue-footed booby | Sula nebouxii | 6 | 26 | 28 | 28 | |||||
| BRBO | Brown booby | Sula leucogaster | 8 | 13 | ||||||||
| DCCO | Double-crested cormorant | Phalacrocorax auritus | 7 | 6 | 15 | 13 | 9 | 6 | 13 | |||
| MAFR | Magnificent frigatebird | Fregata magificens | 15 | 15 | ||||||||
| Pelecaniformes | BRPE | Brown pelican | Pelecanus occidentalis | 25 | 16 | 12 | 10 | |||||
| Charadriiformes (Fam. Laridae) | ROTE | Royal tern | Thalasseus maximus | 31 | 23 | |||||||
| LAGU | Laughing gull | Leucophaeus atricilla | 12 | 31 | 30 | |||||||
| HEGU | Heermann's gull | Larus heermanni | 12 | 6 | ||||||||
- FSI = Farallón de San Ignacio; OHB = Ohuira Bay; NMS = Navachiste-Macapule System; SMB = Santa María Bay; MZB = Mazatlán Bay.
Sampling locations
The sampling locations are situated in the southeast region of the Gulf of California along the coastline of Sinaloa State. The FSI site (25°26′12″N, 109°22′38″W) is a rocky island located 27 km from the northern coast of Sinaloa; it is stripped of vegetation and is the ecosystem most influenced by pelagic conditions 25. The largest colony of blue-footed booby is found there, with approximately 2500 nesting pairs. Colonies of Heermann's gull and brown booby have 2000 and 1200 pairs, respectively. The colony of double-crested cormorant is smaller and varies between 60 and 90 nesting pairs yearly 17. Eggs from these 4 species were collected from FSI. Ohuira Bay is a coastal saltwater lagoon (23°37′46″N, 108°58′54″W). Eggs from double-crested cormorant were collected on Patos Island (25°37′10.8″N, 109°00′45.7″W), which is located inside the bay at a distance of 3 km from Topolobampo town 26. Eggs were collected from a site where approximately 50 nests of double-crested cormorant are found. At the NMS site, eggs from brown pelican and double-crested cormorant were collected on Pájaros Island (25°22′38″N, 108°42′13″W), where 350 nests of double-crested cormorant have been reported 27; during egg sampling, several hundred nests of brown pelican were observed.
The largest coastal lagoon in Sinaloa is the SMB site, extending over 1350 km2 and including several islands, vast mudflats, marshlands, and mangroves 28. Eggs were collected from 3 of the islands. At El Rancho Island (25°09′14″N, 108°22′19″W), eggs from blue-footed booby, royal tern, laughing gull, and Heermann's gull were collected. The colonies of the first 3 species are large at this site, with approximately 3000, 8000, and 2000 nesting pairs reported in 2012, respectively; however, the breeding pattern of Heermann's gull varies, with only 17 nests in the same year 18. In the central area of the bay, eggs from double-crested cormorant, magnificent frigatebird, and brown pelican were collected at El Mero (25°05′34″N, 108°14′58″W) and Pájaros (25°04′44″N, 108°13′52″W) Islands, with approximately 3000, 8000, and 2000 nests, respectively 18. Mazatlán Bay is a semi-enclosed body of water that includes 3 islands and 2 islets located near the coast. Brown pelican eggs were collected from Pájaros Island (23°15′16″N, 106°28′36′W), which is located 0.9 km from Mazatlán City.
Determination of Hg and Cd levels
After collection, the sampled eggs were transported to the Ecotoxicology Laboratory at the Research Center for Food and Development, AC, Mazatlán Unit, where linear dimensions (length and width) were measured, and the egg contents were removed. The egg content was categorized by visual observation according to the following stage of embryo development: no development, initial (embryos without fully developed limbs), intermediate (embryos with fully developed limbs, but with translucent skin that still reveals organs), and advanced (embryos fully formed). The total contents of each egg (white, yolk, and/or embryo) were lyophilized, subsequently ground in a ceramic mortar, and digested to determine the metal content. The moisture content of each sample was calculated by subtracting the final weight after lyophilization from the initial fresh weight, using an analytical balance. First, 0.25 g of the dried and homogenized content of each egg was digested with 5 mL of 50% HNO3. The resulting samples were transferred to a digestion system (CEM model MARSX), where a second digestion was performed by adding 3 mL of H2O2, in accordance with US Environmental Protection Agency (USEPA) method 3052 29. Digested samples were analyzed in an atomic absorption spectrophotometer (PerkinElmer model 1100B). The Hg concentration was determined in a hydride generator following USEPA method 7471A 30, and the Cd concentration was obtained by flame atomic absorption using USEPA method 7130 31. The Hg and Cd concentrations were calculated in µg/g of dry weight and then converted to wet weight using the moisture content of the sample to facilitate comparisons with the literature. For quality assurance/quality control purposes, a blank solution, a duplicate, and a certified reference material for trace metals (DOLT-4 Dogfish Liver) from the National Research Council of Canada were digested with every batch of 10 samples and analyzed with the rest of the samples. Detection limits were 0.0002 μg/g for Hg and 0.5 μg/g for Cd. The quality assurance/quality control results suggest that the average percentage recoveries were 99.8% for Hg and 94.0% for Cd. The maximum percentage relative difference values for Hg were 10.4% and 14.9% for Cd.
Data analysis
Considering that the development stage was not homogeneous and that the results were initially calculated on a dry weight basis, before applying statistical analyses for comparisons among species, sites, and breeding seasons, the variation of egg dry weights of each species was analyzed. This was performed using general linear models, which included the dry weight as the dependent variable, the development stage (without development, initial, intermediate, and advanced) as the factor, and the volume of eggs as the covariate. In 6 species (blue-footed booby, brown booby, double-crested cormorant, magnificent frigatebird, brown pelican, and Heermann's gull), it was found that the dry weights were different between stages of embryonic development. Dry weight decreased gradually between development stages (∼12% between nondevelopment stage of eggs and advanced stages). Therefore, concentrations of Hg and Cd were standardized to estimate the initial development values for each species (using the mean decline in dry wt controlled by egg volume). These standardized concentrations were converted to wet weight, using the moisture content of eggs.
To perform the statistical analysis, below-detection concentrations were set to one-half the detection limit. Concentration values of Hg and Cd were transformed (log x + 1) to minimize the effect of working with a scale involving several orders of magnitude 32. The transformed data were used for normality tests. Data are reported as geometric means; the arithmetic means are presented only for comparisons with other studies. A summary of the statistical tests performed in the present study is shown in Supplemental Data, Table S1. The analyses were applied in the same manner for Hg and Cd concentrations and were included as dependent variables in all cases. Two-way analyses of variance (ANOVAs) were applied to compare the concentrations of these metals in eggs of 3 species (blue-footed booby, brown booby, and double-crested cormorant) on FSI and 6 species (blue-footed booby, double-crested cormorant, magnificent frigatebird, brown pelican, royal tern, and laughing gull) on SMB during 2 breeding seasons (2012 and 2013). One-way ANOVAs were applied to include Heermann's gull only in the interspecific comparison for the 2012 season on FSI and for 2013 season in SMB. A Student's t test was used to compare concentrations of Hg and Cd in eggs of double-crested cormorant and brown pelican in NMS during the 2013 season. The laughing gull eggs collected in SMB were compared using 1-way ANOVAs to determine the differences among 3 sampling periods (2010, 2012, and 2013). Intersite differences in blue-footed booby and double-crested cormorant were analyzed using 2-way ANOVAs during the 2012 and 2013 seasons. One-way ANOVAs were applied to include NMS in the intersite comparison during the 2013 season for double-crested cormorant and for intersite comparison in brown pelican eggs in the same season. Scheffé post hoc tests were applied after the ANOVAs were performed, to identify homogeneous groups. Results with p < 0.05 were considered to be significant. The software Statistica 12.0 (StatSoft) was used to perform the statistical analyses.
RESULTS
Comparison of Hg and Cd levels between species
Greater concentrations of Hg were observed in predominantly piscivorous species, with lower concentrations in both species of gulls. In particular, brown pelican eggs presented the highest concentrations of Hg, followed by brown booby, blue-footed booby, and double-crested cormorant (F7,408 = 18.7, p < 0.001). There were interspecific differences in Cd concentrations: the eggs of magnificent frigatebird and both species of gulls presented the highest concentrations of Cd, whereas the lowest concentrations were detected in the eggs of royal tern and brown pelican (F7,408 = 10.6, p < 0.001; Table 2).
| Parameter | BFBO | BRBO | DCCO | MAFR | BRPE | ROTE | LAGU | HEGU | p |
|---|---|---|---|---|---|---|---|---|---|
| n | 88 | 21 | 69 | 30 | 63 | 54 | 73 | 18 | |
| Hg | |||||||||
| GM | 0.26 B | 0.31 BC | 0.23 BC | 0.24 BC | 0.42 A | 0.21 BC | 0.05 C | 0.18 BC | <0.001 |
| AM | 0.33 | 0.34 | 0.30 | 0.27 | 0.63 | 0.32 | 0.19 | 0.24 | |
| SD | 0.16 | 0.16 | 0.20 | 0.12 | 0.42 | 0.23 | 0.21 | 0.18 | |
| RA | (BDL–0.78) | (0.12–0.91) | (0.01–0.95) | (0.04–0.55) | (BDL–1.87) | (BDL–1.07) | (BDL–1.16) | (0.07–0.62) | |
| ND | 1 | 0 | 0 | 0 | 1 | 1 | 8 | 0 | |
| Cd | |||||||||
| GM | 0.10 ABC | 0.17 ABC | 0.14 ABC | 0.27 AB | 0.09 BC | 0.07 C | 0.30 A | 0.31 A | <0.001 |
| AM | 0.26 | 0.32 | 0.33 | 0.47 | 0.20 | 0.07 | 0.59 | 0.73 | |
| SD | 0.34 | 0.28 | 0.43 | 0.42 | 0.27 | 0.03 | 0.61 | 0.96 | |
| RA | (BDL–1.31) | (BDL–0.86) | (BDL–1.57) | (BDL–1.31) | (BDL–0.81) | (BDL–0.23) | (BDL–2.42) | (BDL–2.70) | |
| ND | 55 | 8 | 33 | 7 | 41 | 51 | 23 | 5 | |
- a Species differences were obtained with one-way analysis of variance. Similar uppercase letters indicate homogeneous groups (p > 0.05) between species. The range of concentrations is shown in parentheses.
- BFBO = blue-footed booby; BRBO = brown booby; DCCO = double-crested cormorant; MAFR = magnificent frigatebird; BRPE = brown pelican; ROTE = royal tern; LAGU = laughing gull; HEGU = Heermann's gull; GM = geometric mean; AM = arithmetic mean; SD = standard deviation; RA = range; BDL = below detection limit; ND = numbers of nondetects.
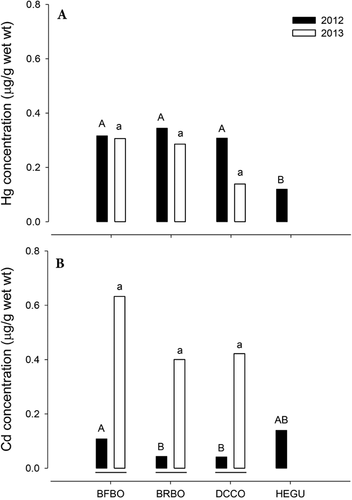
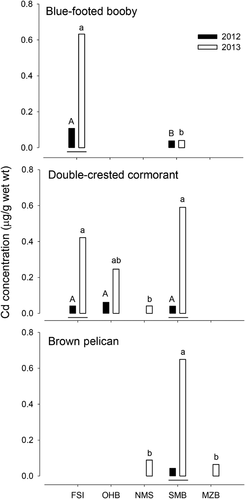
When the same species were compared in the same coastal system, interspecific tendencies similar to those mentioned in the previous paragraph were observed. During 2012 on FSI, the concentrations of Hg were higher in the eggs of blue-footed booby, brown booby, and double-crested cormorant compared with those observed in Heermann's gull (F3,29 = 20.0, p < 0.001), whereas no significant differences in Hg concentrations were found between these species in 2013 (F2,69 = 1.3, p = 0.275). The concentrations of Cd in eggs during 2012 were higher in blue-footed booby and Heermann's gull compared with brown booby and double-crested cormorant (F3,29 = 3.4; p = 0.031). The concentrations of Cd in the 2 species of Sula and cormorants were similar during 2013 (F2,69 = 0.5, p = 0.638; Figure 2).
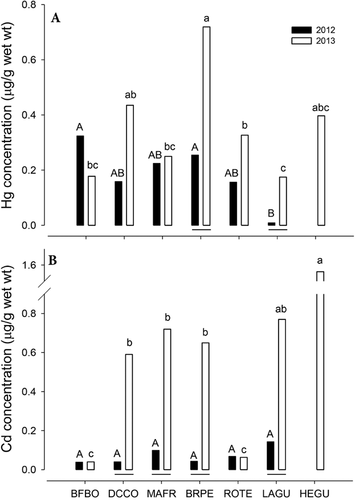
There were significant interspecific differences in Hg concentrations in eggs collected during 2012 on SMB (F5,252 = 8.0, p < 0.001); the lowest concentrations were detected in laughing gull, whereas the rest of the species presented similar values. Differences between species were also detected in 2013 (F6,120 = 12.0, p < 0.001), and a similar tendency to the former case was observed; the lowest values were noted in laughing gull, whereas the highest values were observed in brown pelican and double-crested cormorant. Cadmium concentrations in eggs collected during 2012 were lower in all species (F5,252 = 25.8, p < 0.001). In 2013, the eggs of 5 species (double-crested cormorant, magnificent frigatebird, brown pelican, laughing gull, and Heermann's gull) presented elevated Cd concentrations. These values differed significantly from those observed in royal tern and blue-footed booby eggs (F6,120 = 41.3, p < 0.001; Figure 3). No significant differences were observed between Hg and Cd concentrations in double-crested cormorant and brown pelican eggs during 2013 in NMS (Hg, Student's t test, df = 20 = −1.9, p = 0.070; Cd, Student's t test, df = 20 = −1.5, p = 0.160; data not shown).
Interannual variations of Hg and Cd levels
No interannual significant differences in Hg concentrations in eggs of boobies and cormorants were observed on FSI (F1,69 = 1.3, p = 0.275). In SMB, the eggs of brown pelican and laughing gull presented higher Hg concentrations during 2013 (F1,252 = 8.0; p < 0.001). The interannual differences were more noticeable for Cd concentrations, which presented greater values during 2013 on both FSI (F1,69 = 50.0, p < 0.001) and SMB (F1,252 = 211.5, p < 0.001; Figures 2 and 3). On SMB, Hg and Cd concentrations were measured in laughing gull during 3 breeding seasons; interannual differences were observed for both metals. Mercury concentrations were significantly lower during 2012 compared with 2010 and 2013 (F2,70 = 10.2, p < 0.001), and the Cd concentrations were significantly higher during 2013 compared with the other previously studied seasons (F2,70 = 24.3, p < 0.001; Table 3).
| 2010 | 2012 | 2013 | |
|---|---|---|---|
| n | 12 | 31 | 30 |
| Hg | |||
| GM | 0.23 A | 0.01 B | 0.17 A |
| AM | 0.33 | 0.09 | 0.24 |
| SD | 0.32 | 0.12 | 0.19 |
| Cd | |||
| GM | 0.20 B | 0.14 B | 0.77 A |
| AM | 0.26 | 0.28 | 1.05 |
| SD | 0.20 | 0.33 | 0.65 |
- a Species differences were obtained with one-way analysis of variance. Similar uppercase letters indicate homogeneous groups (p > 0.05) between seasons.
- GM = geometric mean; AM = arithmetic mean; SD = standard deviation.
Comparison between locations
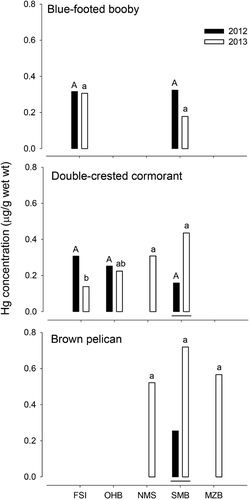
No differences were observed between Hg concentrations in blue-footed booby and double-crested cormorant eggs from different areas during 2012 (F1,84 = 0.1, p = 794; F2,57 = 1.5, p = 0.223, respectively). Similarly, no differences were detected in blue-footed booby (F1,84 = 0.1, p = 794) and brown pelican (F2,35 = 2.4, p = 0.109) eggs during 2013. However, Hg concentrations in double-crested cormorant were significantly greater in the eggs from NMS and lower in the eggs from FSI (F3,37 = 8.7, p < 0.001; Figure 4). The 3 species assessed presented different Cd concentrations depending on the location: for blue-footed booby, the lowest Cd concentrations were found on SMB during the 2 breeding seasons (F1,84 = 30.6, p < 0.001); for double-crested cormorant, no differences were observed between locations during 2012 (F2,57 = 0.8, p = 0.460), but differences were noted during 2013, when Cd concentrations were lower in NMS (F3,37 = 6.1, p = 0.002). Finally, Cd concentrations in brown pelican were significantly greater in the eggs collected on SMB compared with the eggs from NMS and MZB, which presented similar values (F2,35 = 36.3, p < 0.001; Figure 5).
DISCUSSION
Interspecific analysis
In the present study, Hg and Cd concentrations presented interspecific differences associated with the trophic positions. As predicted, Hg concentrations were high in species feeding mainly on fish and low in omnivore species. The relationship between trophic level and Hg content in seabirds' tissues throughout different world regions has been documented 7, 19, 33. Seabirds that feed at higher trophic levels tend to accumulate larger quantities of this metal 7. The Suliformes and Pelecaniformes species of coastal Sinaloa presented the highest Hg concentrations in eggs collected from 2 different ecosystems. On FSI, high Hg concentrations were observed in species of Sula and cormorants, whereas Hg concentrations were low in gulls. In the Gulf of California, brown booby and blue-footed booby feed mainly on sardines and anchovies 34, 35, but double-crested cormorant also feeds on demersal fish 36. The diet of Heermann's gull in the central region of the Gulf of California also consists of sardines and anchovies, but most fish consumed by this species are juvenile 37. This difference could explain why the Hg concentration in its eggs was lower than concentrations detected in Sula and cormorants, and this finding is in accordance with the Hg content found in several pelagic species of fish, which increases with size and developmental stage 38.
Brown pelican eggs presented Hg concentrations up to 2-fold higher than the average concentration for the other species, including piscivorous seabirds, during 2013 on SMB. It has been documented that larger fish normally contain higher Hg content than smaller fish 39, which could explain the greater concentrations found in eggs of brown pelican, as the diet of this species includes fish of up to 160 mm in length on average (range: 97–257 mm 40). The size of the prey can also explain the lower Hg concentrations in royal tern compared with those observed in other piscivorous species, as the tern species are relatively small birds that feed on smaller fish 19. In relation to omnivorous species, laughing gull eggs presented the lowest Hg concentrations, which is attributed to their varied diet, consisting of shrimp, anchovies, and insects in the SMB region (E. González-Medina, National Autonomous University of México, Mazatlán, México, unpublished data).
Similar trends and concentrations related to the trophic level have been reported in multispecific studies conducted elsewhere. In the Portuguese Atlantic, the lowest Hg concentrations were found in eggs of terns (common tern [Sterna hirundo)] and roseate tern [Sterna dougallii], ≈1.0 μg/g dry wt) and gulls (yellow-legged gull [Larus cachinnans], 1.4 μg/g dry wt), whereas higher concentrations were recorded in Bulwer's petrel (Bulweria bulwerii, 1.7 μg/g dry wt), a species that consumes mostly mesopelagic prey 41. In Barnegat Bay (NJ, USA), Hg concentrations were lower in gulls (herring gull [Larus argentatus)] and great black-backed gull [Larus marinus], ≈0.35 μg/g dry wt), intermediate in terns (common and Foster tern [Sterna forsterii]; 1.0 μg/g and 1.7 μg/g dry wt, respectively) and greater in black skimmer (Rynchops niger; 2.0 μg/g dry wt), which feed on larger fish 7.
The prediction that eggs of omnivorous species would contain greater Cd concentrations was also confirmed. High Cd concentrations are common to species feeding on lower trophic levels, including marine invertebrates (mollusks and crustaceans) 15, 19. Two omnivorous species of gulls presented high Cd concentrations on the Sinaloa coast 20, 21. However, the concentrations observed in magnificent frigatebird eggs presented similar values, even though this species feeds mainly on fish. This finding could be attributed to the fact that the 2 species of gulls are kleptoparasited by magnificent frigatebird in the SMB region; however, magnificent frigatebird has an extensive foraging range 42 and a very diverse diet 43, possibly because this species comes into contact with pollutants from other regions. In contrast, the metal concentration in royal tern eggs was extremely low. It has been suggested that metals such as Cd tend not to accumulate in the eggs of some species 16. Nevertheless, other studies suggest that Cd concentrations in females of several species of terns are not related to Cd concentrations found in their eggs 44. This may be the reason why only a few studies have been carried out on the levels of this metal in seabirds' eggs.
Interannual variations
In the present study, interannual differences in Cd concentrations were found in eggs of most seabirds species, with occurrences markedly greater in 2013 than in 2012. In contrast, Hg concentrations were higher in 2013 only in brown pelican and laughing gull. This suggests that interannual variability could be related to drastic changes in oceanographic conditions, specifically when a more intense upwelling such as in 2013 occurs (data not shown). In addition, the influence of local factors such as storms, changes in prey availability, and mainland inputs of sediments and wastewater to coastal ecosystems during rainy seasons could also be related. Further research on the influence of potential sources of metals (e.g., perhaps using stable isotope methodologies), oceanographic and climatological parameters, and foraging ecology of species is desirable to assess the bioaccumulation in eggs.
Spatial variability
Several studies have proposed that widely distributed species of seabirds can be used to identify regional and local differences in metal concentrations 19. The pollutant contents in samples collected in different points of the range of seabird species provide relevant information about changes in food availability and the presence of point sources of pollution 45. In the present study, spatial variability of Hg and Cd concentrations was found in 3 widely distributed seabird species on the Sinaloa coast (blue-footed booby, double-crested cormorant, and brown pelican), but no spatial trends were apparent. In blue-footed booby and double-crested cormorant, it was assumed that eggs from colonies located in coastal lagoon systems situated near agricultural areas would present higher metal concentrations. However, such low values were only observed for Hg concentrations in double-crested cormorant eggs collected during 2013. In this particular case, larger Hg concentrations were observed at NMS and SMB, as these systems are affected by agricultural waste discharges from the Guasave, Évora, and Culiacán valleys 46, 47. However, the aforementioned assumption did not hold for Cd concentrations. The blue-footed booby eggs collected on FSI contained greater Cd concentrations than eggs from SMB. It has been suggested that the bioavailability of Cd in the Gulf of California is conditioned by upwelling events during the winter season 4. For this reason, the spatial variability of Cd concentration in blue-footed booby eggs could be explained by the different oceanographic conditions present in each particular location. The boobies from SMB normally feed near the coast, but the boobies from FSI obtain their food from oceanic waters 35, where the upwellings bring Cd to the sea surface, making it available to seabirds. This condition also could explain the high Cd concentrations in double-crested cormorant eggs collected on FSI during 2013.
Brown pelican eggs were collected in 3 locations of coastal lagoons. Mercury concentrations were similar in all 3 locations, but the Cd concentration obtained from SMB was 3 times greater than the concentrations obtained at NMS and MZB. Santa María Bay is the largest lagoon system of the Sinaloa coast and is permanently connected to the Gulf of California through 2 entrances 28. The lagoon receives water from 3 rivers (Sinaloa, Mocorito, and Tule) that are affected by agricultural, urban, and industrial waste discharges 48. Therefore, it is possible that factors such as the inflow of continental water, hydrodynamic processes, and the water exchange between the lagoon and the ocean cause Cd to be available in higher quantities in this coastal ecosystem.
Brown pelican and double-crested cormorant eggs collected from SMB presented high Cd concentrations, but the Cd concentrations found in blue-footed booby were low. Although the latter species is piscivorous, both their diet and their foraging ecology present characteristics that differ from those of the other 2 species: the foraging range of blue-footed booby is twice that of double-crested cormorant and brown pelican 49-51. Thus, double-crested cormorant and brown pelican obtain their food exclusively from the bay, where greater Cd concentrations are present 5, whereas blue-footed booby can catch their prey in 2 different areas (pelagic zones and coastal areas), which reduces their exposure to metals, and thus lower accumulation in their eggs.
Ecotoxicological considerations for seabirds in Sinaloa
Research suggests that Hg concentrations between 0.5 μg/g and 1.0 μg/g wet weight in seabirds' eggs could cause mortality, malformations, and neurological effects 9. On the coast of Sinaloa, only brown pelican eggs presented Hg concentrations above the levels considered harmful for seabirds. During the 2013 breeding season, 26% of the brown pelican eggs collected in the 3 sampling locations presented values greater than 1.0 μg/g (range: below detection limit to 1.87 μg/g) of this metal. This finding is particularly relevant because only brown pelican populations from Sinaloa have declined in recent years 18; therefore, additional studies are needed to assess the ecotoxicological risk of Hg to brown pelican colonies in the coastal area of Sinaloa.
It has been documented that even Cd concentrations below 1.0 μg/g wet weight can cause chronic effects, such as kidney damage, behavioral alterations, egg-laying inhibition, and perhaps eggshell thinning 8. In the present study, 61% of eggs of the 2 species of gulls collected from SMB during 2013 presented Cd concentrations greater than 1.0 μg/g (Heermann's gull, 1.83 μg/g [range: 0.59–2.54] and laughing gull, 1.05 μg/g [range: 0.06–2.42]), suggesting that at least parts (61%) of these populations are at potential risk of suffering from chronic effects. It may be appropriate to perform studies specifically focused on the health conditions of the species, their reproductive parameters, and any changes in populations in relation to Cd concentrations in eggs. Because shrimp are the main diet for laughing gull during the egg-laying period (E. González-Medina, National Autonomous University of México, Mazatlán, México, unpublished data), it is important to further investigate Cd concentrations in shrimp collected from the areas where laughing gull is feeding (at SMB and marine waters closer to the colony) because of its high commercial and social importance in the region.
Considering this information, implementation of a permanent monitoring program and extension of the study area to the entire Gulf of California is recommended. Such an effort would facilitate determination of distribution patterns on a regional scale and could evaluate the influence of temporal variability in oceanographic and environmental conditions on the availability of these metals on a temporal scale.
Supplemental Data
The Supplemental Data are available on the Wiley Online Library at DOI: 10.1002/etc.3402.
Acknowledgment
The present study was funded by Fondo Mexicano para la Conservación de la Naturaleza, AC (PIE-2012-A-P-C-IGSI-12-12) and Consejo Nacional de Ciencia y Tecnología, México (CONACYT I010/176/2012). CONACYT funded a doctoral scholarship to J.P. Ceyca (317081). C. Covantes, A. Piña, E. González, M. Guevara, M. González, M. Lerma, C. Franco, and F. Santiago provided support during field work. C. Vargas and D. Aguilera helped with sample processing and analysis of metals, respectively. E. Mellink, A. Van der Heiden and L. García provided valuable comments that improved the manuscript. We appreciate the revision of the English language by V. Williams and the comments by 3 anonymous reviewers. Samples were collected according to applicable Mexican laws (Licencias de Colecta Científica SGPA/DGVS/62712/12 y SGPA/DGVS/02923/13, on behalf of M. Betancourt Lozano).
Data availability
Data, associated metadata, and calculation tools are available from M. Betancourt ([email protected]).




