In response: Industry perspective
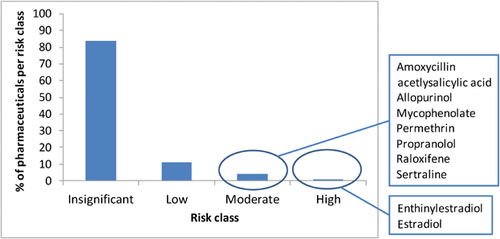
Pharmaceuticals are the most rigorously tested products in the world in terms of assessing their impact on human health. Development of a new medicine can take more than 10 yr, and only 1 in 10 000 new chemical entities progresses from concept stage to market, passing all required efficacy and preclinical and clinical safety tests, including those supporting the environmental risk assessment 1. Since 2006, approximately 200 to 300 new pharmaceutical products have been tested under current regulatory environmental risk-assessment guidelines in the European Union 2, and none has been shown to present a significant risk to the environment through routine patient use. Similarly, according to retrospective risk analyses compiled by the Swedish Association of Pharmaceutical Industries 3, only 2 out of 200 products are categorized as “high risk” to the environment (Figure 1), again suggesting that the risks are low or insignificant for the vast majority of pharmaceuticals. There are, however, 2 exceptions where potential and known risks to the environment have been identified. The first is the synthetic estrogen 17α-ethynylestradiol (EE2), which has been the subject of numerous research projects, including studies on potential adverse effects on wild fish populations 4, 5. This has led to the inclusion of EE2 (together with 17β-estradiol [E2]) on the European Union “watch list” under the Water Framework Directive 6. The second is the anti-inflammatory drug diclofenac, which has been causally linked to the near extinction of 3 species of vulture in Asia 7 because of its veterinary use in cattle and secondary poisoning of vultures. The impact of diclofenac on populations of fish or other aquatic taxa is unknown, but the scale of the impact on vultures has raised sufficient stakeholder concerns such that this compound has also been placed on the European Union watch list. In addition, there are 3000 to 4000 legacy pharmaceutical products on the market that predate the 2006 European Union environmental risk-assessment guideline; and, for many of these, there are little or no data available to assess risks 8. Thus, although most assessed drugs are predicted to present no significant risks to the environment, the above exceptions point toward 3 clear needs for better understanding the risks of pharmaceuticals in the environment. First, we need to ensure that environmental risk-assessment tools are fit for purpose and are able to identify any potential risks. Second, we need tools and approaches to help prioritize and identify whether any of the existing legacy products present a risk. Third, we need to know what further assessment and management options exist if a potential risk is identified. These 3 needs are explored in more detail in the following sections.
Ensuring environmental risk-assessment tools are fit for purpose
Environmental risk assessment generally consists of an exposure assessment, generating the predicted environmental concentration (PEC), and an effects assessment, generating the predicted no-effect concentration (PNEC). Both the PEC and PNEC are needed to characterize risk. The principal environmental exposure pathway for pharmaceuticals is excretion from patients, collection in sewage, sewage treatment, and aqueous discharge to surface waters, because the majority of pharmaceuticals are hydrophilic by design 9. However, there are exceptions to this rule. There are also differences in practices and infrastructure in different countries, which may be important to environmental risk assessment. For example, different countries have different processes and efficiencies of wastewater treatment. Similarly, in the European Union, irrigation of land using wastewater is not considered a significant exposure pathway, but this is commonplace in drought-stressed parts of the world 10. The need for careful evaluation of exposure pathways—including the consideration of local climates, demographics, cultural practices, and infrastructure—is exemplified by the diclofenac/vulture story mentioned earlier.
Within exposure assessment, there is also considerable scope to refine the PEC for each environmental compartment. This is initially based on total concentrations of drug residues in the environment, estimated from product use, and worst-case assumptions of 100% excretion from patients and 0% removal or degradation in sewage-treatment plants 2. In reality, actual environmental concentrations may be much lower than these predictions; but measured data often are lacking, and few studies have sought to evaluate removal efficiencies in sewage-treatment works or to quantify variability 11. Generic models capable of linking influent and effluent concentrations in sewage-treatment works and predicting or extrapolating environmental concentrations of pharmaceuticals potentially offer powerful tools for refining exposure assessments, provided that they are appropriately calibrated with reliable monitoring data. Such data also will support additional refinement of risk assessments concerning metabolites or degradation products 12.
Effects assessment typically focuses on chronic developmental or reproductive end points in model species representing a range of taxonomic groups, trophic levels, and environments 2. Other more targeted end points reflecting the specific mode of action of each pharmaceutical potentially offer increased sensitivity, provided therapeutic concentrations and effects reliably “read across” from mammalian (human) models to wildlife or surrogate test species 13. Assumptions still have to be made concerning the conservation of function of proteins targeted by drugs, along with their secondary targets and associated side effects, which should not be overlooked in wildlife. Nevertheless, the increasing availability of data, including preclinical test data, and rapid scientific development in this area, add to the future potential of mode of action/read-across–based environmental risk assessment of pharmaceuticals 14.
Prioritizing legacy compounds
Given the number of legacy products on the market, the fact that the environmental risk-assessment process is resource-intensive and requires animal testing, and the fact that the vast majority of compounds are likely to be of low or insignificant risk, it is clear that effective risk-based prioritization is needed. One simple approach would be to consider usage volume alongside therapeutic potency. An active pharmaceutical ingredient with high potency and high total usage would obviously be a higher priority than an active pharmaceutical ingredient with low potency and low usage. Although the sensitivities of environmental species do not always match those of the targeted therapeutic end points in humans, the minimum therapeutic doses for active pharmaceutical ingredients can provide some insight into the potential for physiological responsiveness of environmental species, at least in vertebrates. This approach has been applied in principle to generate a shortlist of pharmaceuticals designed to arrest cell division and growth or modulate hormonal and reproductive function—that is, modes of action that might relate to adverse effects in nontarget organisms 15, 16. This mode of action/read-across approach may offer further potential for prioritizing the environmental risk assessment of other legacy pharmaceuticals; however, this potential has yet to be realized. This is because very few empirical studies have related internal doses of pharmaceuticals to specific mode of action end points and adverse effects measured according to traditional apical end points 17. Nevertheless, several toxicological properties in mammals (reproductive and developmental toxicity, mutagenicity, carcinogenicity) and therapeutic targets (hormonal, anticancer, antimicrobial) are known for pharmaceuticals and could be used to support a screening assessment in a prioritization context.
Once a list of prioritized compounds is agreed to, a program of testing can be initiated to generate the data needed to conduct the environmental risk assessment. The commitment to do this will require significant resources, and thus it will be important that all stakeholders agree on the criteria used to prioritize. To this end, the European Federation of Pharmaceutical Trade Associations and the European Commission have just launched a call for proposals under the Innovative Medicines Initiative to develop tools and approaches for environmental risk-assessment screening 18. If successful, this program will not only develop the tools needed but also engage stakeholders across industry, academia, and regulatory groups in determining how this prioritization should be done.
What to do once a risk is identified
Determining what further assessment and management options exist if a potential risk is identified is less about refining the PEC:PNEC (discussed in Ensuring environmental risk-assessment tools are fit for purpose) and more about deciding what to do if a refined PEC:PNEC is greater than 1, as exemplified by EE2. In such cases, there is a need to go beyond the PEC:PNEC approach and consider ecological significance in the receiving environment. The inclusion of EE2 on the EU watch list may result in considerably more data on environmental concentrations but will do little to inform the risk assessment, unless the significance or consequence of the observed environmental concentrations can be established.
A key requirement in chemical and pharmaceutical environmental risk assessment is extrapolation from individual organism to population-level effects, for example, using population modeling since the protection of wildlife populations is the widely stated minimum protection goal in environmental legislation 19. This is rarely done in environmental risk assessments across all chemical sectors, although suitable tools are becoming increasingly available 20. Extrapolating risks based on laboratory tests to field populations could also benefit from a cumulative risk-assessment approach, in which the exposure and effects of pharmaceuticals and other components of environmental mixtures are considered. To some extent these, cumulative effects can be accounted for in population models. However, the inherent variation of the environment and the complexity of sewage effluents containing pharmaceuticals make defining a typical environmental chemical mixture virtually impossible in prospective environmental risk assessment. Instead, effort could focus on the retrospective assessment of hot spots, using sensitive biomonitoring and chemical monitoring tools and improving wastewater treatment in those areas where ecological health is potentially degraded. This may ultimately be the most pragmatic and economically attractive approach for society and may deliver the greatest ecological benefits, without necessarily having to relate adverse effects in the environment to any specific cause, let alone specific chemical.
In summary, the current weight of evidence suggests that the risks to the environment are low or insignificant for the vast majority of pharmaceuticals. Where potential risks have been identified, several tools exist to help refine the environmental risk assessment, particularly with respect to the exposure assessment, and there are several possibilities for the development of screening tools to help prioritize testing of legacy products. Ultimately, the role of environmental risk assessment is to maximize the chances of identifying a risk to the environment before any harm occurs; and, in this respect, further research is needed to understand how we can better utilize the wealth of clinical and preclinical data which are uniquely available for pharmaceuticals, compared with any other class of chemical. If, after undertaking laboratory studies, a potential risk is not excluded, further risk refinement may lead to population-level risk assessment or monitoring in the receiving environment. In this context, pharmaceuticals are no different from any other chemical entering the environment, and accepted, validated tools are needed to assess risk at the population level and to provide reliable indicators of ecological health.
-
A. Ross Brown
-
AstraZeneca, Global SHE
-
Brixham, United Kingdom




