Ecotoxicity of benzo[a]pyrene assessed by soil microbial indicators
Abstract
The ecotoxicity of benzo[a]pyrene (BaP) to soil microorganisms was evaluated using the following microbial indicators: soil microbial biomass, respiration, nitrification, and Shannon index. Two soil types, udic ferrosols and aquic cambisols, were amended with 0 mg/kg, 1 mg/kg, 10 mg/kg, 100 mg/kg, 500 mg/kg, or 1000 mg/kg BaP; incubated at 25 °C; and tested on days 28, 60, and 180. The Shannon index was extremely insensitive to BaP. Microbial biomass and respiration could not be classified as sensitive indicators because of their relatively high 10% effect concentration (EC10) values. Nitrification was the most sensitive indicator in both soils and could be the preferred microbial indicator for testing the ecotoxicity of BaP. Higher toxicity of BaP was exhibited in udic ferrosols than in aquic cambisols, and the ecotoxicity of BaP decreased with incubation time. Extending the 28-d incubation time, which is suggested in the International Organization for Standardization and Organisation for Economic Co-operation and Development guidelines, to 60 d was recommended for future microbial toxicity tests of BaP. On day 28, the EC10 values for microbial biomass, respiration, and nitrification were 71 mg/kg, 43 mg/kg, and 3.4 mg/kg in aquic cambisols and 51 mg/kg, 22 mg/kg, and 1.3 mg/kg in udic ferrosols, respectively. On day 60, these values were 106 mg/kg, 59 mg/kg, and 19 mg/kg in aquic cambisols and 77 mg/kg, 40 mg/kg, and 6.9 mg/kg in udic ferrosols. These values could be used in combination to derive ecotoxicological soil screening levels of BaP. Environ Toxicol Chem 2014; 33:1930–1936. © 2014 SETAC
INTRODUCTION
Benzo[a]pyrene (BaP) is 1 of 16 polycyclic aromatic hydrocarbons (PAHs) on the priority pollutant list of the US Environmental Protection Agency (USEPA). It is a well-known carcinogenic, teratogenic, and mutagenic organic pollutant that accumulates in soils as a result of anthropogenic activities such as industrial spills, sewage irrigation, and atmospheric deposition 1. Because of its high octanol–water partitioning coefficient (log KOW; 6.13) and high molecular weight (252.32), it is resistant to microbial degradation and can persist in soil for decades 2. The occurrence and accumulation of BaP in agricultural soils has prompted concerns about ecosystem health and the maintenance of properly functioning soil ecosystems. Therefore, the derivation of ecotoxicological soil screening levels that can be used to determine safe levels of BaP in soils is urgently needed.
Terrestrial ecotoxicity data are the basis for establishing soil BaP ecotoxicological soil screening levels. The scientific literature and ecotoxicity databases are the main sources of terrestrial ecotoxicity data. Published data are difficult to find, however, and data quality widely vary. For example, Kapustka 3 reviewed more than 325 articles on PAHs, and only 14 met the minimum criteria for useful toxicity data. Two internationally renowned ecotoxicity databases are the USEPA ECOTOX and the National Institute for Public Health and the Environment (RIVM) e-toxBase, but most of the data in these databases pertain to aquatic ecotoxicity. Consequently, valid and useful terrestrial ecotoxicity data are very scarce. The lack of terrestrial ecotoxicity data is the major limitation to the development of ecotoxicological soil screening levels for BaP 4. The key to solving this problem is to conduct systematic terrestrial ecotoxicity tests.
In terrestrial ecotoxicity assessments, the test subjects are typically microorganisms, plants, and invertebrates. Published terrestrial ecotoxicity data for BaP have been reviewed by Jensen and Sverdrup 4 and Sverdrup et al. 5, and microbial ecotoxicity data are particularly scarce. Soil microbes are crucial components of the soil ecosystem and play significant roles in nutrient cycling and transformation, organic matter decomposition, and organic pollutant degradation 6. They can respond promptly to environmental change 7. Changes in the activity and diversity of microbial communities have often been proposed as easy and sensitive indicators to study the effects of environmental pollutant on soil ecology 8. Therefore, it is necessary and feasible to prioritize microbial ecotoxicity tests.
Two key components of microbial toxicity tests, concentration gradient and incubation time, directly affect the quality of the data. However, the different studies of BaP microbial ecotoxicity employ different gradients and incubation times. For example, the number of BaP concentration gradients is set as 2 9 or 3 10, and the test time is set as 28 d 5 or 100 d 9. An inadequate concentration gradient will reduce the validity and utility of the results, and the discrepancy in incubation time makes it difficult to compare the ecotoxicity of BaP in different soils. Therefore, it is very important to standardize microbial ecotoxicity testing methodology, especially the test concentration gradient and incubation time. The International Organization for Standardization (ISO) and the Organisation for Economic Co-operation and Development (OECD) have standardized several microbial ecotoxicity tests (Table 1). A geometric series of at least 5 concentrations and a 28-d incubation time are specified in the ISO and OECD guidelines (ISO 14238 11, OECD 216 12, and OECD 217 13). Conducting BaP ecotoxicity tests according to the detailed ISO and OECD standards is a very important way to ensure the quality of microbial ecotoxicity data.
| Guideline | Year | Title |
|---|---|---|
| OECD 216 | 2000 | Soil microorganisms: Nitrogen transformation test |
| OECD 217 | 2000 | Soil microorganisms: Carbon transformation test |
| ISO 14238 | 1997 | Biological processes: Determination of nitrogen processes |
| ISO 14240 | 1997 | Determination of soil microbial biomass |
| ISO 15685 | 2012 | Determination of potential nitrification |
| ISO 16072 | 2002 | Laboratory method for determination of microbial soil respiration |
| ISO 23753 | 2005 | Determination of dehydrogenase activity in soil |
| ISO/TS 29843 | 2011 | Determination of soil microbial diversity |
- OECD = Organisation for Economic Cooperation and Development; ISO = International Organization for Standardization; TS = technical specification.
Microbial biomass, respiration, nitrification, and microbial diversity are the major microbial indicators in microbial ecotoxicity tests 14. Some microbial indicators, such as microbial diversity 9, respiration 15, and nitrification 5, have been used in BaP ecotoxicity tests. To the best of our knowledge, however, systematic studies of BaP ecotoxicity inferred from all 4 types of microbial indicators and comparisons of the sensitivity of these indicators have not been presented before. Therefore, very little is known about which microbial indicators are more or less sensitive to BaP in a given soil type and whether relative sensitivities differ among soil types.
Following microbial ecotoxicity tests, it is crucial to derive microbial ecotoxicity guidelines from the analysis of the experimental data. To date, nearly all published ecotoxicity data for BaP have been given in the form of the no-observed-effect concentration (NOEC) or the lowest-observed-effect concentration (LOEC) 5. For this reason, the NOEC is used to develop ecotoxicological soil screening levels in most countries, such as European Union member states (including The Netherlands) and Canada. The NOEC has many disadvantages, however, which have been described in Hoekstra and van Ewijk 16 and Chapman et al. 17. The main disadvantages of the NOEC are summarized by Pires et al. 18 as follows: it ignores the dose–response trend, it tends to increase as the precision of the experiment decreases, it must be 1 of the concentrations set in the experiment and is not always obtainable, and it depends on the method of the significance test. The effect concentration causing x% inhibition (ECx) in a dose–response model, such as the logistic model, which addresses many of the objections to the NOEC, is to be preferred over a NOEC 19. The ECx is statistics-based and can be a direct expression of the dose–response relationship between the pollutant and the effect of indicator. Some countries and organizations, such as the USEPA and Oak Ridge National Laboratory, have recently turned to effect concentration causing 10% inhibition (EC10) to develop ecotoxicological soil screening levels. It would therefore be valuable to derive EC10 values as the basis for determining future ecotoxicological soil screening levels for BaP.
Two representative soil types in China with very different chemical and microbial properties, udic ferrosols and aquic cambisols, were chosen as the test soils. The purpose of the present study was to determine the toxicity of BaP to soil microbes based on the detailed ISO and OECD standards; calculate EC10 values of microbial indicators, including soil microbial biomass, respiration, nitrification, and microbial diversity; and compare the sensitivities of microbial indicators to exogenous BaP in both soils.
MATERIALS AND METHODS
Experimental soil
Samples of 2 contrasting soil types, aquic cambisols and udic ferrosols, were collected from the top 20 cm of agricultural soils in Fengqiu, Henan Province (35°00′N, 114°24′E), and Yingtan, Jiangxi Province (28°12′N, 116°56′E), China. Gravel and plant root residues in the sampled soils were discarded. Field moist samples were sieved through a 2-mm mesh and stored at 4 °C. The soils were stored for no more than 1 wk. A portion of the soil samples was air-dried for chemical analyses (Table 2), following the methods described in Lu 20. Aquic cambisols is alkaline (pH 8.22) and has a relatively high content of organic matter (12.97 g/kg), whereas udic ferrosols is acidic (pH 5.16) and has a low content of organic matter (6.77 g/kg).
| Soil | pH (1:2.5 w/w) | Organic matter (g/kg) | BaP (mg/kg) | Microbial biomass (mg/kg) | Respiration (mg/kg/h) | Nitrification (mg NO3−/kg/d) | Shannon index |
|---|---|---|---|---|---|---|---|
| Aquic cambisols | 8.22 | 12.97 | —a | 1535 | 2.64 | 12.66 | 3.30 |
| Udic ferrosols | 5.16 | 6.77 | — | 358 | 0.36 | 6.17 | 3.18 |
- a The data were below the detection limit.
- BaP = benzo[a]pyrene
Soil spiking and incubation
Soils were preincubated at 25 °C for 7 d at 60% of their maximum water holding capacity. Afterward, each soil was divided into 18 aliquots of 500 g (6 BaP levels × 3 replicates). The soils were spiked with 0 mg/kg, 1 mg/kg, 10 mg/kg, 100 mg/kg, 500 mg/kg, or 1000 mg/kg BaP (>97% purity; Adamas-beta) according to the procedure proposed by Sverdrup et al. 5. The spiked soils were placed in 18 glass containers and incubated at 25 °C under aerobic conditions. Soil moisture content was maintained at 60% water holding capacity throughout the incubation by weighing and correcting for any weight loss by adding distilled water.
Microbial indicator analysis
Soil microbial biomass, respiration, nitrification, and microbial diversity were tested on days 28, 60, and 180. Soil microbial biomass was determined by substrate-induced respiration according to ISO 14240-1:1997 21. Basal respiration was determined as the rate of CO2 emission from the soil according to ISO 16072:2002 22. The CO2 concentration was measured by gas chromatography (GC; Agilent 7890A). Soil nitrification was determined using the ISO 14238:1997 standard 11. The nitrogen concentration was measured by a continuous flow analyzer (Skalar San++ System). The Shannon index was determined from phospholipid fatty acid analysis according to ISO TS 29843-2:2011 23. Phospholipid fatty acids were identified by GC (Agilent GC6850-MID).
BaP analysis
Soil samples were collected immediately after soil spiking. The BaP in the samples was extracted using Soxhlet extraction. Two grams of freeze-dried soil and 2 g of dried sodium sulfate were wrapped in filter paper and placed in a Soxhlet extractor. The extractor was then fitted to a 100-mL round-bottom flask containing 70 mL dichloromethane, and the extraction was performed for 24 h at 54 °C. All extracts in the round-bottom flasks were dried by rotary evaporation. The residues were dissolved in 2 mL of cyclohexane. Then, 0.5 mL of the solution was transferred, purified with a silica gel column, and washed with a mixture of hexane and dichloromethane (1:1). The first 1 mL of eluate was discarded. The second 2 mL of eluate was collected, dried by sparging with N2, and then redissolved in 2 mL of hexane for GC-mass spectrometry (MS) determination. Identification and quantification of BaP were carried out with a GC-MS system consisting of an Agilent 7890A GC and an Agilent 5975C MS using temperature-programmable splitless injection and a 30-m DB-5 silica capillary column. The instrumental detection limit of the GC-MS for BaP was 0.86 µg kg−1. Method blanks (solvent) and spiked blanks (soil spiked with standards of EPA610 PAH mixture, LA 96245; Supelco) were also extracted and analyzed. The average recovery and the relative standard deviation for BaP were 88% and 0.11%, respectively. The results of the blanks extracted under the same conditions were below the detection limits, and sample results without recovery ratio correction are presented.
Statistical analysis
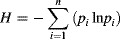
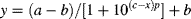
RESULTS
Soil BaP
Chemical analysis showed that the measured initial concentrations were close to the nominal BaP concentrations (Table 3). The measured concentration series could meet the requirements for standardized soil microbial ecotoxicity tests. To study the time effect of BaP on soil microbial indicators, the NOEC and EC10 values on day 28, day 60, and day 180 were all based on the measured initial concentrations.
| Nominal concentration (mg/kg) | Measured concentration (mg/kg) | |
|---|---|---|
| Aquic cambisols | Udic ferrosols | |
| 0 | <0.003 | <0.003 |
| 1 | 0.83 | 0.86 |
| 10 | 8.3 | 8.2 |
| 100 | 84 | 85 |
| 500 | 469 | 463 |
| 1000 | 962 | 975 |
Dose–response curves
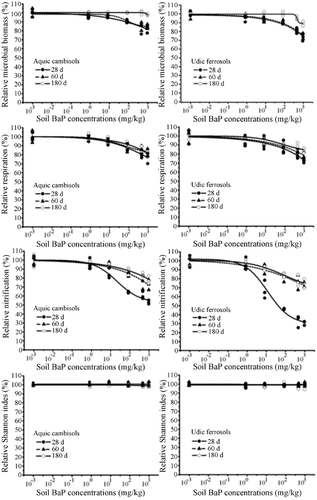
For most soil microbial indicators, the data fit the logistic dose–response curve reasonably well (Figure 1). Exceptions were the microbial biomass on day 180 in aquic cambisols and the Shannon index, for which dose–response curves could not be fitted well when the toxic effects of BaP were extremely low. The negative effects of BaP on most soil microbial indicators increased with increasing BaP concentration. For the Shannon diversity index, however, the dose–response relationship was nearly uniform, and no significant effect (ANOVA, Duncan's, p > 0.05) was observed even at the highest dose (Table 4). In the test concentration range, the maximum inhibitory rate was lower than 50% for all microbial indicators in aquic cambisols, whereas the maximum inhibitory rate for nitrification in udic ferrosols on day 28 was greater than 70%. Figure 1 shows that the toxic effects of BaP decreased with time in different degrees. On day 180 of incubation, soil microbial biomass had almost recovered to the level of the control soil. For nitrification, the toxic effect was reduced by day 60, whereas respiration exhibited only limited recovery by day 60 and 180.
| Soil | BaP (mg/kg) | Shannon index | ||
|---|---|---|---|---|
| 28-d incubation | 60-d incubation | 180-d incubation | ||
| Aquic cambisols | 0 | 3.32 ± 0.04 | 3.29 ± 0.02 | 3.31 ± 0.06 |
| 1 | 3.30 ± 0.08 | 3.30 ± 0.03 | 3.34 ± 0.06 | |
| 10 | 3.32 ± 0.04 | 3.34 ± 0.06 | 3.36 ± 0.05 | |
| 100 | 3.31 ± 0.04 | 3.30 ± 0.07 | 3.31 ± 0.08 | |
| 500 | 3.30 ± 0.06 | 3.30 ± 0.05 | 3.32 ± 0.06 | |
| 1000 | 3.29 ± 0.06 | 3.31 ± 0.09 | 3.31 ± 0.07 | |
| Udic ferrosols | 0 | 3.19 ± 0.05 | 3.20 ± 0.07 | 3.17 ± 0.05 |
| 1 | 3.18 ± 0.03 | 3.17 ± 0.04 | 3.12 ± 0.08 | |
| 10 | 3.12 ± 0.07 | 3.16 ± 0.04 | 3.17 ± 0.03 | |
| 100 | 3.19 ± 0.09 | 3.17 ± 0.02 | 3.13 ± 0.03 | |
| 500 | 3.10 ± 0.08 | 3.16 ± 0.06 | 3.16 ± 0.05 | |
| 1000 | 3.11 ± 0.10 | 3.18 ± 0.08 | 3.16 ± 0.04 | |
Ecotoxicological data for BaP
The ecotoxicological results for BaP varied greatly between the 2 soils (Table 5). On day 28, EC10 values ranged from 3.4 mg/kg to 71 mg/kg for aquic cambisols and from 1.3 mg/kg to 51 mg/kg for udic ferrosols. On day 60, EC10 values ranged from 19 mg/kg to 106 mg/kg for aquic cambisols and from 6.9 mg/kg to 77 mg/kg for udic ferrosols. By the end of the 6-mo incubation, EC10 values varied from 39 mg/kg to >1000 mg/kg for aquic cambisols and from 18 mg/kg to 642 mg/kg for udic ferrosols. It is worth noting that the EC10 value of aquic cambisols was much higher than that of udic ferrosols for the same indicator and incubation time, suggesting that BaP might have a more potent toxic effect on microbes in udic ferrosols. In the case of the same indicator and soil, the EC10 value increased with the incubation time, suggesting that the negative effect of BaP on soil microbial indicators decreased with incubation time. Over the whole incubation period, the EC10 value increased gradually, suggesting that the toxic effect of BaP reached the maximum level during the first month of incubation. In addition, the EC10 value could reflect the sensitivity of the indicators; a smaller EC10 value is associated with a higher sensitivity. Therefore, the most sensitive indicator was nitrification, followed by respiration and then microbial biomass, in both aquic cambisols and udic ferrosols. At the same time, the Shannon diversity index based on phospholipid fatty acid analysis could be considered the least sensitive indicator.
| Soil type | Time (d) | EC10 (r2adj)b | NOEC | ||||
|---|---|---|---|---|---|---|---|
| Biomass | Respiration | Nitrification | Biomass | Respiration | Nitrification | ||
| Aquic cambisols | 28 | 71 (0.77) | 43 (0.83) | 3.4 (0.86) | 8.3 | 8.3 | 0.83 |
| 60 | 106 (0.79) | 59 (0.69) | 19 (0.81) | 8.3 | 8.3 | 8.3 | |
| 180 | >1000 | 141 (0.79) | 39 (0.76) | 469 | 8.3 | 8.3 | |
| Udic ferrosols | 28 | 51 (0.89) | 22 (0.70) | 1.3 (0.92) | 0.86 | 8.2 | 0.86 |
| 60 | 77 (0.89) | 40 (0.75) | 6.9 (0.76) | 0.86 | 8.2 | 8.2 | |
| 180 | 642 (0.76) | 96 (0.74) | 18 (0.75) | 85 | 8.2 | 8.2 | |
- a No value of soil microbial diversity is listed because the highest dose added could not cause a significant (p < 0.05) effect.
- b Percentage of variance accounted for by the dose–response model.
- EC10 = 10% effect concentration; r2adj = adjusted r2 values; NOEC = no-observed-effect concentration.
The NOEC values were also obtained for the microbial indicators (Table 5). However, no NOEC value of soil microbial diversity was determined because the highest added dose did not cause a significant (p < 0.05) effect. The NOEC values did not reflect the dose–response relationship between BaP and the microbial indicators when combined with the dose–response curves. Additionally, the relative sensitivity of the soil microbial indicators and the effect of the ecotoxicity of BaP over time could not be determined from the NOEC values.
DISCUSSION
Sensitivity of microbial indicators to BaP
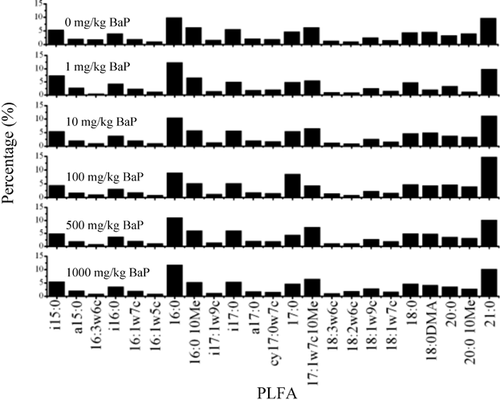
The Shannon index based on phospholipid fatty acid data seemed to be extremely insensitive to exogenous BaP in both udic ferrosols and aquic cambisols when compared with microbial biomass, nitrification, and respiration. We did not find any significant changes in the Shannon index using phospholipid fatty acid analysis. However, the quantities and types of phospholipid fatty acids differed greatly between the BaP-treated soils and control soils. The phospholipid fatty acid profiles in udic ferrosols of day 28 are shown in Figure 2 as an example. Although there were no changes in the Shannon index, BaP actually greatly influenced soil microbial structural diversity. Our finding was consistent with a previous report that the Shannon index calculated from phospholipid fatty acid data did not reflect changes in soil microbial community structure 24. A possible reason is that the number of types of phospholipid fatty acid is less than the number of microbial species. For example, a soil sample contains hundreds of different fungal species 25, but there are only several major types of fungal phospholipid fatty acids. Therefore, information on microbial diversity is partly lost during phospholipid fatty acid analysis. Furthermore, calculation of the Shannon index uses only a small part of the information in the phospholipid fatty acid profile 26 and exacerbates the loss of information in microbial diversity. Therefore, changes in community structure do not necessarily change the Shannon diversity index based on phospholipid fatty acid analysis.
At relatively high EC10 values, soil microbial biomass, as measured by substrate-induced respiration, and respiration could not be classified as sensitive indicators in the 2 tested soils despite being more sensitive to exogenous BaP than was the Shannon diversity index. The low sensitivity of microbial biomass and respiration to exogenous BaP was also detected in 3 other soil types by Hund-Rinke and Simon 27. Therefore, the utility of microbial biomass and respiration as sensitive indicators to exogenous BaP is questionable.
Nitrification was very sensitive to exogenous BaP in both soils, especially on incubation day 28. Nitrification also has been found to be highly sensitive in other PAH ecotoxicity tests. For instance, Sverdrup et al. 28 showed that for 8 polycyclic aromatic compounds, nitrification is the most sensitive indicator. Nitrification is also recommended as a sensitive indicator for soil assessments 29. Another study by Sverdrup et al. 5 demonstrated the opposite result, however, finding that nitrification was particularly insensitive to BaP. It is worth mentioning that the soil used in Sverdrup et al. 5 was pretreated at a high temperature and inoculated with a new microbial community in the form of a soil supernatant. In contrast, the soil used in the present study was freshly collected farmland soil. It is likely that the disturbance of soil microbial physiological and biochemical activities would affect the sensitivity of nitrification. Soil pH is an excellent indicator of soil nitrification capability 30. Nitrification is sensitive to exogenous BaP whether in aquic cambisols (pH 8.22) with high nitrification rates or in udic ferrosols (pH 5.16) with low nitrification rates. Soil nitrification appeared to be an excellent indicator of exogenous BaP, and it had potential for use in other soils with different pH values.
The sensitivity of the 4 microbial tests to exogenous BaP was nitrification > respiration > microbial biomass > Shannon index. This might be the result of these methods detecting different portions of the microbial populations 27. The Shannon diversity index based on phospholipid fatty acid data includes the total soil microbial community, whereas respiration and microbial biomass sample heterotrophic aerobic and facultative anaerobic microorganisms. Nitrification only reflects the activities of the nitrifiers, a smaller and more homogenous population, thus yielding a more pronounced response to exogenous BaP.
Factors influencing the ecotoxicity of BaP
Incubation time has a marked influence on the ecotoxicity of exogenous BaP. We noted that the EC10 values of the microbial indicators increase with incubation time, indicating that ecotoxicity of BaP decreases with time greater than 180 d. When PAHs enter soil, they undergo a number of transport and loss processes with time, such as aging 31 and biological degradation 9. These processes may reduce the ecotoxicity of PAHs. As a high–molecular weight PAH, however, BaP is degraded only with difficulty or not at all because of its low water solubility and high resonance energy 32, 33. Hence, the alleviation of ecotoxicity of BaP during the incubation time may be partly a result of the aging effect. In addition, the soil microbial community has the ability to respond to environmental disturbances 34. The adaptability of the soil microbial community may be another reason for the decrease in ecotoxicity of BaP during the incubation time. Thus, determining a proper incubation time is critical for studying the ecotoxicity of BaP.
In the OECD and ISO guidelines for non-agrochemicals, the test is terminated after 28 d of incubation and the data obtained on day 28 are used to determine the ECx value for the chemical. In the present study, the EC10 values on day 28 were the smallest and therefore most conservative when used to derive the BaP ecotoxicological soil screening levels. Though conservative data help protect the environment under a broad range of possible conditions, they ignore the emergence of the aging effect of BaP and the adaptation of soil microbes with time. Therefore, extending the incubation time to 60 d and using the EC10 values of both day 28 and day 60 in the derivation of BaP ecotoxicological soil screening levels can overcome the above disadvantages.
Among soil properties, organic matter content is closely related to sorption capacity 35. The sorption of organic pollutants onto soil organic matter is one of the most important factors affecting pollutant ecotoxicity 36. Such adsorbed organic pollutants are suggested to be nonbioavailable, thus reducing their toxic effects on soil microorganisms 37. The present study shows that BaP resulted in greater toxicity in udic ferrosols than in aquic cambisols, indicating that organic matter content might be a factor affecting the ecotoxicity of BaP; the organic matter content of udic ferrosols (6.77 g kg−1) is much lower than that of aquic cambisols (12.97 g kg−1). Moreover, the soil ecosystem will be more resistant to disturbance when there is higher biodiversity 34. The microbial biomass, respiration, nitrification, and Shannon diversity index of udic ferrosols were much lower than those of aquic cambisols (Table 2), indicating that the microbial community in aquic cambisols had higher activity and richer diversity. Therefore, the lower toxicity of BaP in aquic cambisols may also be partly a result of its richer microbial diversity and higher microbial activity.
Comparison of EC10 and NOEC values
The EC10 values could replace the dose–response curve as a measure of the relationship between BaP and the microbial indicators. Additionally, the relative sensitivity of the microbial indicators, the difference in ecotoxicity of BaP in different soils, and the time effect of the ecotoxicity of BaP could easily be indicated by the EC10 values. In contrast, there appeared to be no pattern to the changing NOEC values, and they also did not reflect the dose–response relationship between BaP and the microbial indicators. Therefore, the results of the present study reconfirmed that the use of NOEC values to develop ecotoxicological soil screening levels is problematic 16, and it is valid to use EC10 values to derive ecotoxicological soil screening levels for BaP.
CONCLUSIONS
The present study showed that BaP had detrimental effects on microbial biomass, respiration, and nitrification in udic ferrosols and aquic cambisols under our experimental conditions. In contrast, the Shannon diversity index showed no significant changes in either soil, even at 1000 mg/kg BaP. Microbial biomass, respiration, and nitrification were relatively sensitive to BaP, whereas microbial biomass and respiration were not classified as sensitive indicators because of their relatively high EC10 values. Nitrification was the most sensitive microbial indicator in both tested soils and the preferred microbial indicator for ecotoxicity tests of BaP. Benzo[a]pyrene was more toxic in udic ferrosols than in aquic cambisols, and the maximum toxicity level of BaP was detected on day 28 in both soils. Extending the incubation time to 60 d is recommended for future microbial ecotoxicity tests of BaP, and using the EC10 values of days 28 and 60 in combination will improve the derivation of BaP ecotoxicological soil screening levels.
Acknowledgment
The present research was supported by the National Environmental Protection Public Welfare Scientific Research Project (201009032) and the GanPo 555 Talents Program of Jiangxi Province, China.




