Acute photo-induced toxicity and toxicokinetics of single compounds and mixtures of polycyclic aromatic hydrocarbons in zebrafish
Abstract
The present study examined photo-induced toxicity and toxicokinetics for acute exposure to selected polycyclic aromatic hydrocarbons (PAHs) in zebrafish. Photo-enhanced toxicity from co-exposure to ultraviolet (UV) radiation and PAHs enhanced the toxicity and exhibited toxic effects at PAH concentrations orders of magnitude below effects observed in the absence of UV. Because environmental exposure to PAHs is usually in the form of complex mixtures, the present study examined the photo-induced toxicity of both single compounds and mixtures of PAHs. In a sensitive larval life stage of zebrafish, acute photo-induced median lethal concentrations (LC50s) were derived for 4 PAHs (anthracene, pyrene, carbazole, and phenanthrene) to examine the hypothesis that phototoxic (anthracene and pyrene) and nonphototoxic (carbazole and phenanthrene) pathways of mixtures could be predicted from single exposures. Anthracene and pyrene were phototoxic as predicted; however, carbazole exhibited moderate photo-induced toxicity and phenanthrene exhibited weak photo-induced toxicity. The toxicity of each chemical alone was used to compare the toxicity of mixtures in binary, tertiary, and quaternary combinations of these PAHs, and a predictive model for environmental mixtures was generated. The results indicated that the acute toxicity of PAH mixtures was additive in phototoxic scenarios, regardless of the magnitude of photo-enhancement. Based on PAH concentrations found in water and circumstances of high UV dose to aquatic systems, there exists potential risk of photo-induced toxicity to aquatic organisms. Environ Toxicol Chem 2014; 33:2028–2037. © 2014 SETAC
INTRODUCTION
Polycyclic aromatic hydrocarbons (PAHs) are a class of lipophilic compounds containing 2 or more aromatic rings and are widespread environmental contaminants 1, 2. Because of the multiple and varied sources of PAHs, they are usually found in the environment as complex mixtures that have differences in chemical bioavailability, species-specific uptake and elimination, and mode of toxic action. Environmental exposure to multiple PAHs can change the adverse effect or modify the severity of effect through interactions of toxicity pathways at the molecular level. Elucidation of toxicity mechanisms from environmental exposures can be difficult, however, because it can be challenging to separate out effects attributed to a specific chemical component or another in the mixture.
Photo-induced toxicity
Photo-induced toxicity is a phenomenon in which certain PAHs become up to 10 000 times more toxic in the presence of solar ultraviolet (UV) radiation, and inclusion of UV exposure along with PAHs represents a multiple stressor scenario 3, 4. The potential for photo-induced toxicity is closely tied to the dose of UV radiation to the system and can be modulated by aquatic system properties related to water transparency and humic content. When UV radiation enters aquatic systems, the photo-induced toxicity adverse outcome pathway becomes an acute exposure mechanism in which toxicity is related to the internal dose of the PAH in the organism, the intensity of the UV radiation, and the duration of the UV + PAH exposure 5. Photo-induced toxicity occurs when PAHs with a specific molecular structure absorb energy from UV radiation and become excited, causing the outer orbital electrons to be raised to a higher molecular energy orbital. This energy is released when the excited molecule returns to ground state, passing that energy to oxygen molecules in the cell and generating reactive oxygen species (ROS), free radicals, or other reactive products 1, 6. The ROS generated through this process can initiate free radical reactions throughout cells and tissues, which causes oxidative damage leading to adverse effects including cell death, behavioral impairment, and mortality 3, 7, 8. The main targets of ROS compounds in the cell are DNA, lipids, and membrane proteins, and interactions with these targets lead to lipid peroxidation and membrane breakdown, affecting plasma ion balance 1, 6, 9.
Only a subset of PAHs has the ability to react with UV radiation and cause adverse effects through this toxicity pathway. A review of photo-induced toxicity and PAHs by Arfsten et al. 6 identified PAHs with the potential to exhibit photo-induced toxicity based on structural/chemical properties such as the size of the highest occupied molecular orbital and the lowest unoccupied molecular orbital (HOMO-LUMO) gap. Polycyclic aromatic hydrocarbons with HOMO-LUMO gaps in the range of 7.1 ± 0.4 eV are predicted to be phototoxic.
Toxicokinetics and body burden
Factors such as physiological characteristics and life stage make certain aquatic species more vulnerable to photo-induced toxicity, especially organisms with translucent early-life stages (e.g., embryos, larvae). Zebrafish are a model organism for testing the effects of toxicants in the aquatic environment 10. Because larval fish are translucent and respire via diffusion of oxygen through the skin, and because the lipids of their cell membranes have a high potential for accumulation of PAHs, direct UV exposure makes these organisms one of the most sensitive to photo-induced toxicity 11. Because photo-induced toxicity is related to the internal PAH dose and intensity of UV radiation, toxicokinetics and differences in PAH body burden can be useful in assessing risk 12. Toxicokinetic parameters and the steady state/equilibrium of the chemical concentration in the organism can provide insight into internal PAH dose and therefore toxic potential. Toxicokinetic models are important tools that provide essential information for chemical risk assessments and can be used to estimate the body burden (i.e., the amount of a chemical present in an organism at a given point) that may elicit a toxic response, which is particularly useful when evaluating phototoxic responses based on PAH dose 13. Body burden can be expressed through species-specific bioconcentration factors (BCFs), defined as the ratio of uptake (ku) and elimination (ke) coefficients of a specific compound at steady state 14.
Mixtures toxicity
The toxicity of PAH mixtures in the environment relates to the chemical composition of the mixture because each PAH may impart adverse effects through different adverse outcome pathways (AOPs). Each PAH contributes to the overall toxicity, but molecular interactions of individual PAHs can change the toxic effect or modify the toxicity mechanisms to differing or potentially competing toxicity pathways, leading to alternative adverse effects or increased/decreased severity of effect. It was hypothesized that individual chemical information from the published literature could be used to predict which toxicity pathway each chemical would follow and to judge how competing toxicity pathways may interact to modify overt toxicity. For the PAHs examined in the present study, anthracene, carbazole (a polycyclic aromatic compound due to nitrogen substitution in the ring), phenanthrene, and pyrene, there are some similarities and differences in AOPs that make these PAHs representative of an environmental exposure to mixtures. Information on AOPs was sought in the literature and, along with HOMO-LUMO gap information, was used to tentatively predict phototoxic or nonphototoxic AOPs for each PAH. One potential AOP included the aryl hydrocarbon receptor (AHR)-mediated toxicity pathway, which is typically identified with evidence for induction of cytochrome P450 (CYP) enzymes. For example, based on its HOMO-LUMO gap and evidence that it does not induce CYP1A, anthracene was expected to cause photo-induced toxicity in the presence of UV radiation 15. For carbazole, there was no evidence in the literature of a phototoxicity effect; but based on HOMO-LUMO gap, it was anticipated to be nonphototoxic. Data showing carbazole to be a CYP1A inhibitor indicated that carbazole does not activate the AHR pathway and/or is not metabolized through the CYP enzymes 16. This type of analysis was initially conducted to identify mixtures that could represent a simplified model of environmental exposure of PAHs that have multiple AOPs.
Most mixture toxicity testing is done under standard laboratory lighting in the absence of UV radiation, and so photo-induced toxicity has not always been taken into account when assessing risk to aquatic organisms. A handful of studies have addressed the toxicity of mixtures containing phototoxic components in a variety of organisms (in the oligochaete Lumbriculus variegates, the marine amphipod Rhepoxynius abronius, and in the marine diatom Phaeodactylum tricornutum) 17-19. Recently, this approach was used to assess retrospective and contemporary phototoxic PAH risks to fish due to the Exxon Valdez oil spill in Prince William Sound, Alaska, USA 5.
Study objectives
The overall objectives of the present study were to evaluate the acute toxicity data for 4 PAHs individually in the presence and absence of UV radiation. This individual toxicity information was then used to predict the toxicity of mixtures of these PAHs in the presence of UV radiation. This approach first determined 96-h phototoxic median lethal concentration (LC50) in larval zebrafish for each PAH, 2 predicted to be phototoxic (pyrene and anthracene) and 2 predicted to be nonphototoxic (carbazole and phenanthrene). Toxicity of mixtures was assessed using an LC50 ratio (toxic unit) approach. Data were adjusted based on relative phototoxic potency, and a general model was derived for use in predicting photo-induced toxicity effects for mixtures of PAHs.
MATERIALS AND METHODS
Test chemicals
Test chemicals anthracene, carbazole, phenanthrene, and pyrene were purchased from Alfa Aesar. These PAHs were chosen to represent an environmental mixture containing phototoxic and nonphototoxic compounds to assess toxicity interactions of PAHs acting through different AOPs. Each chemical was dissolved in methanol for initial stock solutions of 1 mg/L, wrapped in aluminum foil, and stored at room temperature. Stock solution was added to fish culture water to dilute to nominal exposure concentrations. Concentrations of vehicle never exceeded 1% (v/v). Water quality measures were the same for all tests: temperature of 25.6 °C (standard deviation [SD] = 1.0), dissolved oxygen of 7.5 mg/L (SD = 0.4), salinity at 0.2 ppt (no deviation), and pH at 7.6 (SD = 0.5).
Lighting system
Photoperiod conditions during testing were consistent with ambient light conditions in the laboratory at 16 h light to 8 h darkness. Toxicity tests were conducted under a bank of 78 solar simulating fluorescent bulbs (40W; Durotest Vitalite) and 6 fluorescent blacklight bulbs (F40BLB, 40W; General Electric) suspended approximately 0.75 m above test dishes. Irradiance during exposures was measured with a BIC UV-PAR radiometer (Bioshperical Instruments). The BIC radiometer quantifies downwelling (cosine) irradiance at 3 different UV wavelengths (305 nm, 320 nm, and 380 nm), as well as visible wavelengths of photosynthetically active radiation (400–700 nm). The natural sunlight percentage was estimated by comparison with a sunny day in late summer at 40° latitude, where 305 nm was 1.6%, 320 nm was 1.2%, 380 nm was 7.5%, and photosynthetically active radiation was 6.1% of natural light. Raw irradiance was 0.19 µW/cm2, 7.4 µW/cm2, 2.6 µW/cm2, and 205 µW/cm2 each for 305 nm, 320 nm, 380 nm, and photosynthetically active radiation wavelengths, respectively. Ultraviolet dose (cumulative irradiance) was calculated using the 320 nm irradiance data and was approximately 0.41 kJ/m2 for all experiments. This measurement represents the total cumulative time-weighted UV dose to the system over the 96-h exposure period for 320 nm irradiance. Because LC50s were calculated from each replicate group as a whole for every tested concentration, the exposure period was 96 h for UV dose, and cumulative UV dose was calculated at 96 h, regardless of organism survival.
Analytical methods
Standards and unknown test concentrations for each chemical were quantified using high performance liquid chromatography (HPLC) with a Waters 2695 separations module (Microsorb-MV C-18 column, 3 µm) coupled with a Waters 474 scanning fluorescence detector. Each chemical peak was identified using unique wavelengths for excitation and emission: for anthracene, 251 nm and 405 nm with a retention time of 5.6 min; for carbazole, 293 nm and 360 nm with a retention time of 2.3 min; for phenanthrene, 252 nm and 372 nm with a retention time of 5.9 min; and for pyrene, 235 nm and 390 nm with a retention time of 7.4 min. Solvent was 80:20 acetonitrile (ACN):water with a flow rate of 1 mL/min. Concentrations of test solutions were determined by comparison with linearly regressed standard curves based on the peak areas of at least 3 concentrations of standards and a blank.
Acute toxicity of individual PAHs
Zebrafish (Danio rerio) larvae that were 5 d to 7 d old were exposed to 200 mL of each PAH treatment in glass 500-mL crystallizing dishes (10 individuals per dish; 3 replicate dishes per treatment). Water-only and vehicle (methanol)-only controls were also tested in triplicate. Exposure dishes were floated in water tables with a circulating water bath at 28.5 °C (SD = 1.4). The toxicity tests for each chemical were tested during consecutive weeks. Final concentrations used for testing were identified from available studies in the literature and laboratory range finding tests. Each test was run with at least 4 test concentrations. Exposures were carried out as static tests with 2 daily renewals (at 0 h and 8 h), with an initial 12-h uptake period under no UV radiation. Water changes were conducted by removal of approximately 90% of test solution (∼180 mL) using a syringe and replacement with 180 mL of fresh test solution. Samples for HPLC analysis were taken at 0 h, 8 h, and 16 h, and an Euler integration was used to calculate the time-weighted concentration of compound over time.
Larval organisms were fed Paramecium aurelia ad libitum twice daily just after the water change during testing. Mortality and any sublethal effects (lethargy, defined as no response to stimulus) were assessed twice daily (just after water renewal). Dead fish were removed from test dishes, and death was indicated by the absence of a heartbeat as seen under a microscope. Exposures were carried out until 100% mortality was achieved within a treatment or for up to 96 h. Experiments were terminated if controls experienced more than 10% mortality. Any fish living at the end of each experiment were euthanized with an overdose of MS-222/tricaine methanesulfonate (Argent Chemical). Concentration-response curves using time-weighted measured concentrations and mortality were used to estimate LC50s using a logistic regression model in PROC PROBIT (SAS Ver 9.2).
A no-UV exposure was also conducted near the solubility limits for each PAH and under identical exposure conditions (with the exception of UV) for all other parameters. Because the initial results of the pyrene no-UV exposure test indicated higher than expected toxicity, a full no-UV LC50 was derived. Mortality was assessed and compared with the mortality results from the UV exposures to confirm a photo-enhancement effect.
Bioconcentration factor experimental design
Zebrafish larvae (5–7 d postfertilization) were exposed to 50 µg/L pyrene, carbazole, or phenanthrene or 20 µg/L anthracene (based on solubility limits) in 1 L of test water without UV radiation for the uptake experiment. Experiments were conducted in 1-L glass crystallizing dishes, and exposure dishes were floated in water tables with a circulating water bath at 28.5 °C (SD = 1.4). Initially, 45 zebrafish larvae were added to each replicate, and larvae were sampled at 0 h, 2 h, 4 h, and 12 h for uptake. Water was renewed every 4 h to maintain constant levels of each PAH in solution. At 12 h, remaining larvae were transferred to 1-L clean water for the elimination experiment and sampled at 1 h and 12 h. Each sampled group consisted of 10 larvae (except for the initial 2-h period, in which only 5 larvae were sampled per treatment) and were blotted dry, wet weighed, frozen in liquid nitrogen, and stored at –80 °C. At each larval sample period, a water sample was also retained for chemical concentration analysis.
Tissue processing
Frozen samples were thawed and reweighed in the same treatment groups as previously. Tissues for each treatment were homogenized using a 15-mL Ten-Broeck tissue homogenizer for 60 s after adding 10 g Na2SO4/g fish (a drying agent). This fine powder was dissolved in 5 mL ACN, and contents were transferred to a glass test tube. The homogenizer was rinsed 3 times with additional ACN, which was added to the test tube. Total volume of ACN used for each sample was kept constant at 10 mL. Each sample was then centrifuged for 10 min at 1000 g at room temperature, and 20 µL of the supernatant was injected in an HPLC vial for quantification.
Body burden calculation
Total PAH (µg PAH) was calculated by PAH concentration in extraction (µg PAH/mL, measured via HPLC using standard curve equation and experimental AUC) multiplied by total volume of extraction fluid (mL). Body burden (µg PAH/g fish) was then calculated by total PAH divided by the weight of the fish (g fish).
Toxicokinetic model
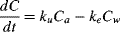 (1)
(1)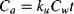 (2)
(2)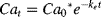 (3)
(3)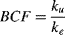 (4)
(4)Data for body burden over time in the uptake and elimination experiments were approximated using least squares linear regression in Excel. The ke was based on Equation 3 and estimated from the slope of the line plotted as the natural log of the body burden over time. The ku was estimated based on a constant infusion model (Equation 2), where the Ca is assumed to be zero and the uptake is based on the concentration in the water. Because the uptake for the first few hours is the most indicative of the uptake flux into the organism, the slope of the line for the initial uptake was divided by the average concentration in the water to derive the ku estimate. Bioconcentration factors were then calculated for each chemical as the ratio of the uptake to the elimination (Equation 4).
Mixtures toxicity testing
A fully factorial experimental design allowed for all possible combinations of the 4 PAHs in single, binary, tertiary, and quaternary mixtures. All mixture treatments were tested in the same experiment. Chemical concentrations for mixture toxicity testing were calculated using ratios of the LC50s: thus, for the single exposures, concentrations tested were aimed to be the LC50s derived for each compound; for the binary mixtures, concentrations were one-half the LC50 concentration for each chemical in the mixture; for tertiary mixtures, concentrations were one-third the LC50 concentration for each chemical in the mixture; and for the quaternary mixture, concentrations were one-fourth the LC50 concentration for each chemical. Concentrations for each mixture are reported as the ∑PAHs in each mixture (Table 1). Vehicle-only controls were also tested in triplicate using the highest vehicle concentration.
| Type | Mixture | Concentration (µg/L) | Mortality (%) | ||
|---|---|---|---|---|---|
| Actual | Expected | Actual | Expected | ||
| Tertiary | ANT–PYR–CAR | 73.9 | 77.9 | 70 | 67.7 |
| Tertiary | ANT–CAR–PHE | 156.3 | 169.3 | 61.3 | 69.7 |
| Tertiary | ANT–PYR–PHE | 80.8 | 94.1 | 66.7 | 63.1 |
| Tertiary | CAR–PYR–PHE | 145.6 | 170.2 | 78.6 | 81.9 |
| Quaternary | ANT–CAR–PYR–PHE | 104.3 | 127.9 | 69.0 | 70.6 |
| Binary | ANT–PYR | 4.0 | 2.3 | 56.0 | 55.0 |
| Binary | PHE–PYR | 125.5 | 140.6 | 70.0 | 76.3 |
| Binary | ANT–PHE | 116.5 | 139.3 | 58.6 | 58.0 |
| Binary | ANT–CAR | 104.7 | 115.1 | 65.5 | 64.9 |
| Binary | PYR–CAR | 106.5 | 116.4 | 80.0 | 83.2 |
| Binary | CAR–PHE | 229.6 | 253.4 | 73.3 | 86.2 |
| Single | ANT | 0.98 | — | 36.7 | — |
| Single | CAR | 229.1 | — | 93.1 | — |
| Single | PYR | 3.7 | — | 73.3 | — |
| Single | PHE | 277.6 | — | 79.3 | — |
- ANT = anthracene; PYR = pyrene: CAR = carbazole; PHE = phenanthrene.
RESULTS
Acute toxicity of individual PAHs
Anthracene, carbazole, phenanthrene, and pyrene were all acutely toxic to larval zebrafish in the presence of UV radiation over the 96-h experimental duration within the concentration ranges tested, and LC50s were derived from concentration-response data. Concentrations of PAHs were found to decrease over time, and time-weighted average concentrations were used to derive LC50s. For example, during anthracene toxicity testing, initial concentrations of 2 µg/L decreased to 0.3 µg/L in 8 h, thus the time-weighted 24-h exposure concentration was 0.75 µg/L. The 96-h UV-enhanced LC50 estimates for anthracene, carbazole, phenanthrene, and pyrene were 1.56 µg/L, 153 µg/L, 271 µg/L, and 2.63 µg/L, respectively (Figure 1). There was no mortality in the vehicle- or water-only controls in the experiments. The dose-response curves were fairly steep for all compounds tested, where slight changes in concentration increased toxicity once a potential threshold was reached.
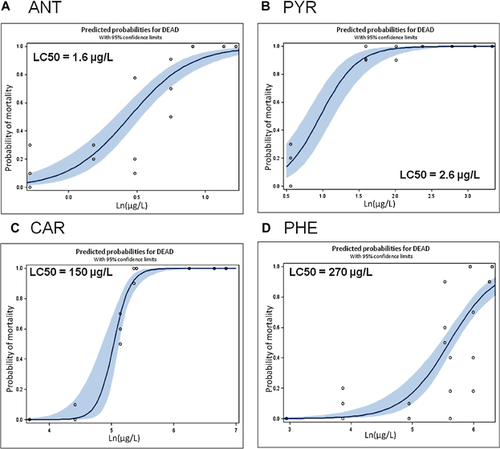
The data indicated that anthracene was the most toxic in the presence of UV radiation followed closely by pyrene. Carbazole and phenanthrene were both almost 2 orders of magnitude less toxic when compared with anthracene and pyrene. However, the no-UV experiment indicated that carbazole and phenanthrene were also moderately phototoxic, because no mortality was seen in the absence of UV radiation at the highest concentration tested for both of these compounds, well above the UV-induced LC50s. There was also no mortality at the solubility limits tested for anthracene in the absence of UV radiation. Because pyrene did exhibit some toxicity in the absence of UV radiation, the derived no-UV LC50 for pyrene was 68 µg/L. Ratios were used to identify relative increases in toxicity due to the presence of UV radiation for each PAH by comparing highest concentration tested with no-UV resulting in no mortality to the UV-induced LC50 values. For anthracene, the presence of UV radiation increased toxicity to at least 18 times the non-UV toxicity. It should be noted that the ratio estimated for anthracene is most likely an underestimate, because non-UV toxicity was not tested above the water-solubility limit. Likewise, the increase in toxicity for pyrene showed a 26-times increase in toxicity in the presence of UV radiation. These ratios were much lower for carbazole and phenanthrene—for carbazole, UV radiation increased the toxicity at least 4.2 times and for phenanthrene UV radiation increased the toxicity at least 2.2 times. The ratios for carbazole and phenanthrene are also likely underestimates of the increase in toxicity, because non-UV toxicity was not tested above the full water-solubility limit (where no toxicity was observed).
A comparison with published literature values of LC50s in fish species for each compound was done to validate the experimental results for UV and no-UV exposures. Overall, it appears that comparisons with literature toxicity values from different fish species and life stages are not sufficient to identify toxicity ranges for the species tested due to sensitivity differences and highly variable reported values. Generally, zebrafish larvae in the current experiments appeared to be more sensitive than other species in the published literature to the toxicants tested, particularly for no-UV exposures. The literature evidence supports the result that anthracene 20 and pyrene 21 are phototoxic. The range of reported literature LC50 values for UV and no-UV exposures to phenanthrene were highly variable, possibly because of differences in fish species' sensitivity, life stage, and exposure regimen, but furthers the idea that literature values may not be ideal for use in comparison of toxicity between species. In this case, the literature was unhelpful in identifying potential for photo-induced toxicity because of the highly contrasting reported values; although most studies did not report any phototoxic effects, some studies did report a weak photo-enhancement 6. This agrees with our data in that phenanthrene exhibited a weak phototoxic effect but was the least phototoxic of all the tested compounds. The database for published toxicity values for carbazole in fish species was scant. Relying on the comparison of the experimental no-UV exposure and the UV-derived LC50, it was determined that carbazole exhibits moderate photo-induced toxicity, as there was no mortality in the no-UV exposure at concentrations tested well above the LC50.
Bioconcentration factors
Bioconcentration factors were approximated for each compound as the ratio of the experimental uptake and elimination coefficients (Table 2). Uptake was lowest in carbazole, but elimination was lowest as well, making the BCF the highest at 3218. Anthracene and pyrene had similar BCFs (2768 and 2615, respectively), but uptake and elimination were much higher for pyrene than for anthracene. Phenanthrene had the lowest BCF of 1215 due to the lowest uptake with relatively high elimination.
| Chemical | ku | ke | BCF | log BCF | RPA |
|---|---|---|---|---|---|
| ANT | 487 | 0.176 | 2770 | 3.44 | 1 |
| CAR | 184 | 0.057 | 3220 | 3.51 | 0.0095 |
| PHE | 306 | 0.252 | 1210 | 3.08 | 0.0058 |
| PYR | 987 | 0.378 | 2620 | 3.42 | 0.674813 |
- a Relative photodynamic activity (RPA) was estimated from the ratio of median lethal concentrations for each compound compared to the most phototoxic compound, ANT.
- ANT = anthracene; PYR = pyrene: CAR = carbazole; PHE = phenanthrene.
A review of BCFs from the published literature was conducted to validate the experimentally approximated values for each compound. The experimentally derived BCFs mostly fell in the middle of reported values: for anthracene (BCF = 2768) the literature values ranged from 675 to 7260 22-24; for phenanthrene (BCF = 1215) the values ranged from 1550 to 8351, and so the experimental value was just below the reported range 25-27; for pyrene (BCF = 2615) the values ranged from 1306 to 4821 22, 26, 28. For carbazole (BCF = 3218), there was only one reported BCF value of 500 in the guppy 22. The experimentally derived value was much greater than the reported value. Because there is so little data for carbazole available in the literature, we were unable to identify a range of BCFs for comparison with our estimated value.
Mixtures toxicity testing
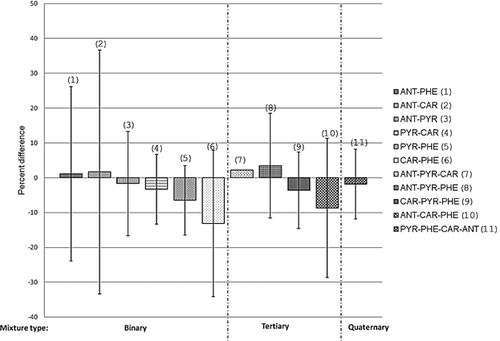
Mortality data for each mixture was predicted based on the mortality data for the single compound in an addition ratio (Table 1), because the initial amount of chemical added to each mixture was a specific fraction of the single PAH LC50. The experimental mortality for the mixtures showed an additive effect, with less than 10% deviation from predicted mortality for all treatment groups except for one (Figure 2). The carbazole–phenanthrene binary mixture slightly deviated from additivity (toward antagonism of effects) at 13% less mortality compared with the additive prediction. However, this difference was not statistically significant. All comparisons between predicted versus actual mortality for mixtures were tested using a one-way analysis of variance (ANOVA), and no significant differences were identified between any of the concentration groups. Our analysis indicates that we obtained overall additive effects for mortality for all treatment groups when compared with single concentration mortality.
Additive model for PAH mixtures toxicity
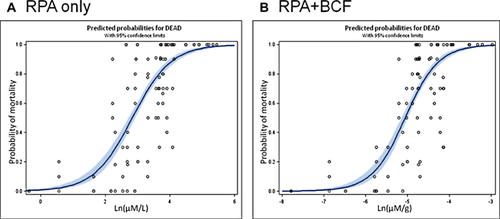
All data from individual LC50 derivation and mixtures toxicity tests were adjusted to compare effects between chemicals in a manner analogous to relative potency factors (RPFs) for phototoxic potential 2, 5. Relative photodynamic activity (RPA) was derived based on the ratio of individual compound LC50s in relation to anthracene, because it exhibited the highest potency, and was used to adjust the concentrations of PAH for differences in potency (Table 2). Experimental BCFs were also used to modify the data to account for chemical concentration in the organism as opposed to concentration in test water. The adjusted data for RPA-only and RPA + BCF were run in the PROC PROBIT model in SAS to identify the best fitting model for all data sets combined (Figure 3). For the RPA + BCF data, the RPA-adjusted data were multiplied by the BCF, which allowed for a comparison of dose-response data between chemicals adjusted for potency and body burden. Figure 3 shows the resulting model estimates for RPA-only and RPA + BCF. The adjustments presented nearly identical lines with similar fit. The logistic regression estimate for RPA-only adjusted data yielded a Wald χ2 value of 216 and a p value of < 0.0001. The logistic regression estimate for RPA + BCF yielded a Wald χ2 value of 226 and a p value of < 0.0001. These estimates indicate that the regressions are nearly identical but that the RPA-only analysis has slightly better fit to the data due to the lower Wald χ2 values.
DISCUSSION
Acute toxicity of individual PAHs
Overall, concentration-response curves were relatively steep once toxic effects began to occur, where small increases in concentrations greatly increased toxicity. This indicated potential for a phototoxicity-related threshold, where these curves were particularly steep for the highly phototoxic PAHs anthracene and pyrene. Because the steepness of the concentration-response curve appeared qualitatively to increase related to phototoxic potential of PAH tested, this could indicate a threshold saturation mechanism involving ROS or some enzymatic saturation. More research into these mechanisms would be useful to further elucidate these relationships.
Physical properties of specific PAHs have been related to toxicity potential through the use of quantitative structure activity relationships (QSARs). Physical characteristics used as predictors of toxicity include number of benzene rings, octanol–water partition coefficient (KOW), solubility in water, chemical stability, and others 29. However, photo-induced toxicity deviates from some of traditional predictive physical properties and shifts to other properties 30, 31. Many studies have identified QSAR predictors for photo-induced toxicity, including molecular size, lipid partitioning coefficients, and other intermolecular forces such as molecular polarizability or Egap (electronic structural property related to the ability for UV absorption) 4, 29, 32, 33. It is widely reported in the literature that a HOMO-LUMO gap in the range of 7.1 ± 0.4 eV is an indicator of potential for photo-induced toxicity 29-31. The HOMO-LUMO gaps for anthracene and pyrene are within this range; however, phenanthrene and carbazole are outside this range. Carbazole and phenanthrene were both identified in the present study as moderately or weakly phototoxic in the presence of UV radiation, with UV radiation increasing carbazole toxicity at least 4.2 times and increasing phenanthrene toxicity at least 2.2 times. Because carbazole is a PAH with a heterocyclic (nitrogen substituted) benzene ring, it is possible that the presence of the 2 valence electrons on the nitrogen may have different excitability characteristics than nonsubstituted PAHs and may be an exception to the HOMO-LUMO rule 30. Phenanthrene was the least phototoxic compound, and the increase in toxicity (at least 2.2 times) was much lower than the highly phototoxic compounds anthracene and pyrene, which increased toxicity at least 18 and 26 times each in the presence of UV radiation. Still, this effect is notable in that photo-induced toxicity could be activated even though the HOMO-LUMO gaps did not exhibit the typical predictive properties. Perhaps identification of a threshold should be designated for photo-enhancement identifying strongly versus weakly photo-enhanced chemicals, such as “over an order of magnitude increase in toxicity” in the range of 7.1 ± 0.4 eV.
The identification of carbazole and phenanthrene as moderately or weakly phototoxic compounds may be a novel finding, but the analysis implies a lack of data for these compounds and particularly for carbazole. Further studies are needed to address this lack of data and also to investigate how photo-modification products may or may not interact with toxicity. For example, photo-modification products including oxygenated PAHs and quinones, among others, showed higher toxicity than the parent compounds when Daphnia magna were exposed to various PAHs 34. The potential role of photo modification was addressed in the present study through the experimental design that allowed for a 12-h uptake period prior to UV exposure in which the organism could accumulate PAHs. Potential photo-modification products from the parent PAHs themselves were not detected and given the concentrations of parent compounds used in the present study, potential toxicity attributed to photo-modified PAHs was unlikely. Identification of photo-modification products for each of the PAHs tested in the present study could be useful in furthering the understanding of photo-induced toxicity.
Bioconcentration factors
Bioconcentration factors can vary depending on species-specific lipid content, because hydrophobic PAHs can accumulate in lipids and variation in lipid content could affect carrying capacity 14. Other factors that can lead to uncertainty and variation in reported BCFs include differences in exposure duration, tissue type tested (whole body vs gills), and organism size, along with water-quality parameters such as dissolved oxygen, turbidity, dissolved organic carbon, and temperature 14. Due to the high variability associated with estimating BCFs, the estimates in the present study may be applicable only to these specific exposure conditions and may not be reliable under environmental exposures where conditions and parameters affecting BCF would be expected to change. Overall, the experimental BCFs were mostly within the range of those reported in the literature for anthracene, pyrene, and phenanthrene, but deviated for carbazole, for which very few data were available.
Additive model for PAH mixtures toxicity
The mixtures toxicity testing in this experiment showed that PAH mixtures exhibited additive toxicity when photo-induced mechanisms were assessed as the AOP. Based on model fit, the RPA-only data were a slight improvement over the RPA + BCF data. Rather than at primarily the gills in young, larval fish, photo-induced toxicity affects the entire surface of the fish 4, and thus total body residue is likely a less important driver of toxicity compared to RPA.
It has been shown previously that PAHs with similar AOPs exhibit mixtures additivity 17-19, 45. Initially, this experiment aimed to identify mixtures interactions that could be representative of photo- and nonphototoxic compounds represented in an environmental mixture. However, all 4 PAHs tested exhibited some measurable photo enhancement, albeit to different severity levels; as such, the interaction mechanism was additive. There was only a slight trend toward antagonism identified for the carbazole–phenanthrene binary mixture, although this was not statistically significant. Trends were investigated to identify possible mechanisms for this slight deviation; it is possible that low photo-induced toxicity for these 2 chemicals may have been driving this effect. However, there were no trends in the data identified for mixtures containing phenanthrene to be either synergistic or antagonistic (Figure 2). Also, the individual exposure for carbazole showed high mortality at 93% (Table 1); however, there were no trends in the data identified for mixtures containing carbazole to be synergistic related to an overprediction of mortality (Figure 2).
The statistical methods employed in other published mixture toxicity studies were highly varied and unsystematic. It is clear that there is a need for standardization for statistically analyzing significant differences in mixtures toxicity testing. In the present study, a mixture of reported methods was applied for which it was assumed that a response within 20% of the predicted mortality meant no difference from additivity, based on the statistical power to detect differences with total number of organisms and replicates; this was validated using one-way ANOVAs. It is possible that large organismal variation in response between replicates could hinder detection of statistically significant differences from predicted and experimental values, and so a more refined and standardized statistical approach could be valuable.
Environmental relevance
Environmental concentrations of most PAHs in heavily polluted areas are below the non-UV LC50s for those individual compounds. However, non-UV LC50 values in the literature are likely orders of magnitude underprotective of photo-induced effects. Polycyclic aromatic hydrocarbons have been detected in groundwater from as low as 0.1 ng/L (in uncontaminated groundwater) to as high as 6100 µg/L (groundwater near an asphalt production plant) 35, 36. Groundwater samples from Washington, USA, contained ∑PAHs up to 870 µg/L and showed that phenanthrene, anthracene, and pyrene co-occurred 36. Levels of carbazole in creosote and other contaminated ground water samples were detected ranging from 0.05 µg/L to 150 µg/L (and measured as high as 4300 µg/L), and co-occurred with pyrene, phenanthrene, and in some cases anthracene 37.
Polycyclic aromatic hydrocarbon concentration combined with UV dose determines the level of phototoxicity; thus, UV penetration into the water column and attenuation of UV is an important factor for phototoxicity in the natural environment. Environment-specific factors influence UV dose, including attenuation of UV radiation, time of day, season of year, characteristics of the water itself (humic content, macrophage population, and so on), and even organism food type 38-40. Ultraviolet light can penetrate surface waters 10 m to 12 m in Lake Michigan (USA) 7, 38 and up to 37 m in Lake Tahoe (USA), depending on water properties and geographical location 41, 42. In Prince William Sound (AK, USA), the potential for photo-enhanced toxicity of PAHs to larval fish was identified based on measured UV radiation 39, 43.
Overall, photo-induced toxicity of PAH in the natural environment should be a consideration in several types of aquatic systems. For example, spawning grounds are usually near-shore, shallow areas that have high UV light exposures and contain a high concentration of susceptible species (transparent organisms such as larvae and embryos). Near-surface pelagic spawners or other species with juveniles or larvae that are buoyant and do not migrate to depth during the daytime hours are susceptible to both surface PAH concentrations and high UV regimes. High-elevation, oligotrophic lakes and streams receive high UV doses, contain oxygen stress–intolerant species (susceptible to oxidative stress), and are often impacted by recreational activities such as boating. Because PAHs can also accumulate in sediments, UV penetration to littoral zone sediments can create potential pathways for phototoxicity for benthic organisms. When taking into account the additive effect of PAH mixtures, there are cases in which environmental exposure to PAHs and UV radiation exceeds toxic values and the potential for photo-induced toxicity in the natural environment exists.
CONCLUSIONS
The original goal of the present study was to use 2 PAHs with non-phototoxic (AHR-mediated) AOPs and 2 PAHs with phototoxic AOPs to investigate how the AOPs interacted in mixtures. Published literature sources were utilized for information on AHR-induction (P450), phototoxicity levels, and HOMO-LUMO gap values for each compound to identify nonphototoxic and phototoxic AOPs. However, after toxicity testing to validate predicted AOPs, all PAHs tested exhibited at least some amount of phototoxicity. As predicted, anthracene and pyrene were highly phototoxic. Carbazole and phenanthrene also exhibited moderate and weak photo-induced toxicity that was not expected; however, the magnitude of the photo enhancement was low as compared with that of pyrene and anthracene. Still, this effect was not predicted based on available literature and the HOMO-LUMO gap values 29, 31. Carbazole and phenanthrene both have a HOMO-LUMO gap outside of the predictive range of 7.1 ± 0.4 eV.
Overall, the phototoxicity of mixtures of the 4 PAHs was additive. A well-fitting toxicity model was generated for predicting toxic effects for mixtures of PAHs after taking into account differences in phototoxic potency between compounds. The inclusion of laboratory-derived BCFs did not significantly improve this model, indicating that across a number of compounds, phototoxic potential (i.e., RPA) is more important than body burden (BCF) for phototoxicity in larval fish. Although there was high variation in mortality response between experimental replicates, the data fit the model well and can be used to predict toxicity in real-world scenarios where PAH compounds and concentrations are varied. Because photo-induced toxicity is the most sensitive mechanism of toxicity for these compounds, future risk assessments should address relative phototoxic potential of all mixture components when predicting toxicity for acute exposure durations. Other environmental factors that can modulate phototoxicity, such as dose of UV radiation, light attenuation in the water column, and differential UV wavelength attenuation, also need to be taken into account on a site-by-site basis 44. Further understanding of how multiple toxicants interact provides a useful tool to predict environmental risk—and refining these estimates based on toxicokinetics and potency can provide realistic predictive models for effects from environmental exposures.
There is currently a need to assess the impact of multiple stressors in aquatic environments, because many systems are heterogeneous and can be impacted from multiple sources. The experiments in the present study identified effects from single and multiple PAHs with the inclusion of UV exposure. Studies have also shown that other compounds such as pesticides and dyes can cause photo-induced toxicity 46-50. The effect of phototoxicity for other aquatic contaminates besides PAHs identifies a potential for adverse effects in aquatic systems well below non-UV exposure guidance. If these effects from dyes and pesticides are also expected to be additive (based on similar AOPs), risk assessment and site remediation should account for all phototoxic contaminants. For example, a simple ∑PAH concentration may not be adequate to address the phototoxic effect. Even calculating ∑photo-toxic PAH based on RPAs may not be sufficient if it does not account for other phototoxic contaminants, such as pesticides in the mixture. Future studies should identify mixture interactions for all environmental contaminates such as dyes, pesticides, PAHs, and others to fully characterize the risk associated with mixtures and multiple stressors. Phototoxic PAH and other phototoxic contaminants in the environment can be adjusted for RPA and UV dose, and then mortality and other effects can be predicted. It is likely that other photo-reactive compounds would be expected to work in an additive fashion and perhaps involve a threshold mechanism. Overall, chemicals that act via the same AOP are expected to have additive effects on environmental receptors, but it is important to include other environmental factors (such as UV) and additional stressors that can alter or affect different AOPs.
Acknowledgment
The authors thank A. Tucker for his help on this project. No conflicts of interest were identified for any of the authors. Funding for this work came in part from the National Oceanic and Atmospheric Administration (NOAA) and from Miami University. The research protocols used were approved by the Institution Animal Care and Use Committee of Miami University.




