Evaluating toxicity of heavy fuel oil fractions using complementary modeling and biomimetic extraction methods
Abstract
The toxicity of chemically dispersed heavy fuel oil (HFO) and 3 distillate fractions to rainbow trout (Oncorhynchus mykiss) embryos was evaluated using the PETROTOX model and a biomimetic extraction technique that involved passive sampling of oil-contaminated test media with solid-phase microextraction (SPME) fibers. Test solutions for toxicity testing were generated using a combination of dispersant and high-energy mixing. The resulting water accommodated fractions (WAF) provided complex exposure regimens that included both dissolved hydrocarbons and oil droplets. The toxicity of the various fractions differed by approximately 3 orders of magnitude when expressed on the basis of WAF dilution. Using detailed compositional data, the PETROTOX model predicted the speciation of hydrocarbons between dissolved and oil droplet phases and explained observed toxicity based on computed dissolved phase toxic units (TUs). A key finding from model calculations was that dissolved hydrocarbon exposures and associated TUs were a nonlinear function of WAF dilution, because dissolved hydrocarbons were largely controlled by the dissolution of oil droplets that were transferred in WAF dilutions. Hence, oil droplets served to “buffer” dissolved concentrations in WAF dilutions at loadings greater than 1 mg/L, resulting in higher dissolved concentrations and TUs than expected based on dilution. The TUs computed at each WAF dilution explained the observed toxicity among the HFO and fractions to within a factor of 3. Dissolved material measured by SPME showed a consistent relationship with model-predicted TUs, confirming the utility of this approach for providing an integrated measure of exposure to bioavailable hydrocarbons. These 2 approaches provide complementary tools for better defining bioavailability of complex petroleum substance. Environ Toxicol Chem 2014; 33:2094–2104. © 2014 SETAC
INTRODUCTION
Petroleum substances encompass a wide range of products that are produced by refineries largely from distillation and processing of crude oil. These substances are used in many applications, including chemical feed stocks, fuels, lubricants, solvents, and asphalts. Most petroleum substances comprise complex mixtures of hydrocarbons within a specified boiling range that vary in carbon number and may include different classes of aliphatic and aromatic structures 1.
Accidental releases of petroleum substances may potentially harm aquatic life. Hydrocarbons that constitute petroleum substances often exhibit a wide range of physical and chemical properties and toxicities. Furthermore, aquatic species may be exposed to petroleum substances through a combination of dissolved and particulate hydrocarbons, such as oil droplets. This complicates hazard and risk assessments because exposures are complex 2.
To assess the aquatic hazard of petroleum substances, different oil-in-water preparation methods are used to generate a water accommodated fraction (WAF) in which a given amount of oil is added to a known volume of water and then gently stirred for a specified period to enable partitioning of soluble hydrocarbons from oil to water. When mixing is terminated, the solution is allowed to settle for a set time and the WAF decanted 3. In some study designs, the WAF prepared at a single oil loading is used as a stock solution to prepare dilutions to which aquatic test organisms are exposed. Because dispersants often are a key oil spill response option, dispersant may be added to the test oil during WAF preparation at a volume ratio that reflects the dosage applied in field response (e.g., 1:20 dispersant to oil). The test procedure used to prepare chemically dispersed oil (chemically enhanced WAF [CEWAF])—including the oil loading selected, amount of mixing energy, mixing and settling times, and use of chemical dispersant—can greatly affect the composition of the WAFs, including the amount of oil that is entrained in the test media as oil droplets 4-6. Often, this complicates comparison of toxicity results for oils among studies with different experimental designs.
Previous work has shown that the development of fish embryos can be impaired by exposure to WAF and CEWAF of crude and refined oils 7-10. Thus, fish embryo toxicity serves as an important endpoint for evaluating chronic risk to the aquatic environment. These chronic effects often are attributed to polycyclic aromatic hydrocarbons (PAHs), which produce developmental abnormalities in fish early life stage tests 11-14. Recent work reported that differences in embryo toxicity among 4 crude oils investigated were correlated with the concentrations of 3- to 5-ring PAHs in the oil 10, 15. However, the hydrocarbon constituents in complex petroleum substances that contribute to fish embryo toxicity remains an active area of research and debate 16.
Recently, an effects-driven chemical fractionation (EDCF) approach was used to identify the petroleum hydrocarbons that contributed most to the chronic toxicity of heavy fuel oil 7102 (HFO) to fish embryos 17, 18. The chronic toxicity of HFO and 3 vacuum distillation fractions were measured by exposure of rainbow trout (Oncorhynchus mykiss) embryos to high-energy CEWAFs over a 24-d test. Fraction 3, enriched in 3- to 4-ringed PAHs, was the most toxic fraction of HFO, consistent with previous reports that PAHs are the predominant cause of the chronic embryo toxicity of oil. Conclusions based on these toxicity tests were confounded by the presence of oil droplets in solution, however, and a lack of standardized analytical methods to discriminate the concentrations of hydrocarbons in true solution from those incorporated in droplets.
The objectives of the present study were to better characterize the dissolved phase hydrocarbon exposures in the study by Adams et al. 17. The present work advances the prior toxicity work by applying new modeling and analytical approaches to explain the differences in fish embryo toxicity reported for these dispersed oil fractions.
To gain insights on WAF composition and toxicity, the PETROTOX model applies a coupled fate and effects model to describe the multicomponent dissolution behavior and toxicity of a complex petroleum substance in a WAF test system 19. The PETROTOX model uses a hydrocarbon block method to relate substance composition to toxicity. The composition and exposure dimensions are used to predict equilibrium concentrations of dissolved-phase hydrocarbons from mass balance equations and Raoult's and Henry's Laws. The acute or chronic toxicity of the WAF components are estimated using the target lipid model (TLM) for a given species-specific endpoint 20. The toxicity of the WAF is computed by calculating the toxic units (TUs) associated with each component and summing TUs across all components, assuming additive toxicity, an assumption well supported for individual- and population-level endpoints (e.g., growth, mortality, and reproduction) 19, 21, 22. An advantage of this modeling approach is that the contribution of each hydrocarbon block to observed toxic effects can be characterized, supporting the EDCF approach. Furthermore, effect assessments from different exposure systems and petroleum substances that are based on TUs have the advantage of being directly comparable across studies. In contrast, assessments based on total concentrations, dilution, or loading schemes often are difficult to compare, given the differential mass distribution (water vs headspace) and multicomponent dissolution behavior of oil constituents.
Another novel approach that holds promise for quantifying dissolved-phase hydrocarbon exposures in WAFs and estimating toxic potency relies on the use of biomimetic extraction with passive samplers. This approach assumes that the passive sampling phase (e.g., polydimethylsiloxane-coated solid-phase microextraction [SPME] fibers) provides a surrogate for target lipid. Thus, total hydrocarbon concentrations on a passive sampler that is equilibrated with a WAF sample provides a proxy of target lipid concentrations that, in accordance with the TLM, should be directly correlated to toxicity. The SPME measurements integrate both the exposure concentration in water and partitioning characteristics of the dissolved hydrocarbon WAF components. This measurement approach has been used previously to estimate the acute toxic potency of WAFs prepared with petroleum products, hydrocarbon resins 23, 24 as well as hydrocarbon-contaminated effluent and porewater samples 25, 26.
METHODS
Test substances
Approximately 4 L of HFO 7102 was fractionated sequentially in 3 steps (low-temperature vacuum distillation, cold acetone extraction, and column chromatography) for subsequent chemical analysis 18 and chronic toxicity testing 17. The present study focused on the low-temperature vacuum distillation fractions, including F2 (boiling point [b.p.] range = 174–287 °C), F3 (b.p. range = 287–481 °C), and F4 (b.p. > 481 °C); there were negligible amounts of fraction F1 (BP < 174 °C, consisting of low-molecular-weight, highly volatile compounds) in HFO 18.
Toxicity testing
In the present study, chemical analyses and models of the toxicity of HFO and its distillate fractions to trout embryos compare predicted values with those measured by Adams et al. 17. In that previous work, embryos were exposed to CEWAF of the parent oil and its fractions, made fresh daily over 24 d, the interval between hatch and swim-up of control embryos. It was assumed that this approach generated toxicity data with essentially constant exposures to be comparable with the standardized toxicity data used to train the TLM. The endpoints evaluated included mortality, blue sac disease, and deformities; additional details on experimental design and discussion of biological endpoints are presented by Adams et al. 17. The response used in modeling for the present study was chronic (24 d) embryo mortality, an endpoint with clear relevance to population-level effects. In addition to the chemical analysis of the present study, total PAH (TPAH) concentrations from Adams et al. 17 were used to estimate the loadings from transferred droplets for the TU calculations, because these concentrations were representative of the exposures experienced by trout embryos.
Characterization of test substances
The neat HFO oil and F2, F3, and F4 fractions were analyzed using 2-dimensional chromatography to determine the carbon number and hydrocarbon classes constituting each test substance. This analysis was performed using an Agilent Technologies 6890 Series gas chromatograph in split mode equipped with a flame ionization detector, following the general approach outlined by Arey et al. 27. Test substances were also subjected to detailed analysis of PAHs (2- to 6-ring parent and alkyl) and n-9 to n-40 saturated hydrocarbons (SHCs), using gas chromatography with low-resolution mass spectrometry with selected ion monitoring. Neat oil SHC concentrations were estimated by normalization of aqueous SHC concentrations to the aqueous total petroleum hydrocarbons (TPH) concentrations that were determined on the highest loading (0.1% v/v).
WAF preparation
The main WAF preparation techniques were based on Adams et al. 17. Briefly, a 10% (v/v) loading of oil (1.6 mL oil and 14 mL water) was added to an inverted 20 mL glass vial with dispersant (COREXIT 9500; 20:1 oil:dispersant ratio, CEWAF) and high-energy mixing. As a control on the use of dispersants, a second set of WAFs was prepared in the present study with high-energy mixing only (physically dispersed WAF [PDWAF]). Vials were vortexed for 5 min and sonicated an additional 5 min. The suspensions were allowed to settle in inverted vials for 1.5 h. A gas-tight syringe was used to remove 10 mL of this initial WAF to a separate glass vial with care to avoid the overlying, nondispersed, oil phase. Varying volumes of this solution were added to glass aspirator bottles containing 0.8 L to 2 L of water to achieve target loadings. The contents of the glass aspirator bottles were stirred for 30 min before collecting samples for analytical characterization. Only CEWAFs were investigated in the toxicity study by Adams et al. 17. To gain further insights on the impact of chemical dispersant on WAF composition, parallel PDWAFs were also included in the present study (i.e., only physical mixing). The PDWAF dosing system followed the same preparation protocol as the CEWAF system, but without the dispersant. The mixing protocol was designed to promote the dispersion of the viscous test substances in the exposure water and to reduce the potential for false negatives in the EDCF 17. The method was not designed to mimic environmental dispersion of oil at spill sites.
In the present study, the term WAF is used generally to describe general trends of the dissolution and aquatic toxicity of hydrocarbon constituents. Use of CEWAF and PDWAF refers to specific results of the toxicity (CEWAF only [17]) and chemical characterization work (CEWAF and PDWAF in the present work).
Analytical characterization of WAFs
Detailed analysis of PAHs and SHCs in each WAF treatment was performed by Alpha Analytical, using extraction with dichloromethane and subsequent analysis of extracts using gas chromatography–mass spectrometry (GC-MS) quantification. These measured concentrations were assumed to represent the total concentration of a given analyte in the exposure systems (i.e., dissolved + droplets). These data were compared with exposure data generated during the toxicity studies 17 and for estimation of the hydrocarbon speciation in WAFs generated in the present study.
Biomimetic extraction
Triplicate 22-mL WAF samples from each treatment were collected in glass autosampler vials, with no headspace, and capped with Teflon®-faced septum lids. The autosampler vials were placed on a LEAP Technologies Combi PAL autosampler (CTC Analytics) configured for automated SPME injections. A 30-µm SPME fiber (Supelco) coated with polydimethylsiloxane (PDMS; 0.132 µL PDMS per fiber) was equilibrated with each sample for 100 min at 30 °C with orbital agitation at 250 rpm.
The SPME fiber and liquid calibration standards (2,3-dimethylnaphthalene) were analyzed on a Perkin-Elmer Autosystem XL gas chromatograph with a flame ionization detector and 15 m × 0.53 mm inner-diameter capillary column with 1.5 μm Rtx-1 stationary phase (Restek). Calibration was achieved by making 0.5-µL injections of increasing amounts of the 2,3-dimethylnaphthalene standard.
An average calibration factor was determined across 3 calibration levels. The average calibration factor was applied to the total GC–flame ionization detector response (total area) for the respective SPME water analysis. The SPME fiber results were normalized to the volume of PDMS and reported as µmol 2,3-dimethylnaphthalene/mL PDMS. The quantitation limit was approximately 0.5 µmol/mL PDMS.
Modeling
Petroleum substances exhibit multicomponent dissolution behavior so that the overall dissolved exposure is both composition- and loading-dependent. The composition and speciation of hydrocarbons in the WAFs were evaluated by comparing aqueous concentrations of PAHs and SHCs to predicted dissolved exposure based on Raoult's Law 21, 22. The speciation of the hydrocarbons in the WAF (e.g., dissolved vs droplet phases) was estimated by comparing the measured composition of hydrocarbons in the WAF (e.g., measured total) with the predicted dissolved phase 28.
PETROTOX 19 was used to relate oil-in-water loading and chemical characterization to observed toxicity data 17 using TU. PETROTOX uses the hydrocarbon block method to model dissolution and toxicity of complex petroleum substances. It relies on a library of representative structures with assigned physicochemical properties (e.g., molecular weight [MW], octanol–water partition coefficient [log KOW], solubility). The TU calculations were used to explain the observed toxicity by integrating the exposure to various dissolved hydrocarbons and predicted partitioning to target lipid. Two pieces of information were needed to calculate TUs. The first set of information was oil composition and loading, which influenced hydrocarbon exposures. Two-dimensional chromatography analyses of the various neat oil fractions were used as inputs for these calculations. The loading (e.g., milligrams per liter of total hydrocarbon) was estimated by scaling the effective dilution factor based on measured TPAH in the stock solution to the available measured TPAH in subsequent dilutions. When TPAH data were not available, the loading was estimated by interpolation.
A key element of the modeling was the conceptual understanding that the entrained droplets continued to re-equilibrate with their new exposure system. This results in a dissolved phase composition and toxicity profile that deviates from the linear dilution of the WAF stock.
The second key element is the TLM equation for calculating chronic toxicity for each WAF component. Chronic toxicity is modeled using an empirical acute-to-chronic ratio applied to all hydrocarbons that are present in the WAF. The embryo endpoint investigated in the present study was chronic lethality, and the median acute-to-chronic ratio for trout (ACR: 3.8; critical target lipid body burden [CTLBB]: 66.1 µmol/g lipid) 20 was used to predict chronic TUs. The TLM has been applied 19, 20 to more than 80 aquatic species and endpoints, including rainbow trout, and was considered suitable for modeling the chronic exposures in the present study. However, the present study represents a first application of this modeling framework to the chronic toxicity of HFO-derived substances to rainbow trout embryos and will provide a basis for identifying areas for model improvement and general knowledge gaps in research characterizing the relationship between chemical composition and effects. The ACR used in the present study was derived empirically for predicting chronic toxicity, and made no assumption related to mode of action for chronic effects. The chronic TLM predictions at TU = 1 corresponded to a 10% effect level (EC10) consistent with the derivation of ACRs within the TLM framework 20.
Given predicted dissolved exposure and chronic effect concentrations, TUs were computed for each component and summed across all components for comparison with the observed toxicity. This assumes that despite the presence of oil droplets, dissolved hydrocarbons determine embryo toxicity. Statistical analysis of dose–response curves was performed using a logistic regression model 29 with Solver in Microsoft Excel and in R 30.
RESULTS AND DISCUSSION
Two-dimensional chromatography characterized the test oils into 5 aliphatic and 9 aromatic structural classes. The F2, F3, and F4 fractions consisted primarily of structures consisting of C9 through C24, C12 through C30, and >C20 carbons, respectively (Supplemental Data, Table S2). The whole oil was composed of approximately 7% of the F2, 25% of the F3, and 66% of the F4 fractions 18. Speciated hydrocarbons in test oils (Supplemental Data, Table S3) were used to confirm the presence of oil in CEWAF and PDWAF treatments, as discussed in the following sections.
WAF chemistry and dosing systems
Predicted dissolved exposures
The overall limits on dissolved exposures for the substances in the present study were reached at a relatively low oil–water loading (∼50 mg/L), consistent with the high-MW compounds typical of the HFO fractions. The magnitudes of the dissolved exposure were very different among the oil fractions (Figure 1). At very low loadings (∼0.02 mg/L), dissolution of the whole oil was approximately 0.003 mg/L and reached a maximum of 2.8 mg/L at very high loadings (>50 mg/L). The F2 fraction was enriched in more water-soluble constituents and reached a maximum dissolved concentration near 10.4 mg/L at high loadings. In contrast, the F3 and F4 fractions were relatively insoluble, with total dissolved concentrations of 0.77 mg/L and 0.21 mg/L, respectively. Although the whole oil was composed of only 7% of the F2, these constituents dissolved and contributed to the higher total dissolved concentrations predicted for HFO. Note that the difference between the predicted dissolved concentration and nominal loading (1:1 line) increased from F2 to F4, reflecting the increasing fraction of oil that was not dissolving and was present in droplet form (Figure 1).
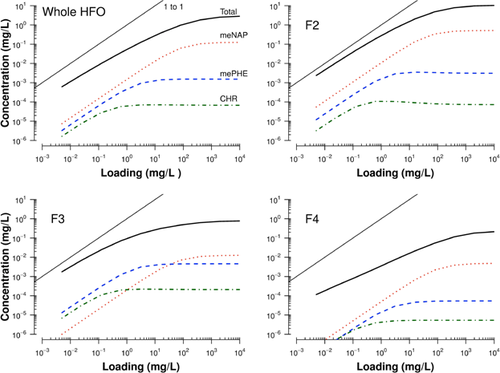
To further illustrate how WAF composition changes depending on oil type and loading, the aqueous concentrations of 2-methylnaphthalene, 2-methylphenanthrene, and chrysene, as predicted by PETROTOX, were plotted in Figure 1. At low loadings of HFO (<1 mg/L), the concentration of each hydrocarbon contributed to the dissolved exposure in a linear manner proportional to both their single-chemical solubility and abundance within the oil (e.g., Raoult's Law). At higher loadings (1–50 mg/L), the predicted solubility limit of chrysene and 2-methylphenanthrene were reached, whereas the aqueous concentration of the more soluble 2-methylnaphthalene continued to increase. The magnitude of the predicted dissolved concentrations varied across the fractions. For example, despite 2-methylnaphthalene having the highest individual chemical solubility of the 3 constituents, at lower loadings of F3, C1-PHE and chrysene concentrations are predicted to be higher because of their greater abundance in this substance than 2-methylnaphthalene.
The range of loadings in Figure 1 spanned the range of oil-in-water loadings used in the toxicity tests performed by Adams et al. 17. In summary, the concentration and composition of dissolved constituents in WAFs were predicted to vary as a function of oil composition and loading.
Comparison with measured WAF chemistry
Measurements of the CEWAF chemistry at the 0.1% v/v loading prepared for the present study were compared with predicted dissolved concentrations of individual constituents (Figure 2). The measured concentrations of individual PAHs (and SHCs) were approximately 2 to 50 times greater than the predicted dissolved exposure, as indicated by data falling below the 1:1 line in the top row of Figure 2. Constituents in the HFO and F4 fractions showed less deviation than the F2 and F3 fractions, probably because of the viscous nature of the former, which were not readily dispersed into aqueous media.
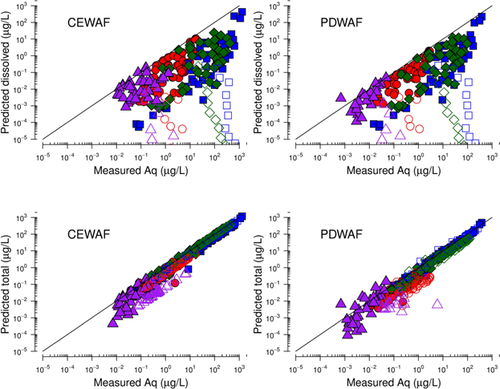
The speciation caused by the partitioning of hydrocarbons between oil and water phases was evaluated using the individual constituent data to minimize potential errors from the experimental and modeling methods. Both CEWAFs and PDWAFs were enriched in 3- to 4-ring PAHs relative to the typically more soluble 2-ring PAHs, as indicated by the increasing ratio of measured aqueous to predicted dissolved concentrations with increasing log KOW (Supplemental Data, Figure S2). The predicted total concentrations showed better agreement with measured data (lower row, Figure 2). The TPH-normalized concentration, or the effective oil composition (Coil), were calculated by dividing the measured aqueous concentrations (Caq) for each constituent, i, by the TPH—for example, Coil,i (µg/mg) = Caq,i (µg/L)/TPH (mg/L)—was compared with the neat oil substance compositions to confirm the presence of entrained oil in the test system. The normalized concentrations were consistent with the chemistry of the neat oil (Supplemental Data, Figure S3), confirming the presence of entrained oil in WAF prepared for all fractions.
Similar observations were noted for the PDWAF dosing systems at the 0.1% v/v loading (Figure 2). Although the concentrations of TPH for F2 and F3 fractions were approximately 4 times lower than in the CEWAF tests, measured aqueous concentrations of individual PAHs were still well above the predicted dissolved concentrations, and the CEWAF composition was indicative of entrained oil. The TPH concentrations for whole oil and F4 fractions in the PDWAF system were approximately 10 times lower than the CEWAF but still 2 to 5 times greater than the predicted dissolved concentrations. However, this trend was less consistent across the range of PAHs shown in Supplemental Data, Figure S2, although the presence of SHCs in the CEWAFs and PDWAFs supported the presence of oil, perhaps at trace concentrations. Similar to the CEWAF results, the PDWAFs were enriched in 3- and 4-ring PAHs, and the TPH-normalized composition of the PDWAFs for these 2 substances was similar to that of the whole oil.
The high-energy mixing involved in CEWAF and PDWAF preparation via vortex and sonication appeared sufficient to generate droplets that were relatively stable in solution. The droplets appeared stable because the chemical measurements that indicated droplet oil were performed over a period of hours to days after WAF preparation and sampling. Chemical dispersants are thought to stabilize droplets, preventing re-coalescence 31, but differences in the behavior of droplets between the 2 WAF systems were not measured. However, when comparing the results between CEWAF and PDWAF solutions, the dispersants had the greatest impact on total aqueous hydrocarbon concentrations of the relatively heavier whole oil and F4 fractions. The TPH-normalized oil concentrations for the dosing systems were similar to the measured oil concentrations and are indicative of oil droplets. The variability in the HFO and F4 tests were attributable in part to concentrations that were near the detection limit but also to the poor dispersion of these highly viscous materials, such that a high proportion of hydrocarbons in the test solution (though generally low) were in the dissolved phase rather than in droplets.
The oil-in-water speciation model 28 was used to estimate the concentration of droplets in these systems (Supplemental Data, Table S5; Figure 2), which provided an indication of the dispersion effectiveness of the different WAF preparation systems. This calculation was performed only where concentration data for individual PAHs and SHCs were available in the WAF, because this was primarily a method for estimating hydrocarbon speciation. Consistent with the TPH measurements and WAF composition discussed earlier in this section, the estimated droplet concentrations were highest for F2 and F3 in the CEWAF and PDWAF systems. Comparatively, the droplet concentrations were lower by approximately 10 to 100 times for whole oil and F4 substances in these 2 systems, likely because of their higher viscosity.
These estimates were based on chemical speciation measured for the neat oil test substances and corresponding CEWAFs or PDWAFs. At low oil loadings, model estimates became uncertain based on noise in the WAF analysis data. Correspondingly, the standard deviations were small for the estimates with obvious entrained oil (Supplemental Data, Table S5) and high for the droplet estimates at low concentrations. The results of the present analysis confirmed that droplets were present in the CEWAF and PDWAF systems for some substances (F3, F2) or at trace to negligible concentrations for others (whole oil, F4).
Predicted toxic units and chronic toxicity
Dissolved-phase hydrocarbons are assumed to be the most bioavailable fraction of oil. The composition of dissolved hydrocarbons in WAF is therefore expected to determine the toxicity toward aquatic species. To illustrate, concentration-loading relationships (Figure 1) were converted to TUs using the TLM. Although 2-methylnaphthalene was typically the more abundant hydrocarbon in WAFs of whole oil, F2, and F4 because of its relatively higher water solubility, it was not a major contributor to toxicity based on the TLM framework (Figure 3).
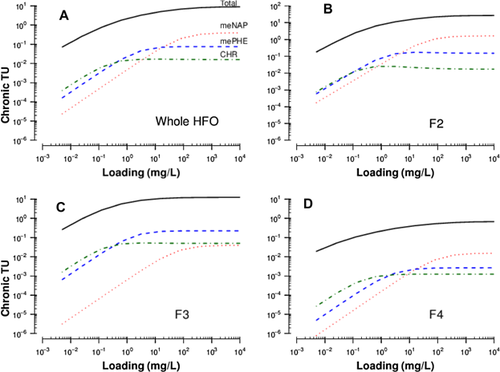
At lower loadings, the more hydrophobic 2-methylphenanthrene and chrysene often contributed more to the predicted toxicity than 2-methylnaphthalene for all oils tested. Only after the solubility limits were reached for 2-methylphenanthrene and chrysene for whole oil, F2, and F4 did 2-methylnaphthalene become a larger contributor. Furthermore, the composition of F3 included many 3- and 4-ring PAHs, which were more hydrophobic than the compounds in F2. Despite the lower predicted dissolved hydrocarbons of F3 WAFs (compared with F2), the maximum chronic TUs were 12 for rainbow trout, which were comparable to the maximum TUs for F2 (28 TUs) and the whole oil (9 TUs). The maximum TUs for the distillate fractions F2, F3, and F4 do not add up to the maximum TUs for the whole oil. After distillation, each of the fractions behaved as a separate substance whose dissolution and toxicity are controlled by the respective loading and composition.
A key goal of the present study was to describe the observed toxicity to trout embryos in terms of toxic units and passively sampled WAFs. Toxicity calculations with PETROTOX were based on measured neat oil chemistry and loadings estimated using measured aqueous TPAH concentrations 17. The underlying principle for toxicity calculations with CEWAF and PDWAF systems is that both dissolved material and oil droplets are transferred from the stock solution to the more dilute loadings. All material (dissolved and droplet) from the initial WAF preparation (e.g., 10% v/v stock) were diluted in the lower loadings and the transferred droplets then re-equilibrated in the new exposure water.
The TPAH concentrations from Adams et al. 17 were used to estimate the loadings from transferred droplets for the TU calculations, because these concentrations were representative of the exposures experienced by trout embryos. The TPAH concentrations were not measured for each of the loadings in the present analysis, because solutions that were toxic were often too dilute for accurate analyses. Hence, nominal loadings were used in TU calculations as scaled by the calculated transfer efficiency where measured TPAHs were estimated from dilutions of known TPAH concentrations in stock WAF solutions.
Differences were found in the TPAH concentrations obtained in CEWAFs prepared in the present study and results reported by Adams et al. 17 (Supplemental Data, Table S4 and Figure S6), likely because of the inter-laboratory variability of the WAF preparation protocol and differences in analytical methods used for TPAH characterization. For example, samples analyzed in the present study were taken directly from WAF preparation volatile organic analysis (VOA) vials, whereas Adams et al. 17 sampled exposure solutions during chronic toxicity tests. Despite the difference in absolute TPAH concentrations, the composition of the TPAH based on the individual PAH analysis (Supplemental Data, Figure S7) remained similar.
Comparison with measured chronic toxicity
The observed mortality of trout exposed to CEWAF spanned approximately 3 orders of magnitude across the different fractions of HFO (Figure 4A) because of the very different nature (e.g., hydrophobicity) of the constituents present. Predicted aqueous concentrations generated using PETROTOX were used to predict toxicity 19. Dissolved-phase TUs were compared with the observed effects data (Figure 4B).
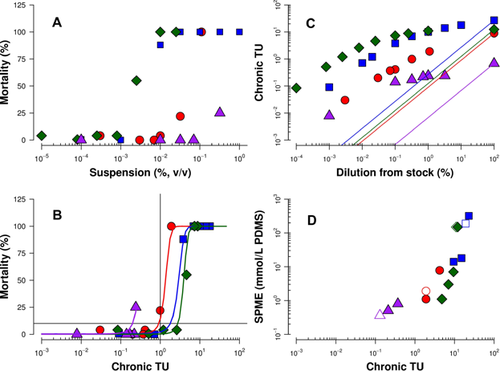
The chronic TUs normalized the different effects from the 4 test oils to within a factor of 3. The modeling approach predicted relatively higher TUs for the lighter F2 and F3 fractions compared with the F4 and whole oil, which is composed of approximately 60% F4 constituents. The differences between these 2 groups of data were within a factor of 2, which is consistent with typical variability in the model framework and experimental toxicity work (factor of 2–5) 20 with petroleum substances. It was, therefore, difficult to identify trends in the model–data comparisons among fractions, given the variability in the comparisons and the small number of fractions analyzed. The exposures of trout embryos to whole oil, F2, and F3 fractions caused mortality that spanned from control levels (<10%) to 100%. The TUs across the exposures ranged from less than 0.001 to 18, with observed dose responses between mortality and TUs that were characteristically steep, typical of nonpolar organics.
Exposures to F4 resulted in 25% mortality at the highest loading, which was associated with only 0.24 TUs. Whether this toxicity was a result of biological variability or exposure to hydrocarbons is not clear. The observation of sublethal endpoints (deformity, blue sac disease) 17 were consistent with exposure to hydrocarbons, but the TU analysis and SPME results (see later discussion) suggested that dissolved-phase hydrocarbons may not have been a large contributor to F4 toxicity or are at least at the lower end of the uncertainties associated in the models and exposure assumptions.
Uncertainty around the TU–mortality comparison was characterized by evaluating the standard errors of the logistic model fit to the data sets for each test oil. This provided a means for establishing confidence intervals around the toxicity predictions and for comparing 1 dataset with another. For example, the chronic TUs associated with 10% mortality (95% confidence intervals in parentheses) were 0.92 (0.32) for whole oil, 1.8 (0.15) for F2, 2.8 (0.97) for F3, and 0.2 (0.25) for F4. The TUs at the observed EC10s were similar, within a factor of 2, with confidence intervals that overlapped or were very similar, except for F4. The similarity in the confidence intervals indicated very subtle differences in the observed exposure–response relationships. Furthermore, the uncertainty associated with TLM predictions (e.g., factor of approximately 3) 20 suggested very little difference among the observed dose responses from the different HFO fractions. Thus, the observed effects were consistently described by this TU approach, which provided a mechanistic means for relating observed toxicity to the chemical composition of the different oil fractions in these complex exposure systems (CEWAFs).
Ideally, the EC10s would be associated with a TU of 1, but there is some uncertainty attributable to assumptions in the modeling or the underlying biological variability in toxicity tests with complex hydrocarbons. In addition, this analysis relied on some estimated exposure concentrations, and losses of material through handling or biodegradation were possible and a likely source of variability. Variability in the oil dissolution model, including the assumption that droplets were in equilibrium with the exposure water, is another source of potential error. However, mortality increased with increasing TUs, and the clear dose responses were generally consistent with theory. Furthermore, the assumption of constant exposures is supported by the reasonably close agreement between the model and observations. However, developing methods that deliver constant exposures of complex substance is needed to minimize variability and to support valid interpretation of dose response data. Hence, variability in toxicity attributable to the estimated exposure concentrations of oil fractions was considered acceptable relative to the other uncertainties discussed previously. The application of PETROTOX to these toxicity data reduced the differences in toxicity (e.g., 1000× on loading basis) among HFO fractions to within a factor of 3.
To further illustrate how the oil dissolution model impacts the interpretation of the toxicity in these exposure systems, the predicted TUs for each loading were plotted at their target dilution from the stock solution (Figure 4C). The curves (as symbols) were shallow, following the general pattern of the dissolved concentration and TU curves in Figures 1 and 3. The calculated TUs based on absolute dilution of the stock solution (e.g., no dissolution) are shown as lines decreasing linearly with increasing dilution of the stock solution. The 2 approaches gave very different profiles and differed by nearly 1000-fold at very dilute loadings. This provides a mechanistic insight for heavy substances that substantial dilutions from a concentration stock may not appreciably alter the dissolved profile because of the solubility characteristics of these substances. This observation is dependent on the composition of the entrained oil as well as the amount of entrained oil, and at present it may be difficult to generalize beyond the results of the present study.
Correlation between toxic units and biomimetic extraction measurements
The use of passive samplers is increasing as a way to provide a more relevant exposure measure for effects assessment and as a potential alternative method to predict toxicity 26. The biomimetic method used in the present study provides an integrated measurement of dissolved hydrocarbons in a complex WAF, which is similar, in principle, to the use of additive TUs. In the present study, the application of this method to the observed effects data was considered qualitative because direct biomimetic extraction measurements were not taken during the toxicity exposures of Adams et al. 17. Instead, the comparisons were made on the basis of predicted TUs calculated from the PETROTOX model.
The biomimetic extraction results for both CEWAFS and PDWAFs at selected loadings for the different oils are provided in Supplemental Data, Table S1. The SPME fiber concentrations of total dissolved, bioavailable hydrocarbons for F2, F3, and whole oil ranged from 0.5 mM to 290 mM PDMS fiber concentrations for F4 CEWAFs, and PDWAFs were low to nondetectable (∼0.5 mmol/L PDMS). Fiber concentrations exhibited a linear trend with predicted TUs over approximately 2 orders of magnitude (Figure 4D). Consistent linear patterns were observed within each dosing system. This suggests that these results were not confounded by fouling of fibers by oil droplets. If fouling were to occur, significant variability in analytical results would be expected. However, replicate measurements of SPME concentrations in CEWAF and PDWAF samples indicated low coefficient of variations of typically less than 20% (Supplemental Data, Table S4).
Furthermore, the SPME chromatograms of CEWAF and PDWAF samples of HFO and the distillate fractions did not resemble the chromatogram for the neat oils but was rather enriched in the more water-soluble components as expected by Raoult's Law. In addition, the pattern of SPME chromatograms changed as a function of loading, with the more water-soluble components enriched at higher loading (Supplemental Data, Figure S8). This is consistent with the expected increased concentration of dissolved-phase components at higher loadings (Figure 1). Based on these observations, the passive sampling method used in the present study appears to provide a viable surrogate for measuring dissolved hydrocarbons in different WAF systems.
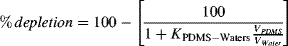 (1)
(1)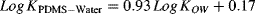 (2)
(2)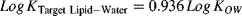 (3)
(3)One goal of the present study was to support the EDCF efforts by identifying important classes of toxic constituents in the HFO and distillate fractions. This was done by summing the contributions of predicted TUs within major chemical classes for each of the petroleum substances evaluated. The calculated TUs for each substance were calculated at the predicted EC10, which corresponds to a TU of 1 and ideally correlates to the onset of chronic toxicity. In the case of F4, in which a TU of 1 was not reached, the TU distribution at the highest loading tested was used (Figure 5).

The modeling suggested that for whole oil, most (60%) of the dissolved TUs were derived from 3 + ring aromatics between log KOW 5 and 7, approximately 25% were from 2 + ring aromatics, and the remaining contributions were from branched monoaromatic and aliphatic constituents in the same range of log KOW. A similar pattern was observed for the F3 fraction, though the distribution reflects a narrow range of log KOW. The TU distribution for F2 is more evenly divided between the 2 ring and 3 + ring aromatic fractions. Approximately half of the TUs in the F4 fraction come from 3 + ring PAHs with log KOW near 6.
To summarize, the TU distributions vary with composition of the neat oils. The patterns observed are specific to these substances and would vary for different petroleum substances categories (e.g., gas oil, kerosene, crude oil). The modeling framework used in the present study allowed the systematic identification of important subsets of hydrocarbon classes for additional research to refine our understanding of the process and constituents that contribute most to the toxicity of these petroleum substances.
Putting the results of laboratory-based toxicity testing in the context of real world exposures is important. In open-ocean spills, the concentrations of dispersed oil are expected to drop below 1 ppm in hours to less than a day 34, thereby limiting the exposure to levels of dissolved hydrocarbons that cause adverse effects. Dissolved hydrocarbons in dilute oil concentrations (<1 ppm) are not likely to be influenced substantially from replenishment by entrained oil droplets (Figure 1). However, that ecological and biological and factors can also modify the risks of oil toxicity must be recognized. Freshwater ecosystems can have more limited volume and relatively more shoreline that can lead to stranding of oil in shoreline habitat and shallow sediments, creating more prolonged exposures.
SUMMARY
The present study had 2 goals: first, to explain the observed effects data for rainbow trout embryos exposed to HFO substances using PETROTOX, and second, to evaluate the application of biomimetic extraction using PDMS-coated SPME fibers as an alternative method for predicting toxicity. The chronic toxicity was consistently described by TUs that were based on the dissolved-phase hydrocarbons, assuming additive toxicity. Although the direct impact of droplets remains an area of ongoing research, their effect seemed to be minor in the present study. We assumed that the effect of the droplets was mainly through re-supply of the dissolved components. The data–model comparisons support our working hypothesis that entrained droplets continued to replenish the exposure system because of the relatively low predicted dissolution of the HFO fractions used in the present work. The SPME analyses provided an integrated measure of dissolved-phase hydrocarbon exposure and correlated well with TUs computed using PETROTOX and shows promise as a simple analytical tool that can be used to predict toxicity.
SUPPLEMENTAL DATA
Tables S1–S5.
Figures S1–S8. (528 KB DOC).




