Chromium resistance of dandelion (Taraxacum platypecidum Diels.) and bermudagrass (Cynodon dactylon [Linn.] Pers.) is enhanced by arbuscular mycorrhiza in Cr(VI)-contaminated soils
Abstract
In a greenhouse pot experiment, dandelion (Taraxacum platypecidum Diels.) and bermudagrass (Cynodon dactylon[Linn.] Pers.), inoculated with and without arbuscular mycorrhizal fungus (AMF) Rhizophagus irregularis, were grown in chromium (Cr)-amended soils (0 mg/kg, 5 mg/kg, 10 mg/kg, and 20 mg/kg Cr[VI]) to test whether arbuscular mycorrhizal (AM) symbiosis can improve Cr tolerance in different plant species. The experimental results indicated that the dry weights of both plant species were dramatically increased by AM symbiosis. Mycorrhizal colonization increased plant P concentrations and decreased Cr concentrations and Cr translocation from roots to shoots for dandelion; in contrast, mycorrhizal colonization decreased plant Cr concentrations without improvement of P nutrition in bermudagrass. Chromium speciation analysis revealed that AM symbiosis potentially altered Cr species and bioavailability in the rhizosphere. The study confirmed the protective effects of AMF on host plants under Cr contaminations. Environ Toxicol Chem 2014; 33:2105–2113. © 2014 SETAC
INTRODUCTION
Chromium (Cr), an essential trace element for glucose metabolism in animals, is also widely used in the chemical industry for electroplating, leather tanning, pigment production, and other processes. In past decades, the extensive use of Cr has resulted in large quantities of Cr being discharged into soil and water bodies around the world. Excessive Cr could be highly toxic for many living organisms, including human beings, animals, plants, and microoganisms 1. As a nonessential element for plants, Cr often interferes with photosynthetic and respiration processes, causes oxidative damage, restrains important enzymatic activities, and even causes plant death 2, 3.
It is well established that the rhizosphere serves as a favorable habitat for soil microorganisms. Some rhizosphere microorganisms establish intimate relationships with plants. A group of ubiquitous soil fungi, arbuscular mycorrhizal fungi (AMF), can form symbioses with the majority of terrestrial plants and improve plant water and nutrient (phosphorus, nitrogen, etc.) uptake especially under stressed conditions 4. Arbuscular mycorrhizal fungi may also improve soil structure by excreting organic compounds such as glomalin 5. In a natural ecosystem, AMF can take an active part in maintaining plant biodiversity and ecosystem stability 6. Many studies have shown that AMF could improve plant performance under heavy metal contamination (such as As, Cu, and Cd) 7-9. For example, AMF significantly improved the growth of Pteris vittata, Coreopsis drummondii, and Trifolium repens in Cu mine tailings 8. In an As-contaminated soil, AMF also enhanced As tolerance of both white clover (Trifolium repens Linn.) and ryegrass (Lolium perenne L.) 9. However, beyond the report on enhanced Cr resistance of sunflower by AMF 10, 11, studies on the role of AMF in plant adaptation to Cr contamination are limited. Moreover, it is important to note that the effectiveness of arbuscular mycorrhizal (AM) symbioses usually varies with plant species 8, even with genotypes of the same plant species 12. To date, however, few studies have addressed the potential influence of plant species (especially plants of different Cr tolerance) when evaluating the importance of AM association in plant adaptation to Cr contaminated environments. Whether AM symbioses can influence Cr speciation in the rhizosphere and further affect Cr uptake by plants is also unresolved, although Cr bioavailability is known to be strongly related to its speciation in soil 13, 14.
As a widely distributed herb in temperate and subtropical regions, dandelion (Taraxacum platypecidum Diels.) has been found to be sensitive to Cr and may serve as an ideal bioindicator of Cr pollution 15, 16. By contrast, bermudagrass (Cynodon dactylon [Linn.] Pers.), another herb commonly found in temperate regions, has been demonstrated to be Cr tolerant 17, 18. Previous studies 19, 20 have demonstrated that AMF could improve bermudagrass growth in As-contaminated soils and inhibit As transport from roots to shoots. However, there are no reports on the role of AMF in dandelion and bermudagrass adaptation to Cr contamination. In the present study, these 2 plant species in association with the AMF Rhizophagus irregularis were grown in soils artificially spiked with different concentrations of Cr(VI), and plant growth, mineral nutrition, and Cr uptake were investigated. The Cr species in soil and its accessibility to plants also were assessed. The aims of the present study are, therefore, to evaluate the importance of AMF in Cr tolerance of different plant species with different Cr tolerance and to elucidate the influences of AM symbiosis on Cr speciation and bioavailability in the rhizosphere. We hypothesized that AM symbiosis can enhance plant Cr tolerance of both plant species. In addition, we also assumed that AM symbiosis can alter Cr bioavailability by influencing Cr speciation in the rhizosphere.
MATERIALS AND METHODS
Growth medium
Soil was collected from Panggezhuang, Daxing district, Beijing (39°36′N, 116°18′E) and had the following physicochemical properties (based on dry wt): pH, (1:2.5 in water) 8.5; 1.02% organic matter; 5.90 mg/kg extractable P (by 0.5 mol/LNaHCO3); 1.37 mg/kg extractable Cr (by 2 mol/L HCl); 1.15 mg/kg extractable Cr (by 0.11 mol/L acetic acid); 0.32 mg/kg Cr(VI) (United States Environmental Protection Agency [USEPA] method 3060A); 0.084% total N (elemental analyzer; Vario EIII, Elementar); and 0.082% total P (digested by HCl + HNO3 + HF + HClO4 [10:5:5:3]). Soil properties were detected by inductively coupled plasma–optical emission spectrometer (ICP-OES; Prodigy, Teledyne Leeman). Total metal concentrations (digested by HCl + HNO3 + HF + HClO4 [10:5:5:3]) were detected by ICP-OES in soil: Cr, 67.3 mg/kg; Zn, 83.3 mg/kg; Cu, 55.5 mg/kg; Mn, 553 mg/kg; and Fe, 22 800 mg/kg. The soil was passed through a 2-mm sieve and then sterilized by gamma rays (20 kGy, 10 MeV electron beam). Before the experiment, basal nutrients of 30 mg/kg P, 120 mg/kg N, and 120 mg/kg K were carefully mixed into the soil.
Host plant
Seeds of dandelion (T. platypecidum Diels) and bermudagrass (C. dactylon [Linn.] Pers) were purchased from Beijing Greatgreen Ecological Technology Development Company. The seeds were surface sterilized with 10% H2O2 for 30 min, washed carefully with Milli-Q water, and then pregerminated on moist filter paper until the emergence of radicles.
Arbuscular mycorrhizal fungus
The AMF R. irregularis Schenck & Smith (BGC AH01; formerly Glomus intraradices) was obtained from Institute of Plant Nutrition and Resources, Beijing Academy of Agriculture and Forestry. The fungus was propagated in pot culture on Sorghum bicolor (L.) Moench in a sandy soil for 10 wk. Inoculum from the pot culture comprised a mixture of spores (approximately 150 spores/g), mycelium, sandy soil, and plant root fragments.
Experimental procedure
We added 0 mg/kg Cr, 5 mg/kg Cr, 10 mg/kg Cr, and 20 mg/kg Cr in the form of K2CrO4 (Cr[VI]) to the soil and then carefully mixed to ensure uniformity. The soil was incubated for 120 d to allow metal equilibrium. For each Cr(VI) addition level, 450 g of amended soil was first put into a pot, then 300 g of soil containing 30 g of fungal inoculum were added for AMF inoculation. For noninoculation controls, 30 g of sterilized inoculum were added together with 10-mL inoculum filtrate to reintroduce soil microbial communities except for AMF. Each of the 8 combinations of Cr addition and mycorrhizal inoculation were sown with either 10 pregerminated dandelion seeds or 10 bermudagrass seeds. Seedlings were thinned 1 wk after emergence to 2 per pot for dandelion and 4 per pot for bermudagrass. There were in total 16 treatments with 4 replicates, each resulting in a total of 64 pots. Each pot was watered daily with deionized water to maintain moisture content of 15% on a dry weight basis (∼55% water-holding capacity).
The experiment was conducted in a controlled growth chamber at a light intensity of 700 µmol m−2s−1, a 16:8-h light:dark photoperiod, a temperature of 25 °C (light) and 20 °C (dark), and 70% relative humidity. The plants were maintained for 80 d before harvest.
Harvest and chemical analysis
The day before harvest, leaf chlorophyll concentrations were recorded using a chlorophyll meter (Model SPAD-502; Minolta). Plant shoots and roots were harvested separately and washed carefully with deionized water. Subsamples of fresh roots were collected for the determination of AM colonization. Dry weights of shoots and roots were determined after oven-drying at 70 °C for 48 h. Dried samples were milled and digested in HNO3 using a microwave-accelerated reaction system (Mars 5; CEM) in a three-step digestion program. The temperature was gently raised to 120 °C over a period of 8 min, held for 3 min, raised to 160 °C over a period of 11 min, held for 7 min, and finally raised to 190 °C over a period of 8 min and held for 20 min. The dissolved samples were then diluted to 50 mL with Milli-Q water. Plant P concentrations were determined by ICP-OES (Prodigy; Teledyne Leeman), and plant Cr concentrations were determined by inductively coupled plasma-mass spectrometry (ICP-MS; model 7500a; Agilent Technologies). Blanks and internal standards of bush leaves (GBW07603; China Standard Research Center) and tea (GBW10016; China Standard Research Center) were used to ensure the accuracy of chemical analysis. Subsamples of fresh roots were cleared in 10% KOH and stained with Trypan blue following a modification of the procedure of Phillips and Hayman 21 in which phenol was omitted from solutions and HCl from the rinse. The root colonization percentage was determined by the method of MYCOCALC software (National Institute for Agricultural Research [INRA]) 22. Soil Cr(VI) concentration was determined according to USEPA method 3060A 23. Soil extractable-P concentrations were determined using the method described by Olsen et al. 24. Soil Cr speciation was determined by a European Community Bureau of Reference (BCR) 3-step sequential extraction procedure 25, in which total soil Cr was made up of 4 Cr species: acid-extractable, reducible, oxidizable, and residual Cr. Briefly, acid-extractable and reducible Cr were extracted separately by 0.11 mol/L acetic acid and 0.5 mol/L hydroxylammonium chloride. The residue soils were digested using 300 mg g−1 hydrogen peroxide and then extracted by 1.0 mol/L ammonium acetate, and this Cr fraction was named oxidizable Cr. The final residues were completely digested by HCl + HNO3 + HF + HClO4 (10:5:5:3), and this Cr fraction was defined as residual Cr. Concentrations of all 4 Cr species were determined by ICP-OES.
Statistical analyses
 (1)
(1) (2)
(2)RESULTS
Root colonization by R. irregularis
Under Cr(VI) addition levels of 0 mg/kg, 5 mg/kg, and 10 mg/kg, roots of inoculated dandelion were extensively colonized by R. irregularis (>70%), whereas 20 mg/kg Cr(VI) significantly decreased the colonization rate to 52.6% (p < 0.001; Table 1). Colonization rates of bermudagrass (16.4–32.3%) were unaffected by Cr(VI) addition, but were much lower (p < 0.001; Table 1) than the corresponding dandelion colonization rates. No mycorrhizal colonization was detected in uninoculated controls for either plant species (Table 1).
| Cr(VI) addition levels (mg/kg) | Root colonization (% of root length) | Root-to-shoot ratio | |||
|---|---|---|---|---|---|
| Noninoculated | Inoculated | Noninoculated | Inoculated | ||
| Plant species | |||||
| Dandelion | 0 | 0 | 75.7 | 1.03 | 0.94 |
| 5 | 0 | 83.9 | 0.84 | 0.51 | |
| 10 | 0 | 83.5 | 0.70 | 0.51 | |
| 20 | 0 | 52.6 | 0.66 | 0.34 | |
| Bermudagrass | 0 | 0 | 32.3 | 0.22 | 0.30 |
| 5 | 0 | 15.2 | 0.23 | 0.25 | |
| 10 | 0 | 19.4 | 0.22 | 0.19 | |
| 20 | 0 | 16.4 | 0.17 | 0.18 | |
| Significance ofa | |||||
| I | ** | * | |||
| Cr | ** | ** | |||
| P | ** | ** | |||
| I × Cr | ** | ns | |||
| I × P | ** | ** | |||
| Cr × P | * | ** | |||
| I × Cr × P | * | ns | |||
- a By analysis of variance.
- I = inoculation; Cr = Cr(VI) addition; P = plant species; ns = not significant.
- * p < 0.01.
- ** p < 0.001.
Plant growth
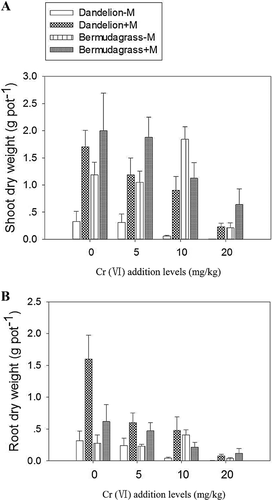
In general, shoot and root dry weights of both plant species decreased with increasing Cr(VI) addition levels in soil, irrespective of inoculation treatments. An exeption was that dry weights of bermudagrass increased under 10 mg/kg Cr(VI) addition compared with non-Cr-addition control (Figure 1; Supplemental Data, Table S1). In addition, Cr contamination also decreased chlorophyll concentrations (p < 0.001; Supplemental Data, Table S2). Bermudagrass indicated a much higher tolerance index than the corresponding rates for dandelion when treated with 10 mg/kg Cr(VI) and 20 mg/kg Cr(VI) (Table 2).
| Plant species | Cr(VI) addition levels (mg/kg) | Mycorrhizalm dependency | Tolerance index | |
|---|---|---|---|---|
| Noninoculated | Inoculated | |||
| Dandelion | 0 | 5.14 | 1.00 | 1.00 |
| 5 | 3.25 | 0.86 | 0.54 | |
| 10 | 13.5 | 0.16 | 0.42 | |
| 20 | 30.8 | 0.02 | 0.09 | |
| Bermudagrass | 0 | 1.79 | 1.00 | 1.00 |
| 5 | 1.83 | 0.88 | 0.90 | |
| 10 | 0.60 | 1.54 | 0.51 | |
| 20 | 3.10 | 0.17 | 0.29 | |
The AMF significantly increased shoot and root dry weights of dandelion and bermudagrass irrespective of Cr(VI) addition levels (p < 0.001; Figure 1; Supplemental Data, Table S1), except that mycorrhiza decreased bermudagrass growth with the addition of 10 mg/kg Cr(VI) (p < 0.05; Figure 1). The interactions between mycorrhizal colonization and Cr(VI) addition were highly significant for both shoot and root dry weights (p < 0.001; Figure 1; Supplemental Data, Table S1). Dandelion showed much higher mycorrhizal dependency than bermudagrass under all Cr(VI) addition levels (Table 2).
Mycorrhizal colonization also decreased the root-to-shoot ratio of dandelion (p < 0.05; Table 1) but had no influence on that of the bermudagrass under Cr(VI) contamination.
Plant phosphorus nutrition
Mycorrhizal colonization, Cr(VI) addition, and plant species all had significant influence on plant P concentrations (p < 0.01; Figure 2; Supplemental Data, Table S1). Generally, shoots of uninoculated bermudagrass had higher P concentrations than the corresponding dandelion shoots (p < 0.001), whereas root P concentrations were similar between the 2 plant species. Shoot and root P concentrations of uninoculated dandelion decreased with Cr(VI) addition (p < 0.001), but no similar trends were found for bermudagrass.
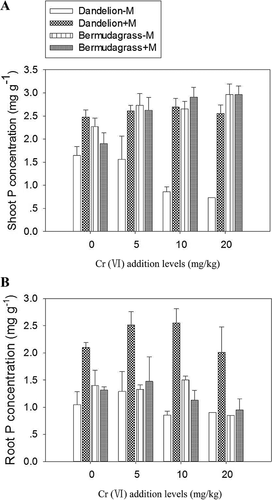
Mycorrhizal colonization markedly increased the shoot and root P concentrations of dandelion (p < 0.001; Figure 2; Supplemental Data, Table S1) but had no significant effects on bermudagrass (Figure 2). Mycorrhizal colonization also increased root P uptake efficiency (estimated as P uptake per unit root dry wt) of dandelion, especially under high Cr(VI) addition levels (p < 0.001; Supplemental Data, Table S3), but had no effects on that of bermudagrass. The mycorrhizal P response of dandelion was also much higher than that of bermudagrass irrespective of Cr(VI) addition levels (p < 0.001; Table 3).
| Cr(VI) addition levels (mg/kg) | %MPR | %MCrR | |
|---|---|---|---|
| Plant species | |||
| Dandelion | 0 | 1044 | 63.3 |
| 5 | 331 | 53.7 | |
| 10 | 3919 | 239 | |
| 20 | 3713 | 300 | |
| Bermudagrass | 0 | 96.1 | −22.7 |
| 5 | 78.7 | −4.9 | |
| 10 | −36.0 | −35.1 | |
| 20 | 172 | 164 | |
| Significance ofa | |||
| Cr | * | * | |
| P | * | * | |
| Cr × P | * | * | |
- a By analysis of variance.
- Cr = Cr(VI) addition; P = plant species.
- * p < 0.001.
Chromium uptake and partitioning
Shoot and root Cr concentrations of both plant species were affected by Cr(VI) addition and mycorrhizal inoculation (p < 0.001; Figure 3; Supplemental Data, Table S1). With increasing Cr(VI) addition levels, shoot and root Cr concentrations of dandelion increased significantly (p < 0.001; Figure 3). In contrast, for noninoculated bermudagrass, shoot and root Cr concentrations remained the same, except that 20 mg/kg Cr(VI) addition increased shoot Cr concentration (p < 0.001).
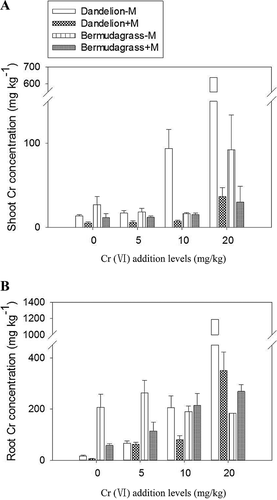
In most cases, the Cr concentrations in inoculated plants were dramatically lower than in the corresponding uninoculated plants (p < 0.01; Figure 3; Supplemental Data, Table S1). Root Cr uptake efficiency (estimated as Cr uptake per unit root dry wt) was also lower for inoculated plants than the corresponding uninoculated plants, irrespective of Cr(VI) addition levels, except for bermudagrass with the addition of 10 mg/kg Cr(VI) (p < 0.001; Supplemental Data, Table S3). In addition, mycorrhizal colonization also decreased the shoot-to-root ratio of Cr content of dandelion under nearly all Cr(VI) addition levels (p < 0.01; Supplemental Data, Table S4) but decreased this ratio in bermudagrass only with the addition of 20 mg/kg Cr(VI) (Supplemental Data, Table S4).
Mycorrhizal colonization did not show significant influences on shoot Cr contents of either plant species but increased the root Cr contents of dandelion at all Cr(VI) addition levels and of bermudagrass at 20 mg/kg Cr(VI) addition level (p < 0.01; Supplemental Data, Table S4). The mycorrhizal Cr response of dandelion was much higher than that of burmudagrass, irrespective of Cr(VI) additions (p < 0.001; Table 3). Bermudagrass even showed negative mycorrhizal Cr response values below 10 mg/kg Cr(VI) addition level (Table 3).
Soil chemophysical properties and chromium speciation
Mycorrhizal colonization increased soil pH but decreased extractable-P concentrations (p < 0.001; Table 4). In general, acid-extractable Cr, reducible Cr, and oxidizable Cr concentrations in soil increased with the increasing Cr(VI) addition levels (p < 0.001; Table 5). The concentrations of different soil Cr species followed the trend, irrespective of experimental treatments: acid-extractable Cr < reducible Cr < oxidizable Cr < residual Cr (Table 5). Among the 4 Cr species in soil, only acid-extractable Cr concentration was significantly affected by AMF inoculation (p < 0.01; Table 5).
| Cr(VI) addition levels (mg/kg) | Soil pH | Soil extractable-P concentration (mg/kg) | Soil Cr(VI) concentration (mg/kg) | ||||
|---|---|---|---|---|---|---|---|
| Noninoculated | Inoculated | Noninoculated | Inoculated | Noninoculated | Inoculated | ||
| Plant species | |||||||
| Dandelion | 0 | 8.19 | 8.60 | 16.5 | 11.5 | 0.57 | 0.39 |
| 5 | 8.19 | 8.58 | 16.2 | 11.6 | 0.41 | 0.33 | |
| 10 | 8.23 | 8.52 | 18.0 | 13.8 | 0.80 | 0.50 | |
| 20 | 8.31 | 8.44 | 19.6 | 15.6 | 1.10 | 0.88 | |
| Bermudagrass | 0 | 8.42 | 8.57 | 13.0 | 13.5 | 0.45 | 0.26 |
| 5 | 8.21 | 8.69 | 16.3 | 11.7 | 0.11 | 0.26 | |
| 10 | 8.70 | 8.49 | 14.4 | 16.7 | 0.12 | 0.46 | |
| 20 | 8.16 | 8.38 | 18.4 | 12.7 | 0.14 | 0.74 | |
| Significance ofa | |||||||
| I | ** | ** | ns | ||||
| Cr | ns | * | ns | ||||
| P | ns | ns | * | ||||
| I × Cr | * | ns | ns | ||||
| I × P | ns | ns | ns | ||||
| Cr × P | ns | ns | ns | ||||
| I × Cr × P | ns | ns | ns | ||||
- a By analysis of variance.
- I = inoculation; Cr = Cr(VI) addition; P = plant species; ns = not significant.
- * p < 0.05.
- ** p < 0.001.
| Cr(VI) addition levels (mg/kg) | Acid-extractable Cr concentration (mg/kg) | Reducible-Cr concentration (mg/kg) | Oxidizable-Cr concentration (mg/kg) | Residual-Cr concentration (mg/kg) | |||||
|---|---|---|---|---|---|---|---|---|---|
| Noninoculated | Inoculated | Noninoculated | Inoculated | Noninoculated | Inoculated | Noninoculated | Inoculated | ||
| Plant species | |||||||||
| Dandelion | 0 | 1.13 | 1.13 | 1.74 | 1.69 | 3.44 | 3.26 | 66.0 | 71.8 |
| 5 | 1.23 | 1.17 | 2.21 | 2.42 | 6.52 | 6.77 | 74.3 | 72.0 | |
| 10 | 1.45 | 1.34 | 3.44 | 2.76 | 9.30 | 8.02 | 70.3 | 66.4 | |
| 20 | 1.90 | 1.83 | 4.43 | 3.90 | 14.9 | 13.0 | 64.3 | 68.6 | |
| Bermudagrass | 0 | 1.12 | 1.08 | 1.62 | 1.78 | 3.52 | 3.74 | 75.8 | 72.1 |
| 5 | 1.22 | 1.20 | 2.33 | 2.28 | 6.00 | 6.08 | 72.1 | 74.2 | |
| 10 | 1.34 | 1.35 | 3.23 | 3.22 | 9.28 | 8.49 | 75.8 | 73.8 | |
| 20 | 1.90 | 1.74 | 3.88 | 4.54 | 14.7 | 15.1 | 69.9 | 75.2 | |
| Significance ofa | |||||||||
| I | ** | ns | ns | ns | |||||
| Cr | *** | *** | *** | ns | |||||
| P | ns | ns | ns | * | |||||
| I × Cr | ns | ns | ns | ns | |||||
| I × P | ns | * | ns | ns | |||||
| Cr × P | ns | ns | ns | ns | |||||
| I × Cr × P | ns | ns | ns | ns | |||||
- a By analysis of variance.
- I = inoculation; Cr = Cr(VI) addition; P = plant species; ns = not significant.
- * p < 0.05.
- ** p < 0.01.
- *** p < 0.001.
DISCUSSION
To the best of our knowledge, the present study is the first to simultaneously investigate the role of AM symbiosis in Cr tolerance of both Cr-sensitive and Cr-tolerant plants. Under Cr(VI) contaminations, both plant species suffered Cr toxicity as indicated by a decrease in plant dry weights and chlorophyll concentrations (especially under the 20 mg/kg Cr[VI] addition level). However, bermudagrass was more tolerant to Cr than dandelion and held a much higher tolerance index (Table 2). Similar to Oryza sativa (or Medicago sativa), which showed biomass increase under 50 µM Cr(VI) treatment 29, 30, a Cr(VI) addition level of 10 mg/kg also stimulated growth of bermudagrass (Figure 1). Different mechanisms of Cr uptake and accumulation by the 2 plant species may partly explain their Cr tolerance: dandelion could easily take up Cr from soil, as its shoot Cr concentrations increased from 13.8 mg/kg to 637 mg/kg with elevated Cr(VI) addition levels, whereas bermudagrass seemed to have developed the strategy to reduce Cr absorption, as its shoot Cr concentrations remained lower than 100 mg/kg at all Cr(VI) addition levels.
Under the experimental conditions, both dandelion and bermudagrass established symbiosis with R. irregularis despite high Cr(VI) addition levels in the soil. This indicated the AMF strain could naturally tolerate Cr contamination, although it did not originate from a Cr-enriched environment. In support of our hypotheses, AM symbiosis did improve plant growth of both plant species under Cr(VI) additions, except for bermudagrass under the 10 mg/kg Cr(VI) addition (Figure 1; Supplemental Data, Table S1). Similar to the results of previous studies on other metals such as Cu, As, and Cd 7-9, the increased plant biomass by AM symbiosis largely diluted Cr in plants (Figure 3; Supplemental Data, Table S1), which may have played a critical role in detoxification of Cr. It should also be noted that there were differences between the mycorrhizal effects on Cr and P uptake by the 2 plant species. For dandelion, the P-uptake efficiency was markedly increased by mycorrhizal colonization, and the mycorrhizal P response were general positive (p < 0.01; Table 3; Supplemental Data, Table S3). In contrast, Cr-uptake efficiency of dandelion was decreased by AMF colonization (Supplemental Data, Table S3). This suggested that the mycorrhizal symbiosis could selectively take up P over Cr, which is in accordance with the previous study that mycorrhizal M. sativa could preferably take up P compared with As 7. For bermudagrass, AM symbiosis did not improve P uptake, but decreased plant Cr uptake efficiency at 0 mg/kg, 5 mg/kg, and 20 mg/kg Cr(VI) addition levels and thus relieved Cr toxicity (Figure 1; Supplemental Data, Table S3). As an exception, the Cr(VI) level of 10 mg/kg stimulated growth of burmudagrass 29, 30, whereas the reduced Cr uptake by AM symbioses may have hampered the stimulation effects” of Cr, which may explain why AMF decreased bermudagrass growth in this case.
The negative mycorrhizal Cr response values of bermudagrass at low and moderate Cr(VI) addition levels suggested that AM symbiosis could decrease Cr uptake by bermudagrass. This is consistent with a previous study 31 that showed AMF could protect white clover by reducing Zn uptake. However, the contribution of AMF to increased Cr contents of dandelion (as the mycorrhizal Cr response value is above zero) was most likely attributable to improved plant growth, although the Cr concentration in dandelion was generally decreased by mycorrhizal colonization. Moreover, we found that AM symbioses decreased the shoot-to-root ratio of Cr content of both plant species at high Cr(VI) addition levels (p < 0.01; Supplemental Data, Table S4), suggesting that AMF could inhibit Cr translocation from roots to shoots, which may also serve as a mechanism of Cr detoxification in mycorrhizal plants.
The decreased shoot-to-root ratio of Cr contents by mycorrhizal colonization implied a potential use of AMF in phytostabilization of Cr contaminated soils. More Cr retained in plant roots may partially result from Cr immobilization by AMF fungal structures (e.g., extraradical mycelium, arbuscules, vesicles) or alteration of Cr species in the rhizosphere. Previous studies have indicated that AMF extraradical mycelium has a high cation exchange capacity (CEC) and can adsorb large amounts of metal ions such as Zn and Cd 32. Extraradical mycelia also hold much higher metal concentrations than those of plants 33, and mycorrhizal roots may exhibit a higher Cr sorption capability than nonmycorrhizal roots 34. Chromium distribution patterns could possibly change on formation of AM associations, just as Kaldorf et al. 35 found that Fe, Ni, and Zn were mainly located in the inner cortical cells containing the fungal structures such as arbuscules and internal hyphae. Furthermore, at the molecular level, AM symbiosis may indirectly affect Cr transporters in plant roots; previous studies have shown that AMF downregulated arsenate transporters in root epidermis 36. More work is needed before this can be confirmed.
Compared with noninoculated controls, inoculated treatments showed higher soil pH but lower extractable-P concentrations (p < 0.001; Table 4). The elevated pH may be attributable to excretion of HCO3− or OH− by mycorrhizal roots because of excessive uptake of anions over cations 37, whereas the depletion of extractable P in soil could be attributed to enhanced P uptake by mycorrhiza. In addition, the nearly exiguous Cr(VI) in soil after plant growth may have resulted from the reduction of Cr(VI) to Cr(III) because of the combined effects of dissolved organic matter, Fe(II), and pH changes 38. However, this does not mean that there is no risk of Cr toxicity to plants, because Cr may exist in other forms with high bioavailability and toxicity to plants 13, 14.
Arbuscular mycorrhizal symbiosis could possibly change metal speciation in the rhizosphere and further affect metal uptake by plants 39. To confirm whether AM symbiosis can influence Cr speciation and bioavailability, different Cr species in soil were determined at experimental harvest. Relationships between concentrations of each soil Cr species and plant Cr concentrations were examined by a stepwise regression analysis. The Cr concentrations in both plants could be mostly explained by acid-extractable soil Cr concentrations (p < 0.01; Table 6). Furthermore, among the 4 Cr species in soil, only the acid-extractable Cr concentration was significantly influenced by AMF inoculation (p < 0.01; Table 5). This happened especially for dandelion, in which the concentration of acid-extractable Cr was lower in the inoculated pots compared with the corresponding uninoculated controls under the addition of 10 mg/kg Cr(VI) (p < 0.01; Table 5). This supported our hypothesis that mycorrhizal symbiosis can alter Cr bioavailability by influencing Cr speciation in the rhizopshere. However, AM symbiosis did not influence soil reducible and oxidizable Cr under the experimental conditions, which differs from the results of previous studies on other heavy metals such as Zn 39. This may be explained by the special chemical characteristics of Cr, because Cr mainly existed in the form of insoluble Cr(OH)3, Cr(III) adhered to soil components, or stable Cr(III) complexes with macromolecular ligands such as humic acids in neutral or alkaline soils 40.
| Plant species | AMF | Plant part | Model | R2 |
|---|---|---|---|---|
| Dandelion | All treatments | Total plant | Y = 50.9XRED – 79.6** | 0.699 |
| Shoot | Y = 80.2XRED – 172** | 0.462 | ||
| Root | Y = 472XAE – 511** | 0.891 | ||
| Noninoculation | Total plant | Y = 63.9XRED – 88.7** | 0.904 | |
| Shoot | Y = 266XAE – 294* | 0.799 | ||
| Root | Y = 94.5XRED – 131** | 0.930 | ||
| Inoculation | Total plant | Y = 149XAE – 158** | 0.948 | |
| Shoot | Y = 46.8XAE – 50.1** | 0.890 | ||
| Root | Y = 468XAE – 514** | 0.928 | ||
| Bermudagrass | All treatments | Total plant | Y = 43.3XAE – 6.19* | 0.285 |
| Shoot | Y = 70.8XAE – 68.7** | 0.501 | ||
| Root | Y = 189XAE – 53.1* | 0.275 | ||
| Noninoculation | Total plant | ns | ||
| Shoot | ns | |||
| Root | ns | |||
| Inoculation | Total plant | Y = 4.21XOX + 8.41** | 0.760 | |
| Shoot | Y = 7.47XRED – 4.63* | 0.542 | ||
| Root | Y = 321XAE – 258** | 0.794 |
- Y = Cr concentrations in different plant parts; X = concentrations of different Cr species in soil; AE = acid-extractable Cr; RED = reducible Cr; OX = oxidizable Cr; RES = residual Cr; ns = not significant.
- * p < 0.01.
- ** p < 0.001.
It should be noted that AMF also may have changed the bioavailability of different Cr species in soil. For example, the reducible Cr concentrations mostly explained the root Cr concentrations of uninoculated dandelion (p < 0.001, 0.930 of R2; Table 6), whereas acid-extractable Cr mostly explained that of mycorrizal dandelion (p < 0.001, 0.928 of R2). These effects became more obvious for bermudagrass, in which no Cr species could explain root Cr concentrations for uninoculated plants and root Cr concentrations were mostly explained by acid-extractable Cr concentrations for mycorrhizal plants (p < 0.001, 0.794 of R2; Table 6). This further suggested that AMF symbioses could change bioavailability of Cr species, which may result from alteration of the pathway by which plants absorb Cr. However, although the BCR 3-step sequential extraction procedure is widely adopted in evaluating metal mobility in soil, it still has some drawbacks in assessing metal bioavailability for the complex processes of metal species transformation in soil and different mechanisms of metal absorption by plants. In addition, the statistical analysis can only provide possible correlations between Cr accumulation in plants and Cr species in soil; more work in vivo and in vitro should be carried out to estimate the accessibility of different Cr species to plants.
CONCLUSIONS
In summary, the present study is the first report on the protective effects of AMF on both a Cr-sensitive plant (dandelion) and a Cr-tolerant plant (bermudagrass) under Cr contamination. Generally, AM symbioses enhanced plant Cr tolerance by growth dilution effects on Cr in dandelion and reduced Cr uptake by bermudagrass. The experimental data also indicate that AM symbioses could potentially influence Cr speciation and bioavailability in the rhizosphere. However, more work is needed to illustrate the mechanisms of enhanced plant Cr resistance by AM symbioses, such as how AMF absorb and tolerate Cr and how the extraradical and intraradical mycelium immobilize Cr in plant roots.
SUPPLEMENTAL DATA
Tables S1–S4. (40 KB DOC).
Acknowledgment
S.-L. Wu and B.-D. Chen contributed equally to this work. This work was supported financially by the Knowledge Innovation Program of the Chinese Academy of Sciences (KZCX2-YW-BR-17) and National Natural Science Foundation of China (41101246). Thanks to O. Patrick from Adelaide University, Australia, for comments on an earlier draft of this paper.




