Critical micelle concentration values for different surfactants measured with solid-phase microextraction fibers
Abstract
The amphiphilic nature of surfactants drives the formation of micelles at the critical micelle concentration (CMC). Solid-phase microextraction (SPME) fibers were used in the present study to measure CMC values of 12 nonionic, anionic, cationic, and zwitterionic surfactants. The SPME-derived CMC values were compared to values determined using a traditional surface tension method. At the CMC of a surfactant, a break in the relationship between the concentration in SPME fibers and the concentration in water is observed. The CMC values determined with SPME fibers deviated by less than a factor of 3 from values determined with a surface tension method for 7 out of 12 compounds. In addition, the fiber–water sorption isotherms gave information about the sorption mechanism to polyacrylate-coated SPME fibers. A limitation of the SPME method is that CMCs for very hydrophobic cationic surfactants cannot be determined when the cation exchange capacity of the SPME fibers is lower than the CMC value. The advantage of the SPME method over other methods is that CMC values of individual compounds in a mixture can be determined with this method. However, CMC values may be affected by the presence of compounds with other chain lengths in the mixture because of possible mixed micelle formation. Environ Toxicol Chem 2016;35:2173–2181. © 2016 SETAC
INTRODUCTION
Surface-active compounds or surfactants are complex and sometimes ionized compounds with both hydrophilic and hydrophobic properties, that is, an easily solvated polar head group in combination with a less solvated apolar hydrocarbon or fluorocarbon tail. Surfactants are widely used in several household and/or industrial applications for their cleaning and solubilizing properties. Technical mixtures of surfactants with different chain lengths and/or charges are currently being used in detergents, paints, polymers, textiles, pesticide formulations, oil recovery, paper industry, hydraulic fracturing fluids, and environmental remediation of soils 1-3. The experimental determination of environmental properties of surfactants such as the octanol–water partition coefficient (KOW) is not straightforward because of the tendency for surfactants to accumulate at interfaces and to emulsify the octanol–water system. One method, which is often used to estimate KOW is the computational method, which uses the ratio of the solubility of surfactants in octanol and water. However, the use of water solubility to determine KOW is complicated by the fact that the solubility of surfactants is neither properly defined nor easy to determine. This is because surfactants have the ability to form micelles in solution above a certain surfactant concentration of monomers, that is, the critical micelle concentration (CMC), although it is possible to introduce a correction factor for the formation of micelles in the octanol–saturated water phase 4, 5. Multiple indirect methods are available to determine the CMC (i.e., electrical conductivity, surface tension, light scattering, spectrophotometry, cyclic voltammetry, nuclear magnetic resonance, and capillary electrophoresis 6), and CMC is often used as a practical surrogate for surfactant solubility to estimate KOW for surfactants. Complications in the measurement of aqueous solubility can arise because of the ability of surfactants to form a micellar phase above the CMC. As a result, traditional methods for measuring CMCs have the potential to underestimate total water solubility of surfactants resulting in potential overestimation of and uncertainty in estimated KOW values. Because the determination of KOW values of (ionic) surfactants is technically very challenging, the CMC value of surfactants may be a more relevant parameter as a predictor of hydrophobicity in environmental risk assessments. For example, a good relationship was observed between CMC values of 16 neutral and 2 zwitterionic surfactants and the liposome–water partition coefficient obtained with isothermal titration calorimetry 7. In general, the development and further testing of alternative property descriptors for surfactants are also relevant for the exploration of models, such as quantitative structure–activity relationships (QSARs) for the prediction of environmental properties (i.e., sorption, bioaccumulation, and toxicity) of surfactants. However, QSARs for surfactants are not very well developed because the behavior of ionized and nonionized surfactants is different from that of other neutral and nonpolar organic compounds.
The occurrence of micelles in aqueous solution can be explained by the high energies required to form a cavity in water caused by the hydrophobic part of a surfactant. These high cavity formation energies are required to compensate for the loss in structure of the hydrogen-bonded water molecules surrounding the hydrophobic moiety 8. This distorted structure of water causes an increase in its Gibbs free energy 9. Because of the amphiphilic nature of surfactants, these compounds tend to concentrate at interfaces, thereby reducing the free energy of water. Reduction of the free energy proceeds via sorption at all available interfaces. When these interfaces are saturated, other physical mechanisms will dominate the behavior of surfactants in water 10. The surfactants will therefore form micelles with the hydrophilic head groups directed toward the water phase 9. The CMC values of surfactants in aqueous solution are affected by several factors: structure of the surfactant (carbon chain length, head group, charge, branching, and type of counter-ion), electrolyte present in solution, organic additives, and temperature 9. As a general rule, however, the CMC values of different surfactant groups (with linear carbon chains) usually decrease in the order of nonionics < zwitterionics ≤ anionics ≈ cationics 2, 9.
An alternative approach to determine CMC for surfactants is the use of solid-phase microextraction (SPME) fibers. Previously SPME fibers coated with polydimethylsiloxane have been used in soil sorption experiments to measure freely dissolved concentrations of polycyclic aromatic hydrocarbons (PAH). The freely dissolved concentrations reached a maximum value when the solubility of the compounds was reached 11. Maximum soil sorbed concentrations were found to be equal to the aqueous solubility of the PAH 12. Polyacrylate-coated SPME fibers were used in a similar way to measure CMC values of nonionic and anionic surfactants in seawater and of cationic surfactants in various environmentally relevant media [13–15]. The CMC is derived when the concentrations in the fiber become constant or reach a breaking point in the fiber–water sorption isotherm because only freely dissolved concentrations of surfactant monomers are measured by SPME fibers. The approach of measuring freely dissolved concentrations of surfactants to derive a CMC value with SPME fibers thus has a clear advantage over indirectly measuring CMC, for example, by determination of surface tension, electrical conductivity, or light scattering 6. For example, the surface tension method does not distinguish between different components in a mixture because the measurement is based on a break in the relationship between surface tension and concentration that is only reflected by the surface tension of the mixture. In contrast, the SPME method measures freely dissolved (monomer) concentrations of each component in the mixture. However, CMC values may be affected by the presence of compounds with other chain lengths in the mixture because of possible mixed micelle formation in solution.
In the present study, CMC or solubility is determined for a selection of surfactants and reference compounds with polyacrylate-coated SPME fibers and compared with values determined using a traditional surface tension method and with literature data. The selected surfactants cover nonionic, anionic, cationic, and zwitterionic surfactants (in total 12 compounds). Two reference substances were used to validate the measurements for nonionic compounds (reference: atrazine) and anionic compounds (reference: pentachlorophenol). Abbreviations and molecular structures of the test analytes are shown in Table 1. Properties and Chemical Abstracts Service registry numbers of the tested compounds are shown in Supplemental Data, Table S1.
 |
- C8EO4 = n-octyl tetraethylene glycol ether; C12EO4 = n-dodecyl tetraethylene glycol ether; C12EO8 = n-dodecyl octaethylene glycol ether; C11CO2 = n-undecyl carboxylate; C12SO4 = n-dodecyl sulfate; C12EO4SO4 = n-dodecyl tetraethoxy sulfate; C12TMAC = n-dodecyl trimethyl ammonium chloride; C16TMAC = n-hexadecyl trimethyl ammonium chloride; C18BAC = n-octadecyl dimethyl benzyl ammonium chloride; C12DMAO = n-dodecyl dimethyl amine oxide; C12DMB = n-dodecyl dimethyl betaine; C12APB = n-dodecyl amidopropyl betaine; ATR = atrazine; PCP = pentachlorophenol.
MATERIALS AND METHODS
Chemicals
Most chemicals were obtained from Sigma-Aldrich: n-octyl tetraethylene glycol ether (C8EO4; 98.7%), n-dodecyl tetraethylene glycol ether (C12EO4; 99.8%), n-dodecyl octaethylene glycol ether (C12EO8; 99.0%), n-dodecyl sulfate (C12SO4; Na-salt; 99.4%), n-undecyl carboxylate (C11CO2; Na-salt; 99.5%), n-dodecyl trimethyl ammonium chloride (C12TMAC; 99.3%), n-hexadecyl trimethyl ammonium chloride (C16TMAC; 100.2%), pentachlorophenol (99.9%), and atrazine (99.1%). The compound n-dodecyl tetraethoxy sulfate (C12EO4SO4; Na-salt; >95%) was supplied by Procter and Gamble. The compound n-dodecyl amidopropyl betaine (C12APB; 90–100%) was supplied by Huntsman Holland. The compounds n-octadecyl dimethyl benzyl ammonium chloride (C18BAC; 24% in water) and n-dodecyl dimethyl amine oxide (C12DMAO; 30% in water) were supplied by Stepan Europe. The compound n-alkyl dimethyl betaine (CxDMB) was supplied by Solvay with the following carbon chain length distribution: C10 (0–2%), C12 (62–68%), C14 (22–28%), C16 (8–12%), and C18 (0–2%). All compounds were solids except for C8EO4 (liquid), C18BAC (24% in water), and C12DMAO (30% in water). Sodium phosphate (NaH2PO4×H2O and Na2HPO4×12H2O), sodium azide, formic acid, and ammonium acetate were obtained from Sigma-Aldrich. Organic solvents (methanol and acetonitrile) were obtained from Biosolve. Ultrapure water was obtained from a Millipore water purification system (>18 MΩ cm; Merck Chemicals).
Fiber–water equilibration and sorption isotherms
Glass optical fibers coated with 35 µm polyacrylate (Vfiber = 15.4 µL/m), 7 µm polyacrylate (Vfiber = 3.3 µL/m), or 100 μm polydimethylsiloxane (Vfiber = 66.7 µL/m) were obtained from Polymicro Industries. Fibers were cut to an appropriate length (4 cm), conditioned at 120 °C for 16 h under a nitrogen flow, and stored in Millipore water until further use 14. Fibers were used only once for each measurement. Equilibrium between fiber and water was tested for all compounds by measuring concentrations of the test analyte in the fiber over time at constant aqueous concentrations (duplicate measurements). The medium composition in all experiments was buffered at pH 7 with 5 mM sodium phosphate, and 1 mM of sodium azide was added to prevent biodegradation of the test analytes. A well-controlled medium composition was used in the present study because the fiber–water distribution of ionic surfactants is highly dependent on pH and ionic strength (e.g., divalent inorganic cations) of the medium 15, 16.
Fiber–water sorption isotherms were obtained by exposing fibers to increasing analyte concentrations at equidistant intervals on a logarithmic scale (10 concentrations in triplicate) at the predetermined equilibration time ranging between 1 wk and 3 wk (Supplemental Data, Table S2). Different intervals were chosen below and above the CMC or solubility. Polyacrylate fibers with a coating thickness of 35 µm were used for all compounds in the equilibration and sorption isotherm experiments except for the sorption isotherm of C11CO2 where 7-µm fibers were used.
To achieve the desired concentrations, compounds were either added with methanol and subsequently evaporated overnight or added by weighing pure compounds. An ultrasonic bath was usually used to solubilize the compounds. Surfactants were added to the medium as individual compounds (no mixtures were used, except for the mixture of C12-16DMB). Glass scintillation vials were filled with minimum headspace volume (∼24.7 mL) to prevent analyte losses to the glass wall and/or liquid–gas interface and closed with a polyethylene-lined cap. The vials were shaken horizontally at 150 rpm at 20 °C in the dark.
At equilibrium, fibers were desorbed in methanol containing 10 mM ammonium acetate for nonionic and anionic compounds 16 or methanol containing 0.5% formic acid for cationic and zwitterionic compounds. Samples from the water phase were reconstituted to the same mobile-phase composition (Supplemental Data, Table S3) used in the liquid chromatographic analysis of individual compounds. Fiber extracts and water samples were diluted to a suitable concentration range for analysis of the test compounds. Fiber extracts were kept at room temperature to allow desorption of the analytes from the fiber. After 1 d, water was added to the fiber extracts for subsequent analysis (80:20, methanol:water, v/v). Both fiber and water samples were stored in a refrigerator prior to analysis and subsequently stored in a freezer at –20 °C. At the end of the experiments, the pH of the water phase was measured. Results of pH measurements are shown in Supplemental Data, Table S2. The cationic surfactants adsorbed on the glass surface was quantified by emptying the vials, subsequently weighing the vials (to correct for remaining water), and adding 3 mL of methanol with 0.1% formic acid to extract all compounds from the glass surface.
The cation exchange capacity (CEC) of 35-μm polyacrylate-coated fibers was determined with Ba/Ca exchange by exposing temperature-conditioned fibers with a length of 5 m in triplicate to 100 mM BaCl2 15. The fibers were shaken for 4 h and subsequently transferred to a solution containing 100 mM CaCl2. After 3 d of shaking, the solutions were acidified with 2% HNO3, and the Ba2+ concentration was measured with inductively coupled plasma-optical emission spectroscopy (Spectro CIROS CCD). The calculated CEC value was subsequently normalized to the volume of the fiber.
Chemical analysis
Both mass spectrometric and ultraviolet (UV) spectroscopic detection methods were used in the analysis of the compounds. All compounds except for pentachlorophenol and atrazine were analyzed on a Perkin Elmer liquid chromatographic system (PE200 series LC), coupled to a triple quadrupole mass spectrometer (MDS Sciex API 3000 MS/MS System; Applied Biosystems). The interface was a Turbo Ion spray source operated at 250 °C to 400 °C (Applied Biosystems). All compounds were optimized for mass spectrometry by direct infusion of standard solutions of the analytes. A solvent delay switch (Da Vinci) was used to prevent inorganic constituents in water samples (sodium azide and sodium phosphate) interfering with the ionization of the compounds and entering the mass spectrometer. Pentachlorophenol and atrazine were analyzed with UV detection on a Shimadzu Prominence high-performance liquid chromatography system (LC20 series). Columns used in the separation of test analytes were the Gracesmart C18 column (150 × 2.1 mm, 5 μm particle size; Grace Discovery Sciences) for C8EO4, C12EO4, C12EO8, atrazine, pentachlorophenol, and C11CO2; the Phenomenex Luna C18 (50 × 2 mm, 3 μm particle size) for C12SO4, C12EO4SO4, C12-16DMB, C12APB, and C12DMAO; and the Phenomenex Kinetex XB (100 × 3 mm, 2.6 μm particle size) for C12TMAC, C16TMAC, and C18BAC. The eluent composition was methanol/water containing 1 mM to 10 mM ammonium acetate for nonionic, anionic, and zwitterionic compounds; acetonitrile and water containing 0.1% formic acid for cationic compounds; and methanol and water containing 3 mM to 5 mM phosphate buffer at pH 3 for the reference compounds. The mass spectrometer setting and UV wavelengths used in the detection of the analytes are shown in Supplemental Data, Table S4. Chromatographic conditions, injection standards for correction of sample volume, and limits of quantification (10 times signal to noise ratio) are shown in Supplemental Data, Tables S3 and S5. Chromatograms were integrated with Analyst 1.4.2 (Applied Biosystems) or LC Solution 1.25 (Shimadzu).
Surface tension measurements
Surface tension (γ in millinewtons per meter) was measured with the ring method 17, which measures the resulting forces during deformations of the surface. The surface tension of the tested compounds was measured at 25 °C by adding aliquots of a stock solution (e.g., 0.5 g/L, 5 g/L, or 50 g/L) to a volume of 50 mL water with a medium composition similar to the medium used in the SPME experiments. Before addition of the compound, an equal volume of water was withdrawn from the solution and the concentration of the test compound recalculated. The CMC value was calculated from the derivative of the surface tension versus concentration profile, that is, the most negative γ value calculated as dγ/dln c. Based on several tests performed with C12SO4, this method was proved to have a higher reproducibility than the traditional intersection method.
Data analysis
The fiber–water sorption isotherms were fitted with Freundlich isotherms to check for linearity of the partitioning and distribution of surfactants between fiber and water. Linear regression was performed on the log-transformed values of the compound concentrations in fiber (cfiber) and water (cwater): log cfiber = log KF + N × log cwater (where KF is the Freundlich coefficient and N is the nonlinearity exponent). When linear partitioning or distribution of analytes between fiber and water applies, KF is equal to the fiber–water partition coefficient (Kfiber = cfiber/cwater) for neutral compounds. Distribution coefficients (Dfiber = cfiber/cwater) were used for ionized compounds. Note that these partition and distribution coefficients may indicate differences in hydrophobicity of surfactants. However, this approach is expected to be valid only within individual (homologous) groups of surfactants.
Average mass balances in the linear phase of the fiber–water sorption isotherm were calculated from the amounts of test analytes measured in fiber, water phase, and glass surface. The mass balances were expressed in percentages relative to the nominal amount added to the experimental system. The average percentage of compound sorbed to the fiber was derived in a similar way. All regressions and statistical analyses were performed with GraphPad Prism Ver 5.00 (GraphPad Software).
RESULTS AND DISCUSSION
Fiber–water equilibration
The logarithmic ratio of concentrations in fiber and water versus time is shown in Figure 1 for representative compounds of the tested surfactant groups. Equilibration curves for all compounds are shown in Supplemental Data, Figure S1. For nonionic surfactants, equilibrium between fiber and water of C12EO4 and C12EO8 was reached within approximately 2 d (Figure 1; Supplemental Data, Figure S1A). Equilibration of C8EO4 was not tested in the present study but should be within a similar time frame as the other alcohol ethoxylates because of its shorter chain length. The neutral reference compound atrazine also had an equilibration time of 2 d (Supplemental Data, Figure S1F). An equilibration time of 7 d was therefore selected for all nonionic compounds. Depletion of the aqueous phase by the fiber ranged from 0.1% to 4.9% (Supplemental Data, Table S6).
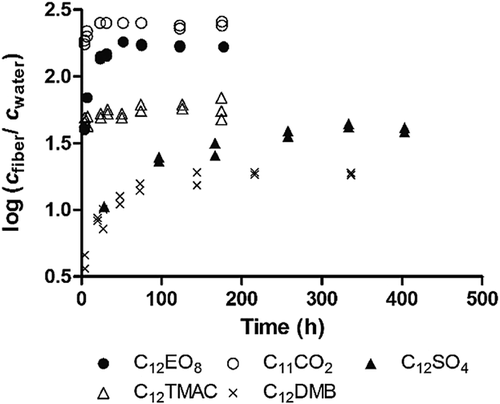
For anionic surfactants, a distinction was made between compounds with a short fiber–water equilibration time (dissociation constant [pKa] values close to 4–5) and a relatively high neutral fraction (see section Fiber–water sorption isotherms) and compounds with a long equilibration time (pKa << 4) and a negligibly low neutral fraction. For C11CO2 (with pKa ∼4.86), equilibrium between fiber and water was reached within approximately 1 d (Figure 1). The reference compound pentachlorophenol (with pKa = 4.35) reached equilibrium within 2 d (Supplemental Data, Figure S1F). An equilibration time of 1 wk was therefore selected for C11CO2 and pentachlorophenol. However, a much longer equilibration time of nearly 3 wk was observed for the anionic surfactant C12SO4 (with pKa = 1.9) compared to that of C11CO2 (Figure 1). The compound C12EO4SO4 did not reach equilibrium within 7 d (Supplemental Data, Figure S1B), but this compound had a similar equilibration time profile as C12SO4. An equilibration time of 3 wk was selected for both C12SO4 and C12EO4SO4. Depletion of the aqueous phase by the fiber was approximately 0.1%.
The cationic surfactants C12TMAC, C16TMAC, and C18BAC reached equilibrium between fiber and water after approximately 7 d (Figure 1; Supplemental Data, Figure S1C). To ensure that equilibrium between fiber and water is reached for all compounds, an equilibration time of 3 wk was selected for all cationic compounds. The aqueous phase was depleted by the fiber for <1% for C12TMAC and C16TMAC and up to 12% for C18BAC (Supplemental Data, Table S6).
For the zwitterionic compound C12DMB, equilibrium between fiber and water was reached within approximately 8 d (Figure 1; Supplemental Data, Figure S1D). Equilibrium between fiber and water for C12DMAO was reached after approximately 13 d (Supplemental Data, Figure S1E). An equilibration time of 3 wk was used for these compounds. Also, C12APB did not show any response on the fiber with increasing fiber lengths (4 cm, 8 cm, and 12 cm of 35-μm polyacrylate fiber). In addition, 7-μm polyacrylate and 100-μm polydimethylsiloxane fibers were used without success. No explanation was found for the lack of affinity of C12APB for the polyacrylate-coated fiber. Depletion of the aqueous phase by the fiber for C12-16DMB and C12DMAO was lower than 1%.
Fiber–water sorption isotherms
The fiber–water sorption isotherms for all alcohol ethoxylates showed linear partitioning to the fiber (Figure 2A) with nonlinearity exponents close to 1; that is, N ranged from 0.91 to 1.02. An overview of the determined regression parameters is shown in Supplemental Data, Table S7. Fiber–water partition coefficients increased by approximately 0.5 log unit per carbon atom and decreased by approximately 0.25 log unit per ethoxylate unit. The presence of ether units in alcohol ethoxylates decreased the affinity for the fiber, which shows that the polyacrylate polymer does not have a large hydrogen bond donating capacity, at least not much higher than the interactions of these compounds with water molecules. This is supported by a more extensive study of polyacrylate sorption data 18. The nonionic reference compound atrazine also showed linear partitioning to the fiber (N = 1.00; Figure 2F). Concentration-independent partitioning of nonionic compounds to polyacrylate-coated SPME fibers (including alcohol ethoxylates) has been observed before 13, 19. Mass balances for all nonionic compounds ranged from 81% to 121% (Supplemental Data, Table S6).
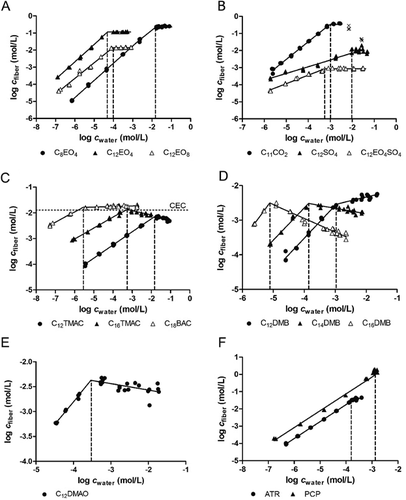
For the anionic surfactant C11CO2, a linear fiber–water sorption isotherm was observed (N = 1.04; Figure 2B). Polyacrylate fibers with different coating thicknesses were used in the fiber–water equilibration (7-μm fibers) and the sorption isotherm experiment (35-μm fibers) of C11CO2. The measured log Dfiber values for C11CO2 at pH 7 were found to be similar, that is, 2.39 for 35-μm fibers and 2.49 for 7-μm fibers in the equilibration and sorption isotherm experiments, respectively. Previous work showed that linear partitioning of the neutral form of C11CO2 (0.72% neutral at pH 7, pKa ∼4.86) dominated sorption to the fiber 16. Also, C11CO2 attained a maximum log Kfiber value at pH values that were 2 log units lower than the pKa value of C11CO2 (100% neutral) 16. The anionic reference compound pentachlorophenol (0.22% neutral at pH 7, pKa = 4.35) also showed linear partitioning to the fiber (N = 1.03 for pentachlorophenol; Figure 2F) in accordance with the results obtained for C11CO2. Mass balances were 110 ± 7% for C11CO2 and 101 ± 14% for pentachlorophenol.
Both anionic surfactants C12SO4 and C12EO4SO4 showed nonlinear distribution between fiber and water (N = 0.39 for C12SO4 and 0.50 for C12EO4SO4; Figure 2B). The reason for this nonlinearity may be that the neutral fraction of C12SO4 (7.94 × 10−4% neutral at pH 7, pKa = 1.9) is negligible compared to that of C11CO2 (0.72% neutral). Because of the much lower neutral fraction of both C12SO4 and C12EO4SO4, these compounds may therefore sorb with additional sorption mechanisms to the fiber, for example, by adsorption of anionic species to the fiber surface or absorption of ion pairs of sodium and surfactant ions into the polymer 16. Polyacrylate fibers with 7-μm coating were used in a previous study to measure C12SO4 in the same medium composition, and a higher response was found for 7-μm compared to 35-μm fibers 16. No plausible explanation could be found for the different responses of the 2 fibers, and the exact sorption mechanism for C12SO4 and C12EO4SO4 therefore remains inconclusive. Irrespective of the sorption mechanism, determination of CMC values for these types of compounds is not expected to be affected by the use of different coating thicknesses. The presence of 4 ethoxylate units in C12EO4SO4 decreased the sorption affinity for the fiber compared to C12SO4 (Figure 2B), similar to the trend observed for alcohol ethoxylates with increasing ethoxylation. Mass balances were 116 ± 14% for C12SO4 and 103 ± 9% for C12EO4SO4.
All cationic surfactants showed nonlinear distribution to the fiber (N = 0.51 for C12TMAC, 0.44 for C16TMAC, and 0.41 for C18BAC; Figure 2C). These compounds probably show specific adsorption to charged functional groups on the fiber surface because negatively charged carboxylic acid groups are believed to occur on the polyacrylate fiber surface 15, 20. Mass balances were 102 ± 8% for C12TMAC and 94 ± 8% for C16TMAC. Addition of concentrations of C18BAC in fiber and water only amounted to 32 ± 9%. However, the mass balance increased to 80 ± 2% when the amount of C18BAC sorbed to the glass surface was taken into account. Above concentrations in water of 6 mg/L C18BAC, the amount on the glass surface became insignificant in comparison with the total amount.
The zwitterionic surfactant mixture of alkyl dimethyl betaines consists of C12DMB (∼65%), C14DMB (∼25%), and C16DMB (∼10%). Surprisingly, the 3 alkyl dimethyl betaines showed linear partitioning to the fiber (N = 0.93 for C12DMB, 0.95 for C14DMB, and 1.22 for C16DMB, for the latter based on only 2 data points; Figure 2D). Linear partitioning to the fiber was also observed for C12DMAO (N = 0.90; Figure 2E). This may be explained by the amphoteric nature of the compounds. Because of the close proximity of the positive and negative charges in the molecular structures, shielding of charges may occur. The zwitterionic surfactants may therefore behave similarly as any other nonionic compound. However, equilibration times for zwitterionic surfactants were much longer compared to those for nonionic surfactants (∼8 d vs ∼2 d). The mass balances of the zwitterionic surfactants were 102 ± 6% (C12DMB), 111 ± 7% (C14DMB), 54 ± 6% (C16DMB), and 101 ± 14% (C12DMAO).
Determination of CMC
The CMC or solubility values were determined with 3 different methods from the fiber–water sorption isotherms (Figure 2). Depending on the trend in the concentrations of the fiber above the CMC value, a calculation method was selected. The CMC or solubility values were calculated by 1) determination of intersection of logarithmic–logarithmic regression lines when concentrations in the fiber are constant or have an upward or downward trend, 2) taking the average concentration in the fiber when precipitation of the compound occurred (i.e., concentrations in the water phase do not increase), or 3) taking the average of the 3 lowest concentrations of test analyte measured in the fiber and calculating the concentration in water with the regression line of the linear phase (on a logarithmic scale). The latter method was selected for compounds that show variable concentrations in the fiber when the CMC value was reached.
The SPME results showed that concentrations of all alcohol ethoxylates in the fiber remained constant above the CMC value (Figure 2A). This suggests that the freely dissolved monomer concentrations in aqueous solutions remained constant above the CMC value. The CMC values of all alcohol ethoxylates were determined from the intersection of linear regression lines of the log-transformed values below and above the CMC value. The CMC values of alcohol ethoxylates were also derived from surface tension measurements versus logarithmic aqueous concentration profiles (Supplemental Data, Figure S2). The CMC values determined with SPME for alcohol ethoxylates were close to the values from surface tension measurements (Supplemental Data, Table S7). For example, the CMC value of C12EO4 determined with SPME (18 mg/L) was close to a value determined with surface tension (34 mg/L). For the neutral reference compound atrazine, both concentrations in the fiber and water phase remained constant because of precipitation of the compound. The solubility of atrazine (39 mg/L) was close to a value from the literature (35 mg/L) 21. No surface tension data could be obtained for atrazine because of precipitation (atrazine is not a surface-active compound).
For the anionic surfactant C11CO2, determination of CMC (or solubility) with SPME was affected by precipitation in water. A “gel-like” precipitate was formed, and the pH value increased from 7 to 9.2 (Supplemental Data, Table S2 and Figure S3). Because the neutral fraction of C11CO2 was more than 100-fold lower at higher pH values, concentrations in the fiber also decreased (× symbols in Figure 2B). The calculated solubility of C11CO2 was therefore not considered reliable (CMC ∼238 mg/L). In addition, the calculated value was much lower than the value from surface tension measurements (1970 mg/L).
For the anionic surfactant C12SO4, constant concentrations in the fiber were not observed above the CMC value, but instead much more elevated concentrations were observed in the fiber (Figure 2B). This suggests that C12SO4 may have precipitated on the fiber surface, and derivation of the CMC value from these data was not considered reliable. The CMC value was calculated from the intersection of the fiber–water sorption isotherm with the 3 lowest concentrations measured in the fiber. The calculated CMC of C12SO4 was much higher (∼3701 mg/L) compared to the value (1150 mg/L) determined by surface tension. In contrast, the compound C12EO4SO4 showed constant concentrations in the fiber (Figure 2B). The CMC value of C12EO4SO4 was 281 mg/L compared to 405 mg/L determined with surface tension. The solubility of pentachlorophenol (658 mg/L) was lower than a value from the literature (1153 mg/L; Figure 2F) 22. No surface tension data could be obtained because of precipitation of this compound (pentachlorophenol is not a surface-active compound).
For the cationic surfactants C12TMAC and C16TMAC, the concentrations in the fiber decreased above the CMC value for both compounds (Figure 2C). No plausible explanation could be found for this behavior. The CMC values were calculated from the intersection of linear regression lines based on logarithmic concentrations in fiber and water. The CMC values determined with SPME for C12TMAC (4467 mg/L) and C16TMAC (125 mg/L) were 1.1 to 1.6 times higher than the values determined by surface tension (4060 mg/L and 80 mg/L, respectively). However, the calculated CMC value of C18BAC (∼1.2 mg/L) was 92 times lower compared to the value determined with surface tension (110 mg/L). The maximum concentration of C18BAC that is reached in the fiber (cfiber = 16.29 mmol/L) is very close to the CEC value determined for 35-μm fibers (CEC = 12 ± 3 mmol/L; Figure 2C). This suggests that the SPME fiber method has a clear limitation for the determination of CMC values for very hydrophobic cationic surfactants. Polyacrylate fibers with 7-μm coating thickness have a CEC value that is 2.7 times higher (CEC = 32 ± 5 mmol/L 15) than that of 35-μm fibers. However, it is not expected that determination of the CMC value of C18BAC would have been possible using fibers with a thinner coating.
For the zwitterionic compounds, the CMC values of the individual compounds of the alkyl dimethyl betaine mixture were reached at increasing concentrations according to chain length in the order of C16DMB < C14DMB < C12DMB (Figure 2D). For C16DMB and C14DMB, concentrations in the fiber decreased above the CMC value, whereas an increase of concentrations in the fiber was observed for C12DMB. The CMC values were calculated from the intersection of linear regression lines based on log-transformed values. The CMC value determined with SPME for C12DMB (376 mg/L) was higher than the value determined with surface tension (260 mg/L). Note that the surface tension method only gives a single CMC value for the same mixture, whereas the SPME method gives results for all individual compounds. The CMC values of C14DMB (56 mg/L) and C16DMB (1.6 mg/L) are logically lower because of their longer chain length. It is not known whether CMC values of the single compounds have been affected by other compounds in the mixture. The CMC values for the individual components in a mixture were also measured with SPME fibers for a mixture of 2 alcohol ethoxylates (C10EO4 and C12EO4; Supplemental Data, Figure S4). The CMC value of C12EO4 measured as a single compound was not affected by the presence of C10EO4 in the mixture. No conclusion could be drawn about the formation of mixed micelles from these experiments. For the zwitterionic compound C12DMAO, concentrations in the fiber decreased above the CMC value (Figure 2E). The CMC value determined with SPME for C12DMAO (67 mg/L) was much lower than the value determined with surface tension (1650 mg/L). The maximum total molar concentrations in the fiber for C12-16DMB and C12DMAO are 5.01 mmol/L and 4.25 mmol/L, respectively, which are lower than the CEC value determined with the fiber (CEC = 12 ± 3 mmol/L; Supplemental Data, Figure S5). Because the exact sorption mechanism of zwitterionics to the fiber is not known, it is unclear whether the CEC of the fiber is reached for these compounds. No definite conclusions can therefore be made about the reliability of the CMC values of the zwitterionic surfactants. The CMC value of C12APB could not be determined because of a lack of sensitivity of the polyacrylate polymer for this compound (486 mg/L determined with surface tension).
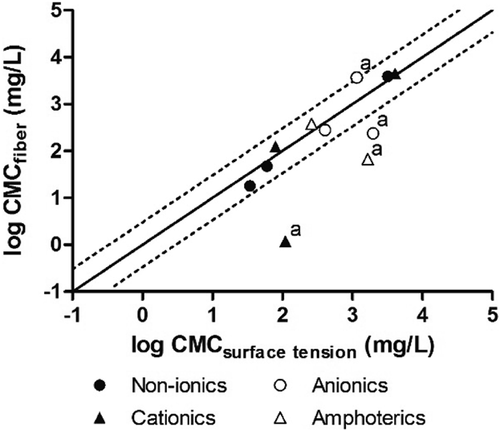
The CMC values determined using SPME deviated by less than a factor of 3 from surface tension measurements, except for C11CO2 (8 times lower), C18BAC (92 times lower), and C12DMAO (25 times lower; Figure 3). The CMC values of all compounds were also in good agreement with values from literature and deviated by a factor of maximum 3, except for C11CO2 (24 times lower), C18BAC (84 times lower), and C12DMAO (7 times lower). The fact that the deviation in values generated using SPME from both surface tension and literature values was systematically lower for the same 3 surfactants only suggests that the discrepancy was the result of systematic failings in the SPME method. Deviation of C11CO2 from surface tension or literature data can be explained by the pH-dependent response of the fiber when the pH of the samples increased after precipitation of the compound. The value determined for C12SO4 with the SPME method is higher than the value determined by surface tension because of precipitation of the compound on the fiber. The relatively low CMC value of C18BAC compared to both surface tension and literature data is caused by reaching the maximum concentration in the fiber (i.e., CEC) before the CMC of C18BAC is reached. The reason for the discrepancy of the CMC of C12DMAO compared to surface tension and literature data is not clear.
SUMMARY
The CMC values of the tested compounds logically decrease with increasing number of carbon atoms, which was observed for alcohol ethoxylates, alkyl trimethyl ammonium chlorides, and alkyl dimethyl betaines. The number of ethoxylate groups in the surfactant molecules shows a contrasting effect on the CMC value. Whereas CMC values of alcohol ethoxylates increased with the number of ethoxylate groups, the CMC value of C12 ethoxysulfate decreased with increasing number of ethoxylate groups (compared to C12 sulfate). However, clear relationships with the number of carbon atoms and/or other structural or functional groups can only be obtained with additional compounds in a homologous group for each class of surfactants.
Most values determined using the SPME method were consistent with both surface tension and literature values. For 3 surfactants only (C11CO2, C18BAC, and C12DMAO), however, CMC values were systematically lower compared to both surface tension and literature-derived values. For C11CO2 and C18BAC, these can be explained by the pH-dependent and concentration-dependent response of the fiber. No explanation can be found currently for the discrepancy of the SPME-derived CMC value for C12DMAO.
The present study's experiments also provided additional information about the sorption processes of different surfactants on the fiber. Whereas linear partitioning occurred for the nonionic surfactants and the reference compound atrazine, nonlinear distribution occurred for the anionic and cationic surfactants. The nonlinear distribution and the relatively long equilibration times of anionic surfactants with a low neutral fraction (C12SO4 and C12EO4SO4) can probably be explained by distribution of ion pairs to the fiber. Anionic compounds with a relatively high neutral fraction (C11CO2 and pentachlorophenol) showed linear distribution between fiber and water. Sorption of the neutral fraction was found to be the predominant sorption process for these compounds. The nonlinear distribution of cationic surfactants can be explained by sorption to cation exchange sites, that is, dissociated carboxylic acid groups on the fiber surface. Linear distribution occurred for the zwitterionic surfactants, which may be caused by shielding of both positive and negative charges on these compounds.
Above the CMC value, compound concentrations in the fiber were constant for all alcohol ethoxylates, C11CO2, C12EO4SO4, and C18BAC. For other compounds, concentrations in the fiber decreased at higher aqueous concentrations in the case of C12TMAC, C16TMAC, C16DMB, C14DMB, and C12DMAO and even increased for C12DMB. The same effect also occurred for C12SO4, which may explain the higher CMC value for this compound compared with the value found in the literature.
Perhaps 1 of the limitations of the present study is the lack of understanding that it has been able to provide on the interaction of individual surfactants when applied in a mixture. Although the data indicate that the presence of 1 compound does not influence the CMC value of another compound in a mixture, no conclusion can be drawn currently on the formation of mixed micelles.
Supplemental Data
The Supplemental Data are available on the Wiley Online Library at DOI: 10.1002/etc.3397.
Acknowledgment
The present study was jointly funded by the Environmental Risk Assessment and Management (Brussels) Task Force of “Hydrophobicity of Surfactants” and the Dutch Technology Foundation STW, which is part of the Netherlands Organization for Scientific Research and partly funded by the Ministry of Economic Affairs. The Faculty of Geosciences at Utrecht University is thanked for the inductively coupled plasma-optical emission spectroscopy measurements. We acknowledge IMETER/MSB Breitwieser MessSysteme (Augsburg, Germany) for the surface tension measurements.
Data availability
Data are available on request from the corresponding author, J.J.-H. Haftka ([email protected]).




