Mercury bioaccumulation in dragonflies (Odonata: Anisoptera): Examination of life stages and body regions
Abstract
Dragonflies (Odonata: Anisoptera) are an important component of both aquatic and terrestrial food webs and are vectors for methylmercury (MeHg) biomagnification. Variations in mercury content with life stage and body regions may affect the relative transfer of mercury to aquatic or terrestrial food webs; however, there has been little research on this subject. Also, little is known about mercury bioaccumulation in different body regions of dragonflies. To address these knowledge gaps, dragonfly naiads, adults, and exuviae were collected at 2 lakes in Kejimkujik National Park, Nova Scotia, Canada, and mercury concentrations in different life stages and body regions were quantified. Mean whole body concentrations of MeHg were substantial in naiads (232 ± 112 ng g−1 dry wt, n = 66), emerging adults (236 ± 50 ng g−1 dry wt, n = 10), and mature adults (231 ± 74 ng g−1 dry wt, n = 20). Mean MeHg concentrations in exuviae (5.6 ± 4.3 ng g−1, n = 32) were 40-fold lower than in naiads and adults. Emerging adults had 2-fold to 2.5-fold higher Hg(II) concentrations than naiads, mature adults, and exuviae. In body regions of both naiads and adults, some abdomens contained significantly higher concentrations of Hg(II) than heads or thoraces, and this trend was consistent across families. Across families, Aeshnidae had significantly higher concentrations of MeHg and total Hg than Gomphidae and Libellulidae, but not higher than Cordulidae. The Hg(II) concentrations were lower in Aeshnidae and Libellulidae than in Gomphidae and Cordulidae. Shedding of exuviae presents a possible mechanism for mercury detoxification, but mercury concentrations and burdens in exuviae are low in comparison with naiads and adults. Dragonfly adults retain a high potential for transferring substantial amounts of MeHg to their predators. Environ Toxicol Chem 2014; 33:2047–2054. © 2014 SETAC
INTRODUCTION
Methylmercury (MeHg) is a bioaccumulative neurotoxin that can impact the health of both aquatic 1 and terrestrial 2 organisms. Elemental mercury (Hg[0]) and divalent mercury (Hg[II]) are atmospherically deposited from natural and anthropogenic sources, and Hg(II) is subsequently transformed into MeHg in many remote freshwater ecosystems 3, 4. Methylmercury biomagnifies through food webs up to 4 times more efficiently than Hg(II) 5, such that the percentage of MeHg relative to total mercury increases at each trophic level 6. Aquatic invertebrates form a significant portion of many food webs and occupy multiple trophic levels, thus making them important biovectors in trophic transfer processes of the mercury cycle.
Aquatic and terrestrial insects are important food sources for higher trophic-level organisms such as fish 7 and birds 2, 8, which have been found with elevated concentrations of MeHg 9, 10. Mercury concentrations reported in insectivorous birds are comparable to those of piscivorous birds in some cases 10-12, suggesting that invertebrates are an important biovector for the movement of MeHg from aquatic to terrestrial systems 13-15. Mercury bioaccumulation in emerging insects has been quantified in chironomids 14 and other aquatic insects 16, but studies mainly focus on insects that are detritivores and herbivores, which often have lower MeHg and percent MeHg than predatory insects 17-19. The role of aquatic invertebrates in the transfer of organic contaminants (e.g., polychlorinated biphenyls) from aquatic to terrestrial food webs has been well studied 13, 20; however, only a few studies have examined the role of aquatic invertebrates in aquatic–terrestrial transfer processes for mercury 11, 15, 21, 22.
Chételat et al. 23 found that invertebrate life stages can play a critical role in the movement of mercury through food webs. Food web structures involving different life stages of Chironomids had a greater impact on MeHg concentrations in Arctic char (Salvelinus alpinus) than inorganic Hg input to the system 23. Mercury concentrations in invertebrates at different life stages may be impacted by several factors, including changes in foraging behavior with seasons and environment, selection of larger prey items with growth, and shedding of exuviae (cuticles of the final naiad instar that are lost during emergence) 17. Sarica et al. 24 showed that blowflies experience a lowering in mercury concentrations following metamorphosis, likely through shedding of exuviae and release of meconium. Kinetic modeling exercises have shown the impact of differing sources of metal contamination on bioaccumulation pathways 25; however, little is known about metal kinetics during invertebrate metamorphosis. More research is warranted on the life-stage–level processing of mercury because it may have a substantial impact on the availability of mercury to higher trophic levels.
Information on mercury distribution among insect tissues is necessary for understanding mercury bioaccumulation processes. Mercury speciation and partitioning in tissues has been examined in mayfly naiads (Hexagenia rigida McDunnough), a burrowing detritivore, in controlled exposure tests with replicate in situ concentrations and conditions 26-28. The tests found that Hg(II) was primarily concentrated in the gut region and gills, whereas MeHg was fairly homogeneous. Distribution of mercury in aquatic insect tissues has potential implications for predators that consume only parts of their prey. Some predators selectively feed on high-energy tissues—body regions of higher energy content such as the abdomen—in cases when the prey cannot be consumed whole or when prey densities are high 29, 30. For example, whirligig beetle larvae (Gyrinidae) pierce-suck haemolymph from their prey 19, and Achaearanea tepidariorum (orb weaver spider) feed only on select parts of prey items caught in their web when there is an overabundance of prey 31. Feeding on only selected body regions of dragonflies could potentially result in differing rates of exposure to mercury in some of their predators, such as spiders.
Dragonflies (Odonata: Anisoptera) are important predatory insects in aquatic 32 and terrestrial ecosystems 33 and have been implicated in mercury food web transfer 22, 34. Dragonfly naiads have been found with MeHg concentrations as high as 1920 ng g−1, which was comparable to concentrations in predatory pumpkinseed fish (Lepomus gibbosus) in the same study, with a median 2310 ng g−1 35. The potential for bioaccumulation of high mercury concentrations in dragonfly naiads is related to many factors including their MeHg exposure and their position in the food web where they feed on invertebrates, larval amphibians, and small fish 36. Dragonfly adults further increase the bioaccumulation potential because they feed on other flying invertebrates. In turn, naiads and adults are preyed on by terrestrial predators such as frogs, spiders 36, and insectivorous birds 37. In a study of bird diets in North America, 10% of 468 bird species were found to feed on dragonflies, and 6 of those species fed on them heavily 37. Mass migrations and seasonal emergence can contribute to periodic high abundances of dragonflies 36, further contributing to the potential significance of this mercury biovector if bird diets shift to dragonflies during these events.
Although dragonflies have been implicated as key biovectors of mercury to aquatic and terrestrial food webs, very few data are available on mercury distribution within life stages and body regions for this taxon. To address this critical knowledge gap, we quantified mercury species, MeHg and Hg(II), in dragonfly life stages and body regions sampled from 2 freshwater lakes in Nova Scotia, Canada. We hypothesized that mercury concentrations would be highest in adult dragonflies because of bioaccumulation of mercury with age, and because adults prey on other flying insects. We also hypothesized that mercury accumulation would be significantly different among body regions because of partitioning of mercury in tissues that are disproportionately distributed in the head, thorax, and abdomen. The data we present in the present study are critical to assessing pathways of mercury transfer between aquatic and terrestrial food webs.
METHODS
Collection methods
Dragonflies were collected from Big Dam East Lake (44.450°N, 65.265°W) and Big Dam West Lake (BDW; 44.458°N, 65.287°W) in Kejimkujik National Park, Nova Scotia, during 24–27 May and 15–19 June 2010. Mercury bioaccumulation is an ongoing problem in Kejimkujik National Park, and these sites have been extensively characterized for physical and chemical properties 4. Big Dam West Lake has higher dissolved organic carbon, lower pH, and higher aqueous MeHg than Big Dam East Lake (Table 1). Dragonfly naiads, emerging adults, mature adults, and exuviae were collected from 10 evenly dispersed shoreline sites (∼100 m apart, 5–10 m radius) at each lake. Dragonfly naiads were sampled using a D-frame dip net (mesh size 500 µm) in the sediment, aquatic vegetation, and leaf debris of the littoral zone (<1 m). Recently emerged dragonflies and their exuviae were collected from exposed rocks and shoreline, and mature dragonfly adults were caught with an aerial butterfly net. Emerging dragonflies were distinguished from mature adults by their soft exterior and shiny wings and, in most cases, their inability to fly. Acid-washed plastic forceps and nitrile gloves were used to collect and seal samples in 15-mL polypropylene tubes. All samples were frozen at –20 °C until analysis.
| Characteristic | Lake | Source | |
|---|---|---|---|
| Big Dam East | Big Dam West | ||
| Catchment area (km2) | 2 | 40 | 57 |
| 58 | |||
| Surface area (km2) | 0.46 | 1.05 | 59 |
| Mean depth (m) | 2.32 | 2.47 | 59 |
| Color (relative color units) | 15.3 | 95.0 | 58 |
| DOC (mg L−1) | 4.8 | 8.3 | 58 |
| pH | 6.1 | 5.1 | 58 |
| MeHg (pg L−1) filtered | 38.0 | 96.0 | 60 |
- DOC = dissolved organic carbon.
Processing and mercury speciation analysis
All samples were processed and analyzed in the Center for Analytical Research on the Environment (CARE) at Acadia University (Wolfville, NS, Canada). Each sample was identified to genus using keys provided by Tennessen 38. Wet weights were measured for each sample (including exuviae); then samples were dried at 60 °C for 72 h, and dry weights were measured. Dried samples were used either as whole bodies or separated into body regions of head, thorax, and abdomen using a dissecting knife cleaned with HCl and Milli-Q water between each dissection. Each whole body or body region was homogenized separately using a mortar and pestle, and 20 mg of homogenate was transferred to a 2-mL polypropylene vial for mercury analysis.
Mercury species measured included MeHg and Hg(II), which were quantified in all dry samples using alkaline digestion, ethylation, and purge and trap gas chromatography–atomic fluorescence spectrometry 2, 39. Total mercury was calculated as THg = Hg(II) + MeHg, and the MeHg percentage was calculated as %MeHg = MeHg/THg × 100. Quality control methods included deionized water field blanks, deionized water method blanks, sample replicates, analytical replicates, and comparative analysis using certified standard reference material, dogfish liver tissue (DOLT-4). All method blanks (n = 11) were below the detection limit (MeHg = 0.40 pg; Hg[II] = 1.20 pg), and all samples were significantly above this limit. Exuviae of less than 10 mg dry weight were pooled within lake site and family (n = 2 or 3) to obtain adequate sample mass and detectable MeHg concentrations. Naiads under 10 mg dry weight were not analyzed. Sample replicates were within accepted norms (% relative standard deviation [RSD] < 10%, n = 5 for MeHg) as were analytical replicates (% RSD < 5%, n = 11 for MeHg). The certified reference material DOLT-4 showed good recoveries (MeHg = 89.3% ± 12%; THg = 102.6% ± 10%; n = 18). The Hg(II) recovery (116.7% ± 10%) in DOLT-4 was calculated by assuming that the difference between THg and MeHg in DOLT-4 was primarily attributable to Hg(II); however, it should be noted that this is not a certified value. Additional spikes of Hg(II) with certified reference materials were used to confirm recoveries and were found to be consistent with certified reference material recoveries stated.
Data analysis
Data were visualized using boxplots and summary statistics calculated to assess trends among families, life stages, and body regions. Statistical analyses followed that of analysis of variance, but because of an unbalanced sample design, general linear models were used. General linear models were created for each mercury species to compare species concentrations among families and body regions, and between life stages. Dry weight was included as a covariate because dry weight and wet weight were significantly related (linear regression: r2 = 0.77, p < 0.001; Supplemental Data, Figure S1), and a nonlinear effect as a result of individual size remained even though mercury concentrations based on dry weight were used as responses. Full models included factors of family, life stage, lake, sampling date (month), and interaction effects between life stage × lake and life stage × month. Factors were removed from models if they were not significant or to keep model comparisons consistent. Log10 transformation of responses removed heterogeneous variances for MeHg, Hg(II), and THg; however, figures were plotted as actual values. Reponses of %MeHg had homogeneous variances and were used as calculated. General linear models were created in R (Ver 3.01) 40, and pairwise comparisons were completed using Tukey's honestly significant difference test with the R package multcomp.
Several dragonfly samples were removed from data analysis (n = 10) in cases when they did not have weight measurements, so mercury concentrations could not be calculated, and when they had higher than expected MeHg or Hg(II) concentrations. Three Hagenius spp. samples had high statistical leverage resulting in a high influence on models and so were considered outliers and removed from analyses. Hagenius spp. samples had high mean dry weights and high variability (222 mg ± 118 mg) relative to all other samples (Table 2). This genus occupies a different trophic level than the other dragonfly species within the present study, and Hagenius spp. eat other dragonflies. One emerging adult was found with a leech attached and had much lower mercury than other samples; it was removed from all analyses.
| No. | Dry weight | Wet weight | |
|---|---|---|---|
| Exuviae | 32 | 12.4 ± 5.8 | 17.9 ± 16.8 |
| (3.30–24.4) | (4.50–79.0) | ||
| Naiads | 66 | 41.2 ± 23.0 | 197 ± 120 |
| (11.0–103) | (63.4–549) | ||
| Emerging adults | 10 | 55.3 ± 29.0 | 203 ± 98.4 |
| (24.7–102) | (121–383) | ||
| Mature adults | 20 | 97.9 ± 36.7 | 284 ± 95.0 |
| (40.2–149) | (142–439) |
- a Table excludes Hagenius spp. samples.
RESULTS
Five dragonfly families were identified: Aeshnidae, Cordulidae, Gomphidae, Libellulidae, and Macromiidae. Gomphidae contained all of the emerging adults (n = 10) across all families and few (n = 3) mature adults. Because dry weights were used in modeling, emerging adults and mature adults were pooled for some summary statistics and all modeling, resulting in only 2 life stage levels (naiads and adults) for modeling (Figures 1 and 2). Samples were pooled across months (May and June) for all analyses because there was no significant difference between the mean monthly responses, and the inclusion of month as a factor did not result in significantly better models. Responses were also pooled across lakes (Big Dam East and Big Dam West) because there were insufficient data points to partition the 2 levels of life stage between lakes that resulted in model convergence when family was also a factor. Differences among families were considered more important than differences in either sampling date (month) or lakes.
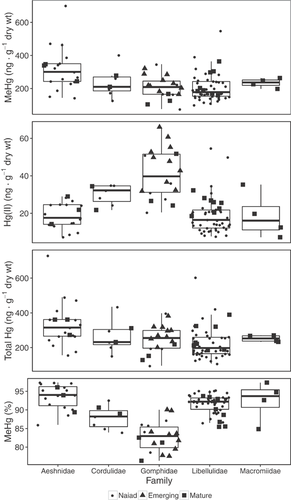
Concentrations of mercury species varied among the 5 dragonfly families pooled across lakes and sampling periods (Figure 1; corresponding mercury burden data in Supplemental Data, Figure S2), yet trends were apparent. Concentrations of MeHg, Hg(II), and THg in naiads were generally more variable than in adults, but variability in %MeHg was generally similar (Figure 1). Gomphidae had comparatively higher median Hg(II) concentrations and lower median %MeHg than all other families. Macromiidae contributed the fewest samples (n = 4) and all were adults; therefore, this family was dropped from modeling. Aeshnidae was composed mainly of naiads (n = 16) with few adults (n = 3). Cordulidae had a low number of both naiads (n = 5) and adults (n = 2), and Libellulidae had the greatest number of naiads (n = 40) and a moderate number of adults (n = 8). Variability was consistent across all families except Macromiidae, where MeHg and THg had low variability compared with the other families.
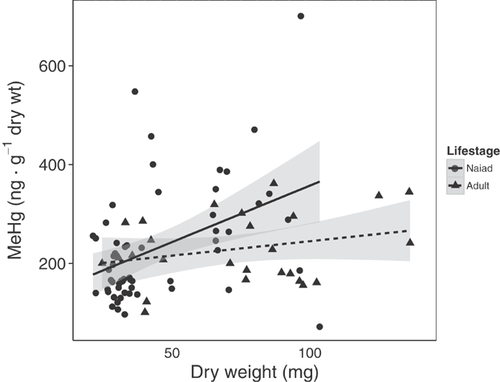
In dragonfly naiads, MeHg concentrations significantly increased with naiad age (instar) (linear regression: r2 = 0.155, p < 0.001), but variation in MeHg concentrations also increased, resulting in only 16% of MeHg concentration accounted for by dry weight (Figure 3). As a result, the older, heavier naiads exhibited both the highest and lowest MeHg concentrations, indicating there would be a high variability in naiad burdens, but also that naiads do increase in mercury burden as they gain mass. Adults showed similar increasing MeHg concentrations with increasing dry weight, but this relationship was not significant, and dry weight explained only 3.4% of MeHg (linear regression: r2 = 0.034, p = 0.183; Figure 3).
Mercury quantification in life stages
Summary trends showed that naiads had whole body MeHg and THg concentrations, as well as %MeHg, similar to that of adults. The Hg(II) concentrations were highest in emerging adults followed by exuviae (Table 3), but all emerging adults were from the Gomphidae family, which also had the highest Hg(II) concentrations for adults (Figure 2). Exuviae had 40-fold lower concentrations of MeHg, 6-fold lower THg, and 7-fold lower %MeHg than naiads and adults, but the second highest concentration of Hg(II) (Table 3). Exuviae contributed the lowest concentrations of MeHg and THg, and the lowest %MeHg consistently among families; however, exuviae contained concentrations of Hg(II) at least as high as those of the life stages (Table 3). Because exuviae had greater than 3-fold lower dry weights in comparison with naiads and adults (Table 2), the total burden of mercury contained in exuviae would be low.
| No. | MeHg | Hg(II) | THg | %MeHg | |
|---|---|---|---|---|---|
| Exuviae | 32 | 5.60 ± 4.33 | 37.4 ± 11.4 | 43.0 ± 13.8 | 12.6 ± 5.69 |
| (1.41–26.2) | (21.3–62.9) | (25.1–81.0) | (5.22–32.4) | ||
| Naiads | 66 | 232 ± 112 | 20.0 ± 10.3 | 251 ± 117 | 91.5 ± 3.72 |
| (75.5–701) | (7.42–54.8) | (96.0–730) | (78.6–97.4) | ||
| Emerging adults | 10 | 236 ± 50.0 | 47.6 ± 11.7 | 284 ± 51.1 | 82.9 ± 4.38 |
| (165–322) | (31.8–66.0) | (201–382) | (77.6–90.1) | ||
| Mature adults | 20 | 231 ± 73.5 | 25.0 ± 9.47 | 256 ± 70.9 | 89.3 ± 5.43 |
| (105–364) | (7.24–51.8) | (129–390) | (76.4–97.3) |
- MeHg = methylmercury; Hg(II) = divalent mercury.
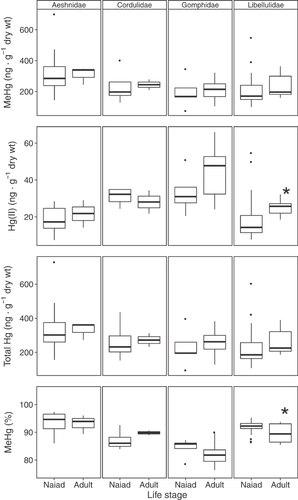
There were only 2 cases across all mercury species in which concentrations between naiads and adults were significantly different within families. Adults had significantly greater Hg(II) concentrations in Libellulidae (p = 0.008), and naiads had significantly greater %MeHg (p = 0.012) in the same family (Figure 2); therefore, naiads and adults were pooled for comparisons among families.
Aeshnidae had significantly greater MeHg concentrations than Gomphidae (p = 0.001) and Libellulidae (p < 0.001), but not Cordulidae (p = 0.252). Cordulidae was not significantly different in MeHg than Gomphidae (p = 0.637) or Libellulidae (p = 0.846), nor was Gomphidae different than Libellulidae (p = 0.904) (Figure 1). The THg results follow those of MeHg because of the high %MeHg in samples. Aeshnidae had significantly greater THg concentrations than Gomphidae (p = 0.015) and Libellulidae (p < 0.001) but not different from Cordulidae (p = 0.420). Cordulidae was not significantly different in THg than Gomphidae (p = 0.803) or Libellulidae (p = 0.669), nor was Gomphidae significantly different in THg than Libellulidae (p = 0.999).
Libellulidae had significantly lower Hg(II) concentrations than Cordulidae (p = 0.005) and Gomphidae (p < 0.001), but not significantly different concentrations than Aeshnidae (p = 0.994). Aeshnidae had significantly lower Hg(II) than Cordulidae (p = 0.021) and Gomphidae (p < 0.001), but Cordulidae and Gomphidae were not significantly different (p = 0.848) (Figure 1). Similar differences were noted in %MeHg. Aeshnidae had significantly greater %MeHg concentrations than Cordulidae (p < 0.001) and Gomphidae (p < 0.001), but these were not significantly different than Libellulidae (p = 0.067). Cordulidae had significantly greater %MeHg than Gomphidae (p = 0.009) yet significantly lower %MeHg than Libellulidae (p = 0.022), and Gomphidae had significantly lower %MeHg than Libellulidae (p = 0.001) (Figure 1).
Mercury quantification in body regions
When mercury concentrations in body regions were analyzed, differences were compared within life stages and families only (Figure 4). Abdomens contained greater Hg(II) concentrations than heads in naiad (p = 0.019) and adult (p = 0.004) Aeshnidae, and adult Gomphidae (p = 0.036), but not in adult Cordulidae (p = 0.702) (Figure 4). Abdomens also contained greater Hg(II) concentrations than thoraces in adult Aeshnidae (p = 0.002), but not in naiads (p = 0.306), and in adult Gomphidae (p = 0.008). No significant differences were found among body regions in adult Cordulidae (p ≥ 0.095 for all comparisons). Conversely, and because of correspondingly higher Hg(II) concentrations, abdomens had lower %MeHg than heads in naiad (p = 0.021) and adult (p = 0.032) Aeshnidae. Concentrations were also lower between abdomens and thoraces in adult Aeshnidae (p = 0.018), and abdomens and thoraces in adult Gomphidae (p = 0.036). All other comparisons of %MeHg among body regions were not significant (p ≥ 0.120) (Figure 4).

DISCUSSION
Mercury transfer to aquatic and terrestrial food webs through aquatic insect emergence is an important aspect of mercury contamination in freshwater ecosystems 2, 41. To our knowledge, the present study is the first to quantify mercury species in both naiad and adult life stages of dragonflies while simultaneously examining the exuviae. We show that dragonflies contain high concentrations of MeHg at both the naiad and adult life stages and thus have potential for transferring considerable amounts of mercury to both aquatic and terrestrial food webs. In addition, low concentrations of mercury in exuviae coupled with their low masses suggest a rather low mercury burden.
Mercury quantification in life stages
Mercury in dragonfly adults has been quantified in recent studies of mercury transfer to aquatic and terrestrial food webs 21, 22, 42. Tsui et al. 42 found that dragonflies collected near a stream had a mean MeHg concentration of 180 ng g−1, which is comparable in the present study to 231 ng g−1 ± 73.5 ng g−1 dry weight for mature adults and 236 ng g−1 ± 50.0 ng g−1 for emerging adults. Clayden et al. 41 found that adult dragonflies (Aeshnidae) varied by 3-fold (170–540 ng g−1 dry wt) in a study of 11 lakes in Kejimkujik National Park. The variations observed by Clayden et al. 4 were attributed to general chemical and physical factors that increase MeHg levels such as low pH and increased dissolved organic matter. Dragonfly naiads from Kejimkujik National Park lakes also show high mercury concentrations 43. Wyn et al. 43 sampled Aeshna umbrosa naiads (n = 12) from 4 Kejimkujik National Park lakes and found that mean MeHg concentrations among lakes ranged between 170 ng g−1 ± 30 ng g−1 and 440 ng g−1 ± 17 ng g−1, which are comparable to the mean MeHg concentrations for naiads shown in the present study (232 ng g−1 ± 112 ng g−1). In comparison, dragonfly naiads from lakes sampled outside Kejimkujik National Park show mean MeHg concentrations ranging from 55 ng g−1 ± 4 ng g−1 (n = 3) in a lake with a partially burned catchment 43 to 615 ng g−1 (n = 3) in a hydroelectric reservoir 18. Thus, Kejimkujik National Park naiads are on the higher end of MeHg concentrations compared with dragonfly naiads from other natural lakes 19, 44.
Overall, the trends reported in the present study are supported and show significant differences in concentrations of MeHg, Hg(II), and THg, as well as %MeHg among families and between some body regions within families, but not between life stages. We found that there were basically no differences in mercury concentrations among naiads, emerging adults, and mature adults, but there were clear and significant differences among families. The fate of mercury during transformation from the naiad to the adult life stage of emerging aquatic insects is not well understood. Rossaro et al. 45 determined that adult midges (Chironomus riparius) contained 40% less total mercury than the larval stage and this loss was attributed to depuration mechanisms. In contrast, Chételat et al. 23 found that adult chironomids in high arctic lakes had 2.9 times higher MeHg than the larval stage, indicating that prey choice may have a considerably negative impact on predators who choose to feed on adult versus larval stages of the same species (especially during seasonal emergence). Furthermore, it is generally accepted that mercury increases with age and trophic position 41, 46, but this does not always appear to be true for aquatic invertebrates. This discrepancy is perhaps attributable to factors such as decreased feeding following emergence, shedding of exuviae, weight loss associated with mating 23, or variations in feeding ecology. For example, Sizmur et al. 47 also observed that MeHg accumulation in polycheate worms from coastal mudflats is influenced more by feeding ecology than trophic position.
Adult dragonflies continue to have a carnivorous diet after emergence 36, and emerging adults have depleted stores of fat 32, which suggest that they should have higher MeHg concentrations than naiads; however, our results do not support this hypothesis. Instead, adults appear to maintain mercury concentrations similar to those of naiads. One possible explanation is that dragonflies are able to limit mercury burdens through depuration during or prior to emergence, but there is currently no published evidence to support this hypothesis for dragonflies. Our results contribute evidence for Hg(II) depuration via dragonfly exuviae; however, there is no evidence for other depuration mechanisms in dragonflies.
Evidence for depuration exists in other insects for mercury as well as other contaminants. In a study on the mayfly Ephoron virgo, Cid et al. 48 showed an increase in THg with larger larval instars until the final instar stage, when it then decreased. This phenomenon was also true for cadmium, but other metals remained at a constant concentration in the final instar stage 48. In dragonfly naiads reported on in the present study, MeHg concentrations increased with naiad age, but variation in MeHg concentrations also increased. Adults showed no increase in MeHg concentrations with increasing dry weight. However, evidence is seen in emerging adults that had higher Hg(II) concentrations than other life stages: over 2-fold higher Hg(II) concentrations than naiads and 1.5-fold higher than mature adults, despite a lack of significant differences in MeHg concentrations among life stages. This finding suggests that weight loss may have a disproportionate impact on Hg(II) concentrations relative to MeHg concentrations. Jones et al. 21 specifically sampled recently emerged dragonflies and reported %MeHg to be 78.5 ± 4.8%, which supports our finding of a 2-fold increase in Hg(II) concentrations in emerging dragonflies over naiads and mature adults. The reason for high Hg(II) concentrations in emerging adults relative to MeHg concentrations (low %MeHg) is unclear, but it is suggestive of effects of weight loss or a mercury transformation mechanism. Low %MeHg can be an indicator for mercury detoxification, as is the case in animal kidneys 49.
Dragonfly exuviae shedding was hypothesized to be a significant method of mercury removal. The MeHg concentrations were 40-fold lower than at other life stages, whereas Hg(II) concentrations were comparable to those of naiads and adults. Interestingly, dragonfly exuviae in the present study had 6 times higher MeHg concentrations than chironomid exuviae 23, reflecting higher mercury in the body. This finding suggests that Hg(II) may have a higher affinity for cutaneous tissues than MeHg. Some research suggests that chitin has a high affinity for Hg(II) 50. Cremona et al. 19 support this finding and stated as unpublished results that dragonfly exuviae had 10-fold lower MeHg than emerging adults. Tsui and Wang showed that molting is not a significant source of mercury efflux; however, molting removed slightly greater amounts of Hg(II) than MeHg 51. Exuviae are extremely light (∼10 mg), and so the burden of MeHg and Hg(II) relative to the total body burden is small. Dragonfly adults maintain considerably high MeHg concentrations and burdens that could be transferred to terrestrial predators.
Mercury quantification in body regions
Body regions were used as a proxy for macroscale analysis of mercury in dragonfly tissues—heads having high concentrations of nervous tissue, thoraces primarily muscle, and abdomens large amounts of gut tissue, as well as gut contents, fat bodies, and reproductive structures 52. There were no significant differences in MeHg or THg concentrations among body regions for naiads or adults, but significant differences were found for Hg(II). Dragonfly abdomens had higher Hg(II) concentrations than heads or thoraces in both life stages. Gut contents were not depurated and may have contributed to mercury concentrations. Homogeneous distribution of MeHg in tissues is probably attributable to its affinity for uniformly present sulphyl-hydryl groups 28, 53. In a study of mercury partitioning in the burrowing mayfly (Hexagenia rigida) using a radiolabeling technique and including gut depuration, Inza et al. 28 found that MeHg was slightly higher in the malpighian tubules of the gut, although it was quite homogenous overall. As for Hg(II), it was found to preferentially accumulate in the gut, with high concentrations in the malpighian tubules 28. These findings confirmed the association of Hg(II) with the gut regardless of depuration of gut contents.
Differential mercury speciation in body regions, such as Hg(II) in malpighian tubules, may indicate a potential mercury detoxification mechanism in aquatic insects, including dragonflies. Malpighian tubules have an equivalent function to kidneys in larger animals such as birds and mammals 52 and kidneys are known to play an important role in mercury detoxification. Kidneys have high MeHg and Hg(II) concentrations, but also low %MeHg 49. Malpighian tubules in Drosophila melanogaster have been associated with high concentrations of the mercury-detoxifying enzyme glutathione S-transferase 54. Dragonfly naiads have been found to have relatively high concentrations of glutathione S-transferase 55, and the final instar stage has a high number (50–70) of malpighian tubules 56. This evidence in other studies coupled with the results of the present study suggests a potential for mercury detoxification in dragonflies through malpighian tubules using detoxifying enzymes. Mercury detoxification has significant implications for understanding the pathway of mercury to terrestrial food webs and the role of dragonflies as vectors of high mercury concentrations to land-dwelling animals such as insectivorous birds.
The present study demonstrates that dragonfly families and body regions are important factors to be considered in the transfer of MeHg through dragonflies to aquatic and terrestrial ecosystems, but that life stage is less important. Our results show that dragonflies contain significant amounts of MeHg; however, further research is warranted on the role of dragonflies in trophic transfer of mercury to aquatic and terrestrial food webs through examination of stable N and C isotope ratios (for an example, see Wyn et al. 43). Examination of the impact of emergence and metamorphosis in a controlled laboratory experiment, including measurement of mercury detoxification enzymes, could help us to better understand the potential mercury depuration or transformation processes in dragonflies.
SUPPLEMENTAL DATA
Figures S1 and S2. (266 KB DOCX).
Acknowledgment
Funding was provided by the Natural Sciences and Engineering Research Council of Canada (NSERC), the Canada Research Chairs (CRC) Program, and the Canada Foundation for Innovation (CFI). We thank S. Edmonds for technical support at the CARE Laboratory and A. Larkin for her assistance with the field sampling.




