Human health risk assessment of triclosan in land-applied biosolids
Abstract
Triclosan (5-chloro-2-[2,4-dichlorophenoxy]-phenol) is an antimicrobial agent found in a variety of pharmaceutical and personal care products. Numerous studies have examined the occurrence and environmental fate of triclosan in wastewater, biosolids, biosolids-amended soils, and plants and organisms exposed to biosolid-amended soils. Triclosan has a propensity to adhere to organic carbon in biosolids and biosolid-amended soils. Land application of biosolids containing triclosan has the potential to contribute to multiple direct and indirect human health exposure pathways. To estimate exposures and human health risks from biosolid-borne triclosan, a risk assessment was conducted in general accordance with the methodology incorporated into the US Environmental Protection Agency's Part 503 biosolids rule. Human health exposures to biosolid-borne triclosan were estimated on the basis of published empirical data or modeled using upper-end environmental partitioning estimates. Similarly, a range of published triclosan human health toxicity values was evaluated. Margins of safety were estimated for 10 direct and indirect exposure pathways, both individually and combined. The present risk assessment found large margins of safety (>1000 to >100 000) for potential exposures to all pathways, even under the most conservative exposure and toxicity assumptions considered. The human health exposures and risks from biosolid-borne triclosan are concluded to be de minimis. Environ Toxicol Chem 2016;35:2358–2367. © 2016 SETAC
INTRODUCTION
Triclosan (5-chloro-2-[2,4-dichlorophenoxy]-phenol) is a chlorinated aromatic (diphenyl ether) compound commonly used as an antimicrobial in pharmaceutical and personal care products 1. As a consumer product ingredient, most triclosan is disposed of in municipal sewer systems, where it receives treatment in wastewater treatment plants (WWTPs) 2. Consequently, triclosan is frequently detected in municipal wastewater and municipal wastewater sludge. For example, surveys conducted by the US Environmental Protection Agency (USEPA) and the Canadian Council of Ministers of the Environment found triclosan in 92.4% and 97% of collected sewage sludge samples, respectively 3, 4. Municipal wastewater sludge (biosolids) can be applied to agricultural land to increase nutrient and organic matter content of the soil, with the aim of improving crop yield and soil tilth 5. For example, the rate of biosolid use for agricultural soil amendment has been estimated to be approximately 36% in the United States (or nearly 2.7 M metric tons in 2004 6) and approximately 39% in the European Union (EU; or nearly 3.9 M metric tons based on data from 2002 to 2008 7). As a result, biosolid amendment of agricultural soils can introduce triclosan into agricultural systems with the potential for subsequent human health exposures.
The occurrence and fate of triclosan in wastewater, biosolids, biosolid-treated soils, and in plants and organisms exposed to biosolid-amended soils, have been the subject of many studies 8-10. In addition, potential ecological risks from exposure to biosolid-borne triclosan have been previously evaluated 11. Finally, several laboratory and field studies have examined the uptake of triclosan into plants grown on biosolid-amended soil and estimated risks from human consumption of those plant tissues 10-13 For example, Prosser and Sibley 13 concluded that consumption of plant tissues containing triclosan grown in soil amended with biosolids likely represents a de minimis risk to human health. Health Canada and Environment Canada conducted an aggregate risk assessment using a margin of safety analysis based on total triclosan in urine measured by the US National Health and Nutrition Examination Surveys and other studies 14. Health Canada and Environment Canada's risk assessment found large margins of safety ranging from 702 to 8562. To the best of our knowledge, a human health risk assessment based on environmental data that considers all potential exposure pathways associated with biosolid-borne triclosan has not been conducted. Understanding human health exposure and risks associated with biosolid-borne triclosan is important, because the beneficial use of biosolids as an agricultural soil amendment is expected to continue being an important practice.
In 1995, the USEPA published a general set of human health exposure pathways as part of the biosolids risk assessment rule (Part 503). The Part 503 rule was developed to provide a comprehensive risk-based framework to protect public health and the environment from potential adverse effects of pollutants that may be present in biosolids 15. The Part 503 methodology is similar to existing methodologies used to evaluate risks from biosolid-borne chemicals in the EU and Canada, as well as general chemical risk assessment guidance 16, 17. Biosolid application rates in the United States are typically higher than outside the United States, such as in the EU and Canada 11. Therefore, risk estimates obtained using the methodology established in Part 503 are considered relevant to countries and regions outside of the United States.
The objective of the present study was to provide a contemporary human health risk assessment for exposure to biosolid-borne triclosan. Specifically, we conducted a multipathway human health risk assessment for biosolid-borne triclosan, following the Part 503 methodology. Human health exposures were quantified using published empirical triclosan exposure estimates or modeled on the basis of published environmental fate and transport parameters of triclosan.
MATERIALS AND METHODS
The Part 503 rule (USEPA) risk assessment framework was used to evaluate potential biosolid-borne triclosan exposures and risks to humans. Literature-based chemical and fate and transport parameters are incorporated into the 4 basic steps of risk assessment: hazard identification, exposure assessment, dose–response evaluation, and risk characterization. The Part 503 rule outlines 14 exposure pathways (9 human and 5 ecological pathways) to characterize potential risk to humans, animals, and plants from the land application of biosolids. As our assessment is limited to human health, the 5 ecological pathways with animals and plants as endpoint receptors were not included.
Exposure pathways and highly exposed individuals
Land application of biosolids to soil may contribute to multiple direct and indirect human health exposure pathways. The human health exposure pathways from the Part 503 rule included in the present study are shown in Table 1. They include both direct (e.g., soil contact) and indirect exposure pathways (e.g., leaching to groundwater). Highly exposed individuals, as defined in Part 503, are modeled to represent the subset of the population at higher risk than the general population. These individuals may include those people who live and/or work on and consume food products from farmlands where biosolids are routinely applied to surface soils 15.
| Exposure pathways | Description |
|---|---|
| Direct soil pathways | |
| Biosolids→soil→human (oral) | Lifetime (adult and child) incidental ingestion of biosolid-amended soils |
| Biosolids→soil→dust or volatilesa→human (inhalation) | Lifetime (adult and child) inhalation (dust or volatilesa) in biosolid-amended soils |
| Biosolids→soil→human (skin)b | Lifetime (adult and child) dermal absorption from biosolid-amended soils |
| Indirect water pathways | |
| Biosolids→soil→surface water→human (oral) | Lifetime (adult and child) incidental ingestion of surface water containing triclosan leached from biosolid-amended soils |
| Biosolids→soil→surface water→human (skin)b | Lifetime (adult and child) dermal absorption of surface water containing triclosan leached from biosolid-amended soils |
| Biosolids→soil→groundwater→human (oral) | Lifetime (adult and child) drinking well water containing triclosan leached from biosolid-amended soils |
| Biosolids→soil→groundwater→human (skin)b | Lifetime (adult and child) dermal absorption of drinking well water containing triclosan leached from biosolid-amended soils |
| Indirect food consumption pathways | |
| Biosolids→soil→plant→human (oral) | Lifetime (adult and child) ingestion of plants grown in biosolid-amended soil |
| Biosolids→soil→plant→animal→human (oral) | Lifetime (adult and child) ingestion of animal products (animals raised on plants grown in biosolid-amended soil) |
| Biosolids→soil→animal→human (oral) | Lifetime (adult and child) ingestion of animal products (animals ingesting biosolid-amended soil) |
| Biosolids→soil→surface water→animal→human (oral) | Lifetime (adult and child) ingestion of fish containing triclosan leached from biosolid-amended soils |
- a The US Environmental Protection Agency (USEPA) 22 considers volatile compounds to be those chemicals with a Henry's law constant ≥ 1 × 10−5 atm-m3/mol and a molecular weight < 200 g/mol. Triclosan has a Henry's law constant of 2.1 × 10−8 atm-m3/mol (modeled at 25 °C; calculated using EPI Suite V4.11, 2012 [USEPA 24] and reported to be between 4.99 × 10−9 and 2.27 × 10−8 atm-m3/mol by Bock et al. 25) and a molecular weight of 290 g/mol, and is therefore not considered a volatile compound.
- b Dermal exposure pathways are not included in Part 503 (USEPA 15), but were included here consistent with relevant USEPA guidance (USEPA 19).
Several USEPA exposure guidance documents have been generated since the development of Part 503 18-22, and many of the exposure assumptions were updated to reflect these changes. Specifically, body weight, exposure duration, exposure frequencies, surface and drinking water ingestion, and food consumption were updated to reflect current USEPA exposure guidance (Table 2). As recommended in Part 503, chemical-specific information is incorporated into a screening assessment using existing data compiled from the peer-reviewed literature where available. The Part 503 rule does not include pathways for evaluating dermal exposure to biosolid-borne chemicals that may be present in soils or water. Dermal exposures are expected to be limited for triclosan, because in vitro absorption studies (i.e., using full-thickness human skin samples) indicate limited dermal absorption rates (7.2–12.0%) 23. Still, to be comprehensive, we included dermal exposures using the models developed by the USEPA 18, 19, 22 and using the upper bound absorption rate of 12% 23. This assumption is expected to provide an overestimate of dermal exposure and risks because it relies on absorption rates determined in studies designed to mimic actual product use conditions. Dermal absorption from soil particles is expected to be lower because of the relatively strong adsorption of triclosan to soil particles.
| Child | Adult | |||
|---|---|---|---|---|
| Parameter | Unit | (1–6 yr) | (18 yr and older) | Reference |
| General parameters | ||||
| Body weight | kg | 15 | 80 | USEPA 21 |
| Exposure duration | yr | 6 | 20 | USEPA 22 |
| Averaging time | d | 2190 | 10 950 | USEPA 15, 22, 54 |
| Frequency parameters | ||||
| Exposure frequency [Soil exposures] | d/yr | 350 | 350 | USEPA 22 |
| Exposure frequency [Surface water exposures] | d/yr | 100 | 100 | General assumption |
| Exposure frequency [Groundwater exposures] | d/yr | 350 | 350 | USEPA 22 |
| Events frequency | Events/d | 1 | 1 | General assumption |
| Ingestion exposure parameters | ||||
| Soil ingestion rate | mg/d | 200 | 100 | USEPA 15, 22, 54 |
| Surface water ingestion | L/h | 0.05 | 0.05 | USEPA 20, 22 |
| Drinking water ingestion | L/d | 0.78 | 2.5 | USEPA 21 |
| (mean) | (mean) | USEPA 20 | ||
| [average 1–7-yr-old] | ||||
| Recreational fish consumption | kg/d | 0.054 | 0.054 | USEPA 22 |
| Fruit/vegetable consumption | kg/d | 0.162 | 0.252 | USEPA 21 |
| Meat consumption | kg/d | USEPA 21 | ||
| Beef | 0.025 | 0.059 | ||
| Pork | 0.016 | 0.031 | ||
| Poultry | 0.032 | 0.064 | ||
| Dairy | 0.384 | 0.245 | ||
| Dermal exposure parameters | ||||
| Dermal surface area [Soil exposures] | cm2 | 2690 | 6032 | USEPA 18, 22 |
| Soil adherence factor | mg/cm2/event | 0.1 | 0.2 | USEPA 18, 22 |
| Dermal surface area [Water exposures] | cm2 | 6378 | 20 900 | USEPA 19 |
Inhalation of dust or volatiles is considered in Rule 503 (Table 1). However, inhalation of volatilized triclosan was not quantified in the present risk assessment. The USEPA 22 identifies volatile compounds as those chemicals with a Henry's law constant ≥ 1 × 10−5 atm-m3/mole and a molecular weight < 200 g/mol. Using these criteria, triclosan is not considered a volatile compound—its Henry's law constant is 2.1 × 10−8 atm-m3/mole (modeled at 25 °C; calculated using EPI Suite Ver 4.11, 2012 24 and reported to be between 4.99 × 10−9 atm-m3/mol and 2.27 × 10−8 atm-m3/mol by Bock et al. 25), and its molecular weight is 290 g/mol. Therefore, risks from exposure to biosolid-borne triclosan via inhalation are considered to be negligible. In summary, with the exception of inhalation exposures (which are considered negligible for biosolid-borne triclosan), all the human exposure pathways included in the Part 503 rule risk assessment and several dermal exposure pathways not included in the Part 503 rule risk assessment (i.e., soil, groundwater, and surface water) were quantified. This resulted in exposure estimates for 10 direct and indirect exposure pathways, both individually and combined (Table 1).
Estimates of triclosan concentrations in biosolid-amended soil
Triclosan concentrations in biosolid-amended soils have been described by several authors [8–11]. For example, Fuchsman et al. 11 conducted a probabilistic assessment of triclosan concentrations in biosolid-amended soils based on biosolid samples from WWTPs. The biosolid triclosan data were almost exclusively representative of United States' WWTPs (93 of 96 WWTPS sampled). Triclosan concentrations in biosolids from non-United States' WWTPs are predicted to be lower than in the United States, based on lower concentrations in WWTP influent 11. The authors fitted a distribution to the reported triclosan biosolid concentrations. Similarly, the authors fitted a distribution to a large published compilation of reported United States' biosolid application rates (note that application rates in Europe and Canada are more restrictively regulated and expected to be lower). Next, the biosolid triclosan concentration and application rate distributions were used to predict triclosan concentrations in biosolid-amended soils (i.e., mixed soil zone): 0.21 (50th percentile), 1.2 (80th percentile), 2.6 (90th percentile), and 4.5 mg triclosan/kg dry weight (95th percentile) 11. The 50th percentile and 95th percentile were used in the present risk assessment to represent average and upper bound triclosan concentrations in biosolid-amended soil (Table 3). Given the approach used by Fuchsman et al. 11, the upper bound (95th percentile) exposure estimate used in the present risk assessment represents a combination of the highest reported biosolid triclosan concentrations and the highest reported biosolid application rates. In addition, we assumed typical agricultural tillage rather than no-till practices because predicted triclosan concentrations in biosolid-amended soil were much greater for tilled soils than for no-till soils (e.g., the 50th percentile and 95th percentile model predictions by Fuchsman et al. 11 for no-till soils are 0.026 mg/kg and 0.34 mg/kg dry wt, compared with 0.21 mg/kg and 4.5 mg/kg dry wt for tilled soils, respectively).
| Parameter | Value | Notes | Range of literature Values | Reference(s) |
|---|---|---|---|---|
| Triclosan biosolid-amended soil concentration (mg/kg dry wt) | 0.21 4.5 | 50th percentile 95th percentile | Modeled 50th to 95th percentiles (0.21–4.5 mg/kg) | Fuchsman et al. 11 |
| Soil partitioning coefficient (log Kd) | 1.54–2.91 | Range of reported values | Mean log Kd of 2.31 (range = 1.54–2.91) in biosolid-amended soils | Cha and Cupples 26; Agyin-Birikorang et al. 27 |
| Organic-carbon partitioning coefficient (log KOC) | 4.12–4.37 | Range of reported values | Mean log KOC = 4.26 (range = 4.12–4.37) in biosolid-amended soils | Agyin-Birikorang et al. 27 |
| Biotransfer factor (BTF) | 0.195 | Estimated value | None identified | USEPA 30 |
| Plant bioaccumulation factor (BAF) (mg/kg dry wt tissue per mg/kg soil dry wt) | 0.4 | Conservative worst-case approximation of bioaccumulation for all the plants based on field studies | Range of reported BAFs (<0.001–0.4) | Pannu et al. 10 |
| Fish bioconcentration factor (BCF) (L/kg wet wt) | 7900 | Highest estimate from modeled or measured BCFs | Range of reported BCFs (642–7900a) | EPI Suite V4.11 (USEPA 24); Schettgen et al. 55 |
| Molecular weight (g/mol) | 289.55 | EPI Suite V4.11 (USEPA 24) | ||
| Dermal permeability coefficient of compound in water (Kp), (cm/h) | 0.0692 | Modeled value; Conservative worst-case approximation for dermal absorption | Range of modeled Kp values (0.00261–0.0692) | EPI Suite V4.11 (USEPA 24) |
- a Note that the upper-end BCF value of 7900 (from Schettgen et al. 55) was found to be highly uncertain and overestimated, compared with other experimentally derived BCF values and QSAR estimates, as a result of missing information on test preparation, the high variation in the recovery of triclosan in the fish tissue, and the high concentrations of methanol used (REACH submission EC 222-182-2 for triclosan).
Estimated triclosan concentrations in groundwater and surface water
Several indirect exposure pathways (Table 1) assume that triclosan may leach from soils to soil porewater and migrate to groundwater and surface water. The USEPA's Part 503 rule 15 and the USEPA's Regional Screening Level guidance 22 include models for estimating leaching from soils into groundwater and surface water. These models estimate concentrations using environmental partitioning coefficients (e.g., soil–water partition coefficient [Kd], soil organic carbon–water partitioning coefficient [KOC]). These parameters are available for triclosan from the published literature. For example, Cha and Cupples 26 conducted leaching experiments with triclosan and reported a log Kd of 1.54 L/kg to 1.74 L/kg and a log KOC of 3.60 L/kg to 3.80 L/kg. Agyin-Birikorang et al. 27 examined the retention and release characteristics of triclosan in biosolid-amended soils and reported an average log Kd of 2.31 (range = 1.90–2.91) and an average log KOC of 4.26 (range = 4.12–4.37). Wu et al. 28 reported that triclosan can persist in soils for several days to months and although amendment with biosolids increased the sorptive strength, it had no detectable effect on degradation. Both groundwater and surface water modeled concentrations using the USEPA's Regional Screening Level guidance, groundwater leaching, and the Rule 503 surface water algorithms were compared with reported environmental concentrations to determine whether modeled concentrations are representative of measured estimates of triclosan in varying environmental media. Both minimum (1.54) and maximum (2.91) log Kd values were used to estimate groundwater and surface water concentrations. By using the lower bound log Kd value (1.54) in the soil for groundwater and surface water modeling, the modeled groundwater triclosan concentrations were 96% higher than when the upper bound log Kd value was used (surface water concentrations were 1% higher). The higher groundwater and surface water concentration estimates (i.e., using the lowest Kd value) were used in the risk assessment to provide an upper bound estimate.
Estimated triclosan concentrations in plant and animal tissue
Bioconcentration (BCF) and bioaccumulation factors (BAF) in fish and plants have been published for triclosan (Table 3). Upper bound BCF and BAF values, as reported in the literature, were used to calculate an upper bound estimate of triclosan concentrations in fish and plants. This resulted in bioaccumulation estimates that are expected to overestimate actual bioaccumulation and therefore serve as conservative inputs to the risk assessment. For example, no evidence has been found of triclosan bioaccumulation in mammals, because it is quickly metabolized 14, 29, and the upper bound reported fish BCF value that was used is considered an overestimate compared with other reported values (Table 3). Similarly, studies have shown that the potential for triclosan uptake into vegetables grown under normal farming conditions is very low 12, 13, in contrast with uptake factors derived from greenhouse studies concluding that the potential for uptake into crops is more significant 9, 10. Furthermore, no studies measuring triclosan concentrations in agricultural animals were found in the literature. As a result, we relied on the USEPA's simplified biotransfer models to estimate uptake of triclosan into farm-raised animal products. The primary limitation of this model is the potential to overestimate biotransfer for chemicals that are metabolized 30. In mammals, triclosan is metabolized to glucuronide or sulfate conjugates in the liver 23. Thus, the estimate of biotransfer in animal products is considered a conservative upper bound assumption.
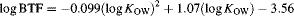
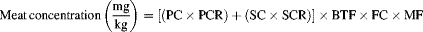
Hazard assessment
Several reviews have characterized the human toxicology of triclosan 23, 31-33. The USEPA 32 reviewed existing animal data and determined that triclosan is “not likely to be carcinogenic to humans” according to the USEPA's cancer guidelines. This classification was also reported by the EU 33. Rodricks et al. 23 reviewed existing carcinogenicity, mutagenicity, and genotoxicity studies. For in vivo carcinogenicity bioassays, existing data (based on studies with mice, rats, and hamsters) indicated that treatment-related tumors were found only in the liver of male and female mice. However, application of the Human Relevance Framework demonstrated that these tumors arose by way of peroxisome proliferator-activated receptor α activation, a mode of action not considered to be relevant to humans. Results from in vitro and in vivo mutagenicity and genotoxicity assays taken together did not indicate that triclosan (or related metabolites) was mutagenic or genotoxic 23. Accordingly, based on the above reviews, triclosan is not considered a human carcinogen, and risks are based on noncancer endpoints.
Several studies report data for multiple noncancer health endpoints following triclosan exposure 23, 31-33. Endpoints include alterations to hematological parameters and kidney and liver effects. The USEPA has not developed an oral reference dose for triclosan in its Integrated Risk Information System database. However, the USEPA used a no-observed-adverse effect level (NOAEL) of 30 mg/kg/d to derive an oral reference dose in the 2008 Reregistration Eligibility Decision of triclosan 34. This NOAEL and other previously published toxicity reference values were compiled and ranged from 12 mg/kg/d to 47 mg/kg/d (Table 4). Rodricks et al. 23 conducted a detailed assessment of the toxicological database for triclosan, following USEPA benchmark dose guidance 35 and considering over 50 health endpoints, and developed a lower bound benchmark dose level of 47 mg/kg/d. The benchmark dose approach involves dose–response modeling to obtain dose levels corresponding to specific response levels (e.g., 10%) and incorporates and conveys more information than the NOAEL or lowest-observed-adverse effect level (LOAEL) 35. Although this methodology is used in both the United States and the EU, no other regulatory agency has published detailed benchmark dose evaluations of the supporting literature to derive toxicity values for triclosan. As shown in Table 4, other available toxicity values for triclosan are based on NOAELs. Because NOAELs are driven by the dose selection used in the underlying toxicity study, they reflect a conservative estimate of the lower end toxicity threshold (i.e., the actual NOAEL might be higher). Therefore, to ensure conservative risk estimates and explore a range of potential margin of safety estimates, the upper bound was based on the benchmark dose level of 47 mg/kg/d (from Rodricks et al. 23) and a lower bound based on a NOAEL of 12 mg/kg/d (from the European Commission 33) of triclosan noncancer toxicity reference values.
| Effect level (mg/kg/d) | Study | Source |
|---|---|---|
| 12 | Reported a NOEL (12 mg/kg/d) for hematological parameters and relative spleen weights in rats exposure orally for 52 wk | European Commission 33 |
| 25 | Based on a NOAEL (25 mg/kg/d) for maternal and developmental toxicity in mice | Norwegian Scientific Committee for Food Safety 56; RIVM 57; Health Canada and Environment Canada 14 |
| 30 | Based on a NOAEL (30 mg/kg/d) reported for clinical signs of toxicity in baboons following chronic oral exposure | USEPA 32 |
| 40 | Based on a NOAEL (40 mg/kg/d) reported from a 2-yr carcinogenicity study in rats based on clinical chemistry and liver histopathology | NICNAS 29 |
| 47 | Based on a lower bound benchmark dose level (BMDL = 47 mg/kg/d) with the best fitting model from numerous toxicity studies. The final value was based on male hamster kidney nephropathy | Rodricks et al. 23 |
- BMDL = benchmark dose level; NOAEL = no-observed-adverse-effect level; NOEL = no-observed-effect level.
Several recent studies have evaluated potential effects of triclosan on thyroid function 36-46. The USEPA indicated in their 2008 reregistration of triclosan that there is some evidence that triclosan disrupts thyroid homeostasis but concluded it is not readily apparent from the available toxicology database for triclosan that the effects observed are the direct result of perturbations of thyroid homeostasis 34. As a result, the USEPA did not use the cited studies to estimate triclosan risks. In its work plan for the upcoming reregistration of triclosan, the USEPA again cited a number of recent studies that have looked at effects on the thyroid, including 2 benchmark dose level values based on a 20% reduction in serum total thyroxine (T4) levels in rats exposed by oral gavage 47. For example, the USEPA cited a study by Crofton et al. 42 that derived a benchmark dose level of 35.6 mg/kg/d, following a 4-d exposure and a study by Zorilla et al. 38 that derived a benchmark dose level of 7.23 mg/kg/d, following a 30-d exposure. A recent literature review by Witorsch 39 found the Zorilla et al. 38 benchmark dose level to be among the lowest compared with other published benchmark dose level values for a 20% reduction in serum T4 levels in rats, for example, 8.07 mg/kg/d 48, 35.6 mg/kg.d 42, and 25.2 mg/kg/d to 104 mg/kg/d 37, 44, 46. Witorsch 39 further concludes that studies show triclosan exposure consistently produced a decrease in serum T4 in rats without any consistent change in other thyroid-related effects. However, there is currently no scientific consensus on whether reported changes in serum T4 following triclosan exposure are either adverse or the result of a direct endocrine mode of action. Similarly, both the EU Scientific Committee on Consumer Safety and the US Federal Insecticide, Fungicide, and Rodenticide Act (FIFRA) Scientific Advisory Panel considered the effects of triclosan on thyroid hormone homeostasis in rats and their relevance to humans. The Scientific Committee on Consumer Safety regarded a decrease in T4 levels following exposure to triclosan in rats as a biochemical effect marker that is not appropriate for human health risk assessment because of the major differences between humans and rats in thyroid hormone physiology and regulation 48. The FIFRA Scientific Advisory Panel noted that additional causal data are needed before a 20% decrease in T4 levels can be used as a point of departure for a risk assessment 48. Given this, we did not make further adjustments to our lower end benchmark dose level value (i.e., 12 mg/kg/d).
Risk assessment
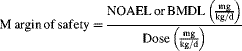
RESULTS
Predicted triclosan concentrations (at the 50th and 95th percentiles) and corresponding daily dose exposure estimates (mg/kg/d) are reported in Table 5. The margin of safety estimates for each exposure pathway and using a range of toxicity benchmarks are presented in Table 6. We examine the modeled environmental triclosan concentrations and margin of safety estimates throughout the Results section.
| Predicted triclosan concentrations | Daily triclosan intake (mg/kg/d) | |||||
|---|---|---|---|---|---|---|
| Exposure pathway | Receptor | Unit | 50th percentile | 95th percentile | 50th percentile | 95th percentile |
| Direct soil pathways | ||||||
| Soil (incidental ingestion) | Adult | mg/kg dry wt | 2.10E–01 | 4.50E + 00 | 2.52E–07 | 5.39E–06 |
| Child | mg/kg dry wt | 2.10E–01 | 4.50E + 00 | 2.68E–06 | 5.75E–05 | |
| Soil (dermal exposure) | Adult | mg/kg dry wt | 2.10E–01 | 4.50E + 00 | 3.64E–07 | 7.81E–06 |
| Child | mg/kg dry wt | 2.10E–01 | 4.50E + 00 | 4.33E–07 | 7.81E–06 | |
| Indirect water pathways | ||||||
| Surface water (incidental ingestion) | Adult | mg/L | 2.22E–08 | 4.75E–07 | 4.34E–12 | 9.29E–11 |
| Child | mg/L | 2.22E–08 | 4.75E–07 | 1.90E–11 | 4.07E–10 | |
| Surface water (dermal exposure) | Adult | mg/L | 2.22E–08 | 4.75E–07 | 7.34E–13 | 1.57E–11 |
| Child | mg/L | 2.22E–08 | 4.75E–07 | 9.80E–13 | 2.10E–11 | |
| Groundwater (incidental ingestion) | Adult | mg/L | 6.02E–03 | 1.29E–01 | 2.06E–04 | 4.42E–03 |
| Child | mg/L | 6.02E–03 | 1.29E–01 | 2.81E–04 | 6.03E–03 | |
| Groundwater (dermal exposure) | Adult | mg/L | 6.02E–03 | 1.29E–01 | 4.96E–07 | 1.06E–05 |
| Child | mg/L | 6.02E–03 | 1.29E–01 | 5.03E–07 | 1.08E–05 | |
| Indirect food consumption pathways | ||||||
| Produce consumption | Adult | mg/kg wet wt | 1.68E–02 | 3.60E–01 | 5.80E–05 | 1.24E–03 |
| Child | mg/kg wet wt | 1.68E–02 | 3.60E–01 | 1.63E–04 | 3.49E–03 | |
| Beef consumption | Adult | mg/kg wet wt | 1.14E–02 | 2.43E–01 | 9.15E–06 | 1.96E–04 |
| Child | mg/kg wet wt | 1.14E–02 | 2.43E–01 | 1.70E–05 | 3.64E–04 | |
| Pork consumption | Adult | mg/kg wet wt | 7.03E–03 | 1.51E–01 | 2.96E–06 | 6.35E–05 |
| Child | mg/kg wet wt | 7.03E–03 | 1.51E–01 | 6.74E–06 | 1.44E–04 | |
| Chicken consumption | Adult | mg/kg wet wt | 2.18E–04 | 4.67E–03 | 1.92E–07 | 4.12E–06 |
| Child | mg/kg wet wt | 2.18E–04 | 4.67E–03 | 4.22E–07 | 9.04E–06 | |
| Dairy consumption | Adult | mg/kg wet wt | 3.28E–03 | 7.02E–02 | 1.10E–05 | 2.36E–04 |
| Child | mg/kg wet wt | 3.28E–03 | 7.02E–02 | 7.54E–05 | 1.62E–03 | |
| Fish consumption | Adult | mg/kg wet wt | 1.75E–04 | 3.75E–03 | 1.30E–07 | 2.78E–06 |
| Child | mg/kg wet wt | 1.75E–04 | 3.75E–03 | 5.67E–07 | 1.21E–05 | |
| All pathways | ||||||
| Cumulative exposure | Adult | – | – | 2.88E–04 | 6.18E–03 | |
| Cumulative exposure | Child | – | – | 5.47E–04 | 1.17E–02 | |
| Toxicity benchmark (mg/kg/d)a | Margin of safetya (lower estimate: 12 mg/kg/d) | Margin of safetya (upper estimate: 47 mg/kg/d) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Exposure pathways | Receptor | Lower estimate | Upper estimate | 50th percentile | 95th percentile | % of total risk | 50th percentile | 95th percentile | % of total risk |
| Direct soil pathways | |||||||||
| Soil (incidental ingestion) | Adult | 12 | 47 | 4.77E + 07 | 2.22E + 06 | 0.09 | 1.87E + 08 | 8.71E + 06 | 0.09 |
| Soil (dermal exposure) | Child | 12 | 47 | 4.47E + 06 | 2.09E + 05 | 0.49 | 1.75E + 07 | 8.17E + 05 | 0.49 |
| Adult | 12 | 47 | 3.29E + 07 | 1.54E + 06 | 0.13 | 1.29E + 08 | 6.02E + 06 | 0.13 | |
| Child | 12 | 47 | 2.77E + 07 | 1.54E + 06 | 0.07 | 1.08E + 08 | 6.02E + 06 | 0.07 | |
| Indirect water pathways | |||||||||
| Surface water (incidental ingestion) | Adult | 12 | 47 | 2.77E + 12 | 1.29E + 11 | <0.01 | 1.08E + 13 | 5.06E + 11 | <0.01 |
| Child | 12 | 47 | 6.32E + 11 | 2.95E + 10 | <0.01 | 2.48E + 12 | 1.16E + 11 | <0.01 | |
| Surface water (dermal exposure) | Adult | 12 | 47 | 1.63E + 13 | 7.63E + 11 | <0.01 | 6.40E + 13 | 2.99E + 12 | <0.01 |
| Child | 12 | 47 | 1.22E + 13 | 5.71E + 11 | <0.01 | 4.79E + 13 | 2.24E + 12 | <0.01 | |
| Groundwater (incidental ingestion) | Adult | 12 | 47 | 5.82E + 04 | 2.72E + 03 | 71.54 | 2.28E + 05 | 1.06E + 04 | 71.54 |
| Child | 12 | 47 | 4.26E + 04 | 1.99E + 03 | 51.43 | 1.67E + 05 | 7.79E + 03 | 51.43 | |
| Groundwater (dermal exposure) | Adult | 12 | 47 | 2.42E + 07 | 1.13E + 06 | 0.09 | 9.48E + 07 | 4.42E + 06 | 0.09 |
| Child | 12 | 47 | 2.38E + 07 | 1.11E + 06 | 0.09 | 9.34E + 07 | 4.36E + 06 | 0.09 | |
| Indirect food consumption pathways | |||||||||
| Produce consumption | Adult | 12 | 47 | 2.07E + 05 | 9.66E + 03 | 20.12 | 8.10E + 05 | 3.78E + 04 | 20.12 |
| Child | 12 | 47 | 7.38E + 04 | 3.44E + 03 | 29.73 | 2.89E + 05 | 1.35E + 04 | 29.73 | |
| Beef consumption | Adult | 12 | 47 | 1.31E + 06 | 6.12E + 04 | 3.17 | 5.14E + 06 | 2.40E + 05 | 3.17 |
| Child | 12 | 47 | 7.06E + 05 | 3.30E + 04 | 3.10 | 2.77E + 06 | 1.29E + 05 | 3.10 | |
| Pork consumption | Adult | 12 | 47 | 4.05E + 06 | 1.89E + 05 | 1.03 | 1.59E + 07 | 7.40E + 05 | 1.03 |
| Child | 12 | 47 | 1.78E + 06 | 8.31E + 04 | 1.23 | 6.98E + 06 | 3.26E + 05 | 1.23 | |
| Chicken consumption | Adult | 12 | 47 | 6.24E + 07 | 2.91E + 06 | 0.07 | 2.45E + 08 | 1.14E + 07 | 0.07 |
| Child | 12 | 47 | 2.84E + 07 | 1.33E + 06 | 0.08 | 1.11E + 08 | 5.20E + 06 | 0.08 | |
| Dairy consumption | Adult | 12 | 47 | 1.09E + 06 | 5.09E + 04 | 3.81 | 4.27E + 06 | 1.99E + 05 | 3.81 |
| Child | 12 | 47 | 1.59E + 05 | 7.43E + 03 | 13.77 | 6.23E + 05 | 2.91E + 04 | 13.77 | |
| Fish consumption | Adult | 12 | 47 | 9.26E + 07 | 4.32E + 06 | 0.04 | 3.63E + 08 | 1.69E + 07 | 0.04 |
| Child | 12 | 47 | 2.12E + 07 | 9.88E + 05 | 0.10 | 8.29E + 07 | 3.87E + 06 | 0.10 | |
| All pathways | |||||||||
| Cumulative exposure | Adult | 12 | 47 | 41 628 | 1943 | 163 044 | 7609 | ||
| Cumulative exposure | Child | 12 | 47 | 21 921 | 1023 | 85 855 | 4007 | ||
- a See Table 4 for description of toxicity benchmark values.
Estimated triclosan concentrations in groundwater and surface water
Concentrations of triclosan in groundwater and surface water were modeled following the algorithms presented in the USEPA's Rule 503 guidance and based on the assumption that triclosan concentrations in biosolid-amended soils were 0.21 mg/kg (50th percentile) or 4.5 mg/kg (95th percentile), respectively 11. We investigated a range of partitioning coefficients to characterize triclosan concentrations in groundwater and surface water. Estimated triclosan concentrations in groundwater varied by almost 10-fold. At the modeled 50th percentile soil triclosan concentration, the predicted groundwater concentration ranged from 0.0003 mg/L to 0.0060 mg/L, depending on the range of soil partitioning coefficients (i.e., 1.54–2.91; Table 3). At the modeled 95th percentile soil triclosan concentration, the predicted groundwater concentration varied from 0.0055 mg/L to 0.13 mg/L. In studies simulating runoff following application of biosolids to an agricultural field, triclosan concentrations ranged from 0.000026 mg/L to 0.00011 mg/L 50, 51, at least 3 orders of magnitude lower than our predicted groundwater concentrations. Thus, our modeled estimates significantly overestimate groundwater triclosan exposures compared with field-based measurements. Although these modeled estimates are appropriate for deriving upper end risk estimates in the context of the present study, they are clearly not reflective of real-world biosolid-borne triclosan concentrations in groundwater and surface water.
Estimated triclosan concentrations in surface water were less variable than the estimates for groundwater. At the 50th percentile soil triclosan concentration, the predicted surface water concentrations ranged from 2.19 × 10−8 to 2.22 × 10−8 mg/L, depending on the soil partitioning coefficient (i.e., 1.54–2.91; Table 3). At the 95th percentile soil triclosan concentration, the surface water concentration varied from 4.69 × 10−7 to 4.75 × 10−7 mg/L. Measured surface water triclosan concentrations reported in the literature ranged from below the detection limit to 0.0023 mg/L 52. However, these higher surface water concentrations of triclosan reported in the literature are typically found near WWTP effluent, are often biased toward locations susceptible to contamination, and also capture other sources of triclosan, beyond the contribution from biosolids. Accordingly, land application of biosolid-borne triclosan is predicted to be a relatively insignificant source of triclosan in surface water.
Estimated triclosan concentrations in plant and animal products
Based on a field study by Prosser et al. 53, triclosan uptake into edible plant tissues grown in triclosan-contaminated biosolids is expected to be negligible. However, to conservatively estimate upper bound risks, we applied an upper bound soil-to-plant BAF (0.4 g dry wt plant tissue/g dry wt soil; Table 3) to estimate triclosan concentrations in fruits and vegetables. Pannu et al. 10 described this BAF as a conservative worst-case approximation, as the maximum BAF from field studies was 0.16. The concentration estimates were converted to wet weight by assuming an 80% moisture content. The corresponding 50th percentile and 95th percentile plant triclosan concentrations estimates were 0.017 mg/kg and 0.36 mg/kg wet weight, respectively. The maximum triclosan concentrations measured in edible plant tissue grown in triclosan-amended biosolids and soil available in the literature ranged from 24.8 ng/g to 49.8 ng/g dry weight 53, or 0.0050 mg/kg to 0.010 mg/kg assuming an 80% moisture content. The modeled plant triclosan concentrations are over 30 times higher than the field estimates reported by Prosser et al. 53. Other field studies have similarly shown very low triclosan uptake into edible plant tissue, when grown under realistic farming conditions 12, 13, in contrast to short-term greenhouse or laboratory studies that do not adequately reflect triclosan degradation and loss from the time of application to crop harvest.
The biotransfer rate for triclosan from soil to animal products was estimated at 0.195 d/kg, based on the methods provided by the USEPA 30. The corresponding beef, pork, chicken, and dairy tissue concentrations are provided in Table 5 based on the 50th percentile and 95th percentile soil triclosan concentrations. No studies measuring triclosan concentrations in agricultural animal products were found for comparison. Still, our modeled tissue concentrations are expected to significantly overestimate actual tissue concentrations, because triclosan has been shown not to bioaccumulate in mammalian tissue 14, 29.
Fish tissue concentrations were estimated using modeled surface water concentrations and a fish BCF. A range of BCFs are reported in the literature (Table 3); we selected the highest reported BCF to provide an upper bound estimate of triclosan concentrations in fish tissue. The corresponding 50th percentile and 95th percentile fish tissue triclosan concentrations estimates are 0.00018 mg/kg and 0.0020 mg/kg wet weight, respectively. Measured triclosan concentrations in fish tissue available in the literature ranged from below detection limits to 0.031 mg/kg wet weight 52. Similar to measured surface water concentrations of triclosan, these higher measured fish tissue triclosan concentrations are reflective of other sources of triclosan, beyond contributions from biosolids alone, as modeled in the present study. Therefore, biosolids are not expected to be a significant source of triclosan in fish tissue, and the modeled triclosan fish concentrations given in the present study are only considered appropriate for the purpose of conservatively estimating biosolids-borne risks via the fish consumption pathway.
Risk characterization
Adults
The cumulative average daily doses of triclosan ranged from 2.88 × 10−4 mg/kg/d to 6.18 × 10−3 mg/kg/d for the 50th percentile and 95th percentile soil triclosan concentrations, respectively (Table 5). Using the most conservative log Kd of 1.54 and the 95th percentile soil triclosan concentrations, groundwater ingestion was the largest contributor to overall exposure (72% of the total intake), followed by produce consumption (20% of total intake). When a higher log Kd of 2.91 and the 95th percentile soil triclosan concentrations were used, produce consumption was the largest contributor to overall exposure (64%), followed by dairy consumption (12%), and the groundwater ingestion contribution was reduced to 10%. Lower and upper margins of safety estimates were all well above 100 for any combination of exposure or toxicity benchmark level. The margin of safety for cumulative exposure ranged from 1943 (lower end based on 95th percentile exposure) to 163 044 (upper end based on 50th percentile exposure; Table 6).
In addition, risks were estimated using upper bound measured values of triclosan in surface water and fish tissue reported in the literature, which were significantly higher than what we modeled based on biosolid-borne contributions alone. Still, cumulative margins of safety were well above levels of concern, ranging from 1936 (95th percentile exposure) to 150 874 (50th percentile exposure). Thus, large margins of safety are predicted for adults exposed to biosolid-borne triclosan.
Children
The cumulative average daily dose of triclosan for children ranged from 5.47 × 10−4 mg/kg/d to 1.17 × 10−2 mg/kg/d for the 50th percentile and 95th percentile soil triclosan concentrations, respectively (Table 5). Using the most conservative log Kd of 1.54 and the 95th percentile soil triclosan concentrations, groundwater ingestion was the largest contributor to overall exposure (51% of the total intake), followed by produce consumption (30% of total intake), and dairy consumption (14% of total intake). When a higher log Kd of 2.91 and the 95th percentile soil triclosan concentrations were used, produce consumption was the largest contributor to overall exposure (59%), followed by dairy consumption (27%), and groundwater ingestion contribution was reduced to 4%. Lower and upper margins of safety estimates were all above 100 for any combination of exposure or toxicity benchmark level (Table 6). The margin of safety for cumulative exposure ranged from 1023 (lower end based on 95th percentile exposure) to 85 855 (upper end based on 50th percentile exposure; Table 6).
When the risk models were run using literature-based measured triclosan concentrations for surface water and fish tissue, the cumulative margins of safety were still above levels of concern, ranging from 1015 (95th percentile exposure) to 72 401 (50th percentile exposure). Thus, large margins of safety are predicted for children exposed to biosolid-borne triclosan.
DISCUSSION AND CONCLUSIONS
A conservative multipathway human health risk assessment was conducted to estimate potential exposures and risks to biosolid-borne triclosan. We estimated exposure to triclosan using upper bound concentrations for biosolid-amended soils and conservative modeled exposure estimates in groundwater, surface water, and food products. For example, we assumed that all soil ingestion (i.e., 100 mg/d for adults and 200 mg/d for children) was from areas with biosolid-amended soils. In addition, we assumed that all produce consumed was derived from agricultural land amended with triclosan-containing biosolids. Furthermore, there is significant variability in the environmental partitioning coefficients (log Kd and KOC and BAFs and BCFs) that have been reported for triclosan. In all cases, we relied on the upper bound values reported in the literature to model triclosan concentrations in groundwater, surface water, and produce. These resulted in exposure estimates for groundwater, surface water, or plant and animal products that exceeded typical concentrations reported in field studies in the literature. Even relying on these upper bound exposure assumptions, large margins of safety (>1000 to >100 000) were calculated for cumulative exposure from all pathways.
A range of noncancer toxicity reference values (i.e., range of 12–47 mg/kg/d) was used in the present study's risk assessment. As discussed in the section Hazard assessment, some benchmark dose levels have recently been reported for triclosan based on a 20% reduction in serum T4 levels in rats (Zorilla et al. 38; Witorsch 39; Crofton et al. 42); Paul et al. 37, 44, 46). Based on currently available data, both the USEPA and the EU Scientific Committee on Consumer Safety determined that this endpoint is not appropriate as a point of departure for a risk assessment. However, even reported benchmark dose level values for thyroid effects below our lower end benchmark dose level (e.g., 7.23 mg/kg/d 42) would result in large margins of safety (>600 to >13 000 for 95th and 50th percentile exposures, respectively) in the present study's risk assessment and therefore would not impact its conclusions. This provides significant confidence in the conclusion that biosolid-borne triclosan does not pose adverse human health effects, even in highly exposed individuals.
Triclosan is frequently detected in biosolids as a result of its use as an antimicrobial in consumer products. Upper bound measured concentrations of biosolid-borne triclosan and conservatively modeled concentrations of triclosan in groundwater, surface water, and produce were used in a multipathway risk assessment consistent with the USEPA's Part 503 biosolids rule. The present study's modeled biosolid-borne triclosan concentrations exceeded upper bound reported measurements for all media, where available, illustrating that these are indeed conservative estimates. Estimated exposures from biosolid-borne triclosan resulted in large margins of safety using a range of toxicity values. The present study clearly demonstrates that biosolid-borne triclosan is not a significant contributor to human health exposure and risk.
Acknowledgment
The Colgate-Palmolive Company funded the present study.
Data availability
All data are available from the cited references included in the manuscript. Further information is available from the corresponding author ([email protected]).




