Cardiac structural and molecular alterations in rodent models of temporal lobe epilepsy
Abstract
Objective
Cardiac structural and molecular changes are prevalent in people with chronic epilepsy, possibly contributing to an increased risk of premature mortality. However, understanding of the underlying pathophysiological mechanisms is limited. Here, we investigated the subacute and chronic changes in cardiac structure and ion channel/exchanger expression in different rodent models of temporal lobe epilepsy (TLE).
Methods
Two models of TLE were used: the kainic acid-induced post-status epilepticus (KASE) model in Wistar rats and the electrical self-sustained status epilepticus (SSSE) model in C57BL/6J mice. Heart tissue was collected at subacute (7 days post-SE) and chronic (12–16 weeks post-SE) timepoints from both models. Histological analysis for cardiac fibrosis and qPCR of ion channel/exchanger mRNA expression was performed.
Results
Increased cardiac fibrosis was found in the KASE rats at the subacute (p = 0.016) and chronic (p = 0.003) timepoints compared with sham rats. In chronically epileptic KASE rats, mRNA expression analyses showed that NaV1.5 and NCX1 were reduced in the septum (p = 0.026 and p = 0.020, respectively) compared with shams. In SSSE mice, NaV1.5 was decreased in the right atrium (p = 0.039), and CaV3.2 and NCX1 were increased in the left ventricle subacutely (p = 0.033 and p = 0.003, respectively), and NaV1.5 was increased in the septum at the chronic timepoint (p = 0.008), compared with the non-epileptic sham group.
Significance
Cardiac alterations at structural and molecular levels were found in both experimental rodent epilepsy models, subacutely post-SE and during the chronically epileptic timepoint. The presence of similar cardiac changes across the models, despite being different species and having different modes of epilepsy indication, suggests that these changes are a direct or indirect result of the seizures.
Plain Language Summary
Epilepsy may lead to heart problems, which could raise the risk of early death, but the exact causes are unclear. This study examined heart changes in two rodent models of epilepsy. In rats, heart scarring and stiffness (fibrosis) increased both shortly after seizures and during chronic epilepsy, and the ability to produce key heart proteins was altered. In mice, similar changes in heart proteins appeared in different heart areas. These findings suggest seizures can directly or indirectly cause harmful heart changes. Understanding these effects might help improve care for people with epilepsy and reduce related heart risks.
Key points
- Cardiac alterations at structural and molecular levels were found in two epilepsy models with different mechanisms of induction.
- Increased cardiac fibrosis was found at both subacute and chronic epileptic timepoints in KASE rats, but not in SSSE mice.
- Cardiac fibrosis is positively correlated with SE severity subacutely in KASE rats.
- Expression changes of NaV1.5, CaV3.2, and NCX1 in the heart were present both subacutely and chronically in the two models.
1 INTRODUCTION
Compared with the general population, people with epilepsy (PWE) are at a 20-fold higher risk of experiencing sudden unexpected death.1 Sudden death in PWE may be attributed to epilepsy-induced centrally mediated neurovegetative shutdown and cardiorespiratory arrest during or following epileptic seizures.2 The presence of cardiovascular comorbidities or arrhythmias is also believed to be a contributing factor to the elevated mortality observed in PWE.3 In a recent retrospective cross-sectional study, PWE reported a poorer diet, less physical activity, and increased frequency of cardiovascular diseases, such as coronary heart disease, myocardial infarction, and stroke, compared with individuals without epilepsy.4
Studies have reported an increased risk of structural cardiac abnormalities in patients with chronic epilepsy.5, 6 Examples of severe cardiac fibrosis in post-mortem assessments of PWE have been reported,7, 8 though it is still uncertain how prevalent this pathological finding is in people with chronic epilepsy.9 Studies in animal models have reported evidence of cardiac fibrosis,10 hypertrophy,11 and echocardiography changes12 related to epilepsy. Thus, the concept of the ‘epileptic heart’ has emerged to describe the chronic damage to the heart due to catecholamine toxicity, and hypoxemia during recurrent seizures, leading to electrical and mechanical myocardial dysfunction.13
There is growing evidence of altered ion channel expression in animal models with chronic epilepsy leading to cardiac arrhythmias and sudden death.14 The alterations in ion channels could result from inherited vulnerability to both epilepsy and cardiac dysfunction or from acquired cardiac channelopathies in epilepsy.15 Specifically, seizures have been related to gene and protein expression changes in voltage-gated potassium channels, voltage-gated sodium channels, T-type calcium channels, hyperpolarization-activated cyclic nucleotide-gated channels, and cardiac sodium-calcium exchanges.15 These ion channels play critical roles in physiological and pathological functions, such as signal transduction and pace-making in cardiomyocytes, which further influence electrophysiology and cardiac function.16 There is evidence from short- and long-term cardiac monitoring studies of an increase in clinically significant cardiac arrhythmia in people with chronic epilepsy.17-19
Despite the extensive literature on descriptive cardiac comorbidities in epilepsy, relatively few studies have explored the progressive structural and molecular cardiac pathology during the development of acquired temporal lobe epilepsy (TLE) and the consistency between models and different mechanisms of epilepsy induction. In this study, we examined the time course of structural cardiac abnormalities and changes in ion channel and ion exchanger expression during the subacute timepoint post-SE and in the chronic epilepsy period using two complementary rodent models of TLE, the kainic acid-induced post-status epilepticus (KASE) rat model and the self-sustained status epilepticus (SSSE) mouse model.
2 METHODS
2.1 Animals
A total of 47 male Wistar rats and 34 male C57BL/6J mice were used for the study. The animals were individually housed on a 12:12 light/dark cycle with food and water ad libitum under a temperature (22 ± 2°C) and humidity (55 ± 10%) controlled environment. All the procedures of this work were approved by the Alfred Medical Research & Education Precinct Animal Ethics Committee (E/2058/2020/M, E/1853/2018/M and E/1873/2018/M), with adherence to the Australia Code of Practice for the care and use of animals for scientific purposes.
2.2 Study timeline
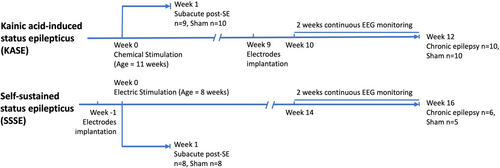
In both models, we evaluated the cardiac abnormalities acutely post-SE (subacute, 1-week post-SE) and at chronically epileptic timepoints (chronic, 12 weeks post-SE for rats and 16 weeks post-SE for mice). Figure 1 summarizes the experimental paradigm for the study.
2.3 Generation of kainic acid-induced status epilepticus (KASE) rats
The post-SE model of TLE was generated using a repeated low dose of kainic acid (KA) protocol.20 Eleven-week-old Wistar rats (n = 47) were randomly assigned to KASE or sham controls. Animals in the KASE group (n = 27) received 5 mg/kg i.p. KA (Enzo Life Science, USA). If no self-sustained seizure activity was observed with at least five class 5 seizures of Racine, another i.p. dose of 5 or 2.5 mg/kg was administered every 45 minutes up to a maximum of 30 mg/kg Animals were monitored for behavioral seizures based on the Racine scale.21 SE was allowed to persist in animals for 4 h, at which time the rats received an injection of diazepam (5 mg/kg i.p.; Mayne Pharma, Adelaide, Australia) to terminate the SE. The KA dose and each animal's time spent in tonic–clonic seizures (min) were documented. Eight animals died during SE. Shams (n = 20) were handled similarly but received saline injections instead of KA; thus, they did not develop epilepsy.
2.4 Electrode implantation in KASE rats
Electroencephalographic (EEG) electrodes were only implanted in chronically epileptic animals and shams (n = 20). Animals were anesthetized with isoflurane (5% induction, 1–2% for maintenance, Ceva isoflurane, Piramal Enterprises Limited, India) with pre- and post-surgical analgesia to alleviate pain (buprenorphine 0.05 mg/kg, s.c, Tasmanian Alkaloids, Pty Ltd., Australia). EEG surgery was performed to implant six EEG electrodes at 9 weeks post-SE, as previously described.22 Four burr holes were drilled through the skull without penetrating the dura in the frontoparietal region on each side of the skull (AP: ±1.7; ML: −2.5) and the temporal region anterior to lambda on each side (AP: ±5.6; ML: left 2.5). Four epidural stainless-steel screw recording electrodes (EM12/20/SPC, Plastics One Inc., USA) were screwed into the burr holes. In addition, ground and reference electrodes were implanted on each side of the parietal bone above the cerebellum.22 Electrodes were plugged into a multichannel pedestal fixed onto the skull using acrylic dental cement (Vertex Dental, The Netherlands).
2.5 Generation of self-sustaining status epilepticus (SSSE) mice
Seven-week-old C57BL/6J mice (n = 34) were surgically implanted with three extradural screw electrodes (two served as ground/reference and one over contralateral parietal cortex as an active electrode) and one bipolar stimulating electrode (E363/3-2TW/SPC, PlasticOne, USA) implanted into the right ventral hippocampus (anteroposterior: −3.00; mediolateral: −3.00; and dorsoventral: 2.80 from bregma).23 Animals were anesthetized with isoflurane (5% induction; 1–2% for maintenance). Presurgical analgesia was administered to alleviate pain (Carprofen 5 mg/kg, s.c.; Rimadyl, Pfizer, Australia and buprenorphine 0.5 mg/kg, s.c.; Tasmanian Alkaloids, Pty Ltd). The electrodes were fixed onto the animal's skull with dental acrylic. After the animals recovered from implantation surgery, SE was induced by electrical stimulation (90 minutes duration, 50 Hz, 1-second duration, 1-ms alternating current pulses) of the ventral hippocampus in 21 animals.23 To confirm the occurrence of SSSE on EEG, the stimulation was paused for 1 minute every 9 minutes. Mice were closely monitored for an additional 150 minutes, after which the SE was terminated by diazepam (5 mg/kg, i.p.; Mayne Pharma, Adelaide, Australia). Six animals died during SE. The number of tonic–clonic seizures during SE was documented. Sham groups were handled the same way but did not receive electrical stimulation.
2.6 EEG recordings and analysis
Video-EEG recordings were performed continuously for 2 weeks before the endpoint for both the KASE rat and SSSE mice at chronic timepoints. EEG was acquired using a Compumedics Grael amplifier with Profusion 3 or 4 software (Compumedics, Melbourne, Victoria, Australia). The signal was unfiltered, digitized at a 512 Hz sampling frequency, and analyzed using automated rodent seizure detection software (Assyst) as previously described.24 Annotated seizure events were verified manually by two independent researchers. One SSSE animal was eliminated from the following experiments and analysis as it did not show spontaneous epileptic seizures during video-EEG monitoring.
2.7 Heart tissue collection
After video-EEG recording, animals were humanely anesthetized with 5% isoflurane and oxygen (2.0 L/min) followed by intraperitoneal injection of Lethabarb (1 mL, Virbac). For post-mortem molecular analysis, the hearts of KASE rats (1 week post-SE n = 9; 1 week sham n = 10; 12 weeks post-SE n = 10; 12 weeks sham n = 10) and SSSE mice (1 week post-SE n = 8; 1 week sham n = 8; 16 weeks post-SE n = 6; 16 weeks sham n = 5) were collected after euthanasia. Fresh heart tissue was rinsed with ice-cold saline, blot-dried on sterile gauze, and quickly weighed on a sterile Petri dish. The heart weight (HW), body weight (BW) and the HW/BW ratio were measured in the animals. The heart was separated in the middle through the transverse plane into top and bottom halves. The top half was dissected into the left atrium (LA), right atrium (RA), left ventricle (LV), right ventricle (RV) and septum, snap frozen with liquid nitrogen, and stored at −80°C for gene expression analysis. The bottom half was fixed directly in 10% neutral buffer formalin overnight and embedded in paraffin for further histological analysis.
2.8 Quantitative polymerase chain reaction (qPCR) assessment of gene expression
Total RNA was extracted from the collected heart tissue using RNeasy Fibrous Mini Kit (QIAGEN, Australia) by QIAcube (QIAGEN, Australia) as per the manufacturer's instructions. Extracted RNAs were stored in RNase-free water (QIAGEN, Australia) at −80°C. RNA concentration and integrity were directly measured by QIAgility (QIAGEN, Australia) as per manufacturer's instructions. Total cDNA was synthesized with QuantiTech Reverse Transcription Kit (QIAGEN, Australia) per the manufacturer's instructions. mRNA transcript levels were determined using Taqman gene expression assays from Thermofisher Scientific (Australia) following an in-house protocol (HCN2 Assay ID Rn01408572_mH; HCN4 Assay ID Rn00572232_m1; NaV1.5 Assay ID Rn00565502_m1 and Mm01342518_m1; CaV3.2 Assay ID Rn01460348_m1 and Mm00445382_m1; KV7.1 Assay ID Rn00583376_m1; NCX1 Assay ID Rn04338914_m1 and Mm01232254_m1) as described previously.25 The mRNA expression for each sample was normalized to stably expressed housekeeping genes including Ywhaz (Assay ID Mm03950126_s1), GAPDH (Rn01749022_g1), UBC (Rn01789812_g1 and Mm02525934_g1), and TBP (Rn01455646_m1 and Mm01277042_m1).
2.9 Histochemical staining and fibrosis quantification
Heart tissue was sent to Monash Histology Platform (Alfred Centre) for sectioning (4 μm thickness) and Picrosirius Red Staining. An image of stained sections was acquired with 40x magnification under a microscope (BX51 Fluorescence Microscope, Olympus, Center Valley, PA, USA). Images were processed using an automated image analyzer (ImageJ version 1.52p, National Institute of Health, USA) to quantify fibrosis in a blinded manner. Red-highlighted pixels were selected by adjusting the threshold with the Red-Green-Blue (RGB) filtering to identify the collagen and whole heart areas. Perivascular regions and staining artifacts were manually removed. The fibrosis level was quantified by the percentage area of collagen within the total heart area for each image as described previously.12
2.10 Statistical analysis
The outcomes assessed were heart weight, cardiac fibrosis levels, and cardiac ion channel and ion exchanger expression. Significance between groups was evaluated by GraphPad Prism 8.0.2 (GraphPad Software Inc., CA, USA). The normality of these parameters was tested using the Shapiro–Wilk test. Normally distributed data (heart weight to bodyweight ratio, fibrosis level at the subacute timepoint in KASE animals, fibrosis level at chronic timepoint in SSSE animals, ion channels/exchanger expression except CaV3.2 mRNA in the septum of KASE animals at chronic timepoint) were analyzed using an unpaired t-test. In contrast, data that did not have Gaussian distribution (fibrosis level at subacute timepoint in SSSE animals, fibrosis level at chronic timepoint in KASE animals, CaV3.2 mRNA in the septum of KASE animals at chronic timepoint) were analyzed using the Mann–Whitney test. Pearson's correlations were performed between cardiac structural changes (HW/BW and fibrosis) and epilepsy profile (SE severity, seizure frequency). Data are presented as mean or mean ± standard error of the mean (SEM) with statistical significance set at p < 0.05.
3 RESULTS
3.1 HW/BW in KASE rats and SSSE mice
Changes in the bodyweight and HW/BW ratio were found at the subacute timepoint post-SE in both models, as presented in Figure 2. Subacutely, the bodyweight in the post-SE animals was significantly lower compared to the sham animals (376.0 ± 50.76 vs. 434.0 ± 21.35, p = 0.0041, and 25.75 ± 2.079 vs. 28.49 ± 1.045, p = 0.0050). The HW/BW was significantly higher in the KASE rats compared with shams (0.280 ± 0.004 vs. 0.328 ± 0.012, p = 0.0008, Figure 2C) and was significantly higher in the SSSE mice compared with shams (0.446 ± 0.019 vs. 0.551 ± 0.026, p = 0.0053, Figure 2F). There was no difference in HW/BW at the chronic timepoint in both KASE and SSSE models (0.269 ± 0.007 vs. 0.273 ± 0.005, and 0.402 ± 0.025 vs. 0.455 ± 0.014, Figure 2I,L, respectively).
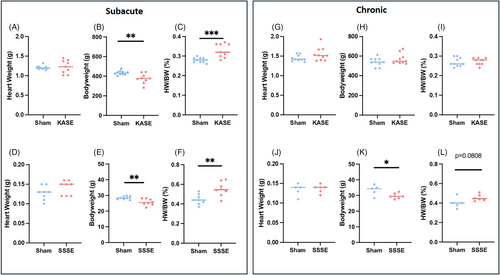
3.2 Cardiac fibrosis is increased in KASE rats at subacute and chronic timepoints
Analysis of the heart histochemistry revealed significant cardiac fibrosis at both subacute (6.200 ± 1.186 vs. 1.980 ± 0.109, p = 0.0016, Figure 3A,B) and chronic (3.190 ± 0.520 vs. 1.460 ± 0.099, p = 0.0027, Figure 3C,D) timepoints in KASE rats compared with sham animals. However, no statistically significant differences were observed in heart collagen deposition in SSSE animals at 7 days (1.300 ± 0.089 vs. 1.188 ± 0.143, Figure 3E,F) and 16 weeks (0.955 ± 0.195 vs. 0.780 ± 0.163, Figure 3G,H) post-SE.
3.3 Subacute fibrosis is positively correlated with SE severity in KASE animals
We then evaluated the relationship between cardiac changes and seizure/epilepsy outcomes. At the subacute timepoint, the SE severity was measured by the time spent in tonic–clonic seizures (class IV-V Racine) in KASE rats. In contrast, in SSSE mice, it was measured by the number of tonic–clonic seizures. For the chronic timepoints, the average number of spontaneous seizures per day was 2.47 (range: 0.14–7.15) in KASE rats and 1.83 (range: 0.57–5.00) in SSSE animals. In KASE rats, at the subacute timepoint, there was a positive correlation between the HW/BW ratio and SE severity (r = 0.7537, p = 0.0190) and a positive correlation between the percentage of fibrosis and SE severity (r = 0.7643, p = 0.0165); there was no significant correlation between the frequency of spontaneous seizures or cardiac fibrosis at chronic timepoints in KASE animals (Figure 4). There was no significant correlation between cardiac measures and epilepsy in SSSE mice. The correlation between cardiac fibrosis and KA dose was investigated to evaluate the peripheral effect of systemic injection of KA. We found no correlation between the KA dose during induction and subacute or chronic cardiac changes (Figure S1).

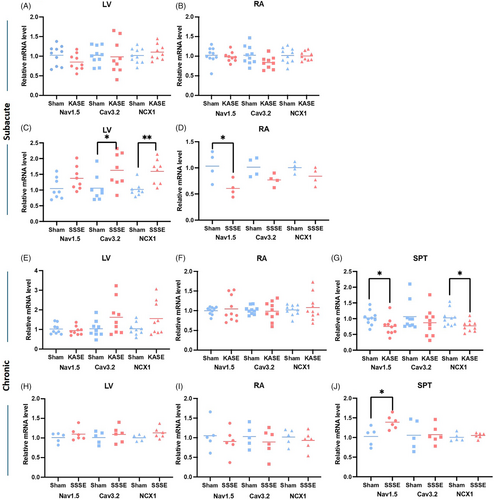
3.4 Cardiac ion channel and exchanger mRNA expression changes at both subacute and chronic timepoints
To investigate key ion channel and ion exchanger expression, we analyzed NaV1.5, CaV3.2, and NCX1 mRNA expression in subsections of cardiac tissue from animals culled at both the subacute and chronic epileptic timepoints post-SE. Both KASE and SSSE models of acquired epilepsy demonstrated expression changes in ion channels and ion exchangers (Figure 5). Chronic KASE rats showed reduced NaV1.5 and NCX1 mRNA expression in the septum (p = 0.0261 and p = 0.0198, respectively) compared with the sham. Interestingly, in the SSSE mice, CaV3.2 and NCX1 mRNA levels were significantly increased in the LV (p = 0.0327 and p = 0.0025, respectively), while the NaV1.5 was decreased in the right atrium (p = 0.0388) at the subacute timepoint. However, only an up-regulation of NaV1.5 mRNA level in the septum was observed at the chronic timepoint in this model (p = 0.0166).
4 DISCUSSION
This study of two different and complementary animal models of acquired TLE, involving different species and modes of epilepsy induction, revealed several important cardiac alterations at both the subacute timepoint post-SE and at the chronic epilepsy phase. Of importance, extensive cardiac fibrosis was demonstrated in the KASE model at subacute and chronic timepoints. At the molecular level, we found that the mRNA expression of NaV1.5, CaV3.2, and NCX1 was altered at different timepoints post-SE in both models, suggesting that both acute severe seizures (i.e. SE) and chronic epilepsy may result in acquired cardiac channelopathies which have the potential to result in altered cardiac electrophysiological function and increased risk of cardiac arrhythmias.
Multiple studies have reported cardiac hypertrophy of various degrees in patients who have suffered an epilepsy-related death.26-28 However, there has been relatively limited data on cardiac hypertrophy or dilated cardiomyopathy in patients after SE. The cardiac complications related to SE, which is accompanied by excessive release of catecholamines and stress hormones that can have an adverse cardiac effect, can pose a significant clinical challenge, particularly in individuals with pre-existing compromised cardiac function.29 Additionally, a previous study found that the difference in heart weight after SE was present in both wet and dry heart weights, suggesting that the transformation is not caused by edema but rather due to changes in the cardiac structure.30 Our study found that in both models of acquired TLE, there was a substantial increase in the normalized heart weight. However, this change is largely affected by the changes in body weight. As we have observed in this study, post-SE animals can experience a significant amount of weight loss or weight gain, which could confound the analysis. For small animals, using tibia length to normalize the heart weight and performing histological analysis on the myocardial architecture would be considered alternatives.31, 32
On the other hand, we found no evidence of increased normalized heart weight at 12 weeks post-SE timepoint, in agreement with results described on a similar KA-induced epilepsy model, suggesting that the post-SE cardiac hypertrophy resolves in the chronically epileptic period.11 Interestingly, this previous study did report cardiac hypertrophy in rats at 7–11 months post-SE, which was correlated with a decreased standard deviation of the NN intervals (SDNN), suggesting the hypertrophy arises from altered autonomic tone.11 A previous study using the intrahippocampal KA-induced SE rat model also observed changed autonomic activity, characterized by an immediate elevation in plasma noradrenaline after status epilepticus.10 Noradrenaline has been reported to temporally induce cardiac hypertrophy and enhance the cardiac troponin T expression in the sarcomere in vitro.33 Future studies may investigate the mechanism of cardiac hypertrophy and stress cardiomyopathy through β-adrenergic activation in status epilepticus and chronic epilepsy.
Cardiac fibrosis is a significant health concern globally and is commonly associated with arrhythmogenesis, impaired heart relaxation and contraction, and progression to heart failure and serious cardiac arrhythmias.34 The cardiac fibroblasts are essential for maintaining hemostasis in the extracellular matrix (ECM). Still, the cardiotoxic effect of catecholamine might induce an abnormal buildup of ECM proteins, including collagen.35 Here, we found increased collagen levels in the KASE animals at both acute timepoints post-SE and chronic epilepsy compared with shams, suggesting an epilepsy-induced structural cardiac pathology. Moreover, at the acute timepoint, we found a correlation between cardiac fibrosis and the time the animal spent in tonic–clonic seizures during SE. This supports the idea that cardiac structural changes may be attributed to recurrent catecholamine surges and myocardial ischemia.36
Similarly, recent work from our group demonstrated a positive association between cardiac fibrosis and seizure frequency during the chronic epileptic stage of KASE rats.12 The present study confirmed the finding of increased cardiac fibrosis in chronically epileptic rats post-KASE, but did not find significant cardiac fibrosis in chronic epileptic mice post-SSSE. This could be explained by an overall lower seizure frequency in the SSSE mice compared with the KASE rats. The sympathetic surge secondary to seizures may not be frequent enough in these animals to result in cardiac myofilament damage, but increased myocardial micro injuries after recurrent convulsive seizures may still induce subtle fibrotic lesions and facilitate future cardiac arrhythmias and cardiac function-related mortality.37 A study also showed that contraction band necrosis was associated with the disarray of cardiomyocytes in the hearts of patients with increased sympathetic tone.38 Unfortunately, there are a limited number of animal studies that have investigated the relationship between acquired epilepsy and cardiac structural changes. Whether mouse models are suitable for fibrotic injury secondary to epilepsy or stress-induced cardiomyopathy in epilepsy remains unknown. A previous study using an intracortical kainate mouse model of TLE showed that cardiac anatomy was unchanged after 48 hours and 30 days of SE.39 This further emphasizes the importance of investigating structural and functional cardiac changes under variable conditions in different animal models of epilepsy.
The peripheral effect of systemic KA injection was also evaluated. The cardiac structural changes in the KASE model at both acute and chronic timepoints were unrelated to the KA dosage administered, indicating that KA does not have a direct cardiotoxic effect that could confound the correlation analysis between epilepsy and cardiac changes. Furthermore, this is supported by our recent work, where the absence of cardiac fibrosis in the KA-resistant rats who were given comparable doses of KA but did not develop SE or become chronically epileptic.12 In contrast, cardiac fibrosis was observed in the animals who went into SE and were confirmed to be epileptic at the chronic timepoint.12
Electrical instability secondary to cardiac channelopathies can be present in the form of altered cardiac rate, rhythm, and repolarization function, in PWE.17 We showed here that the sodium channel NaV1.5, the T-type calcium channel CaV3.2, and the cardiac sodium-calcium exchanger 1 (NCX1) were differentially expressed across the two different TLE models. NaV1.5 is abundant in cardiac cells and plays a significant role in generating electrical signals. Its dysfunction is associated with prolonged cardiomyocyte depolarization and sinoatrial (SA) node dysfunction.40 Several SCN5A variants have also been reported in Sudden Unexpected Death in Epilepsy (SUDEP) cases,41, 42 and some of these variants have been previously diagnosed with long QT syndromes.43 The downregulation of cardiac NaV1.5 channels may also be attributed to recurrent myocardial ischemia and oxidative stress resulting from ictal sympathetic overactivity.15 This corresponds with the decreased NaV1.5 mRNA level we found at the subacute timepoint in the SSSE mice. However, we observed an increased NaV1.5 mRNA expression in the septum of the SSSE animals at the chronic epileptic stage. One potential explanation is that increased mRNA expression compensates for the decreased NaV1.5 protein level, or that both increases and decreases in cardiac expression of this ion channel can be pro-arrhythmogenic; however, further studies are required to explain the inconsistency.
T-type Ca2+ channels play an important role in generating sustained Ca2+ entry for the depolarization of neuronal cells and pacemaker activity.44 Calcium channel CaV3.2, encoded by the CACNA1H gene, may influence the pacemaker cell function in both the brain and heart but also affect cardiac morphology and coronary artery function.45, 46 NCX1 is also involved in intracellular calcium regulation, creating a net inward electrical current to depolarize the cell membrane and maintain a stable rhythm.47 Mutation in the SLC8A1 gene, which codes for NCX1, can cause salt-sensitive hypertension by disrupting the exchange of Na+ and Ca2+ ions.48 Moreover, Ca2+ overload in the cardiomyocytes induces oxidative stress, resulting in apoptosis and necrosis.49 In our study, CaV3.2 and NCX1 mRNA expression was upregulated in the LV of the SSSE animals at the subacute timepoint, which might contribute to a subtle acute cardiomyopathy in this model. The upregulated NCX1 might be a compensatory mechanism in balancing the Ca2+ flow. At the same time, it can also be a sign of overall cardiac malfunction as the NCX1 level was upregulated in heart failure.50 Another study in KASE rats reported that the NCX1 expression in the whole heart was decreased 2 weeks after SE but returned to normal levels during chronic epilepsy.14 However, we found a decreased NCX1 mRNA level in the septum in the KASE model at the chronic epileptic timepoint. Decreased NCX1 expression might lead to increased intracellular Ca2+ levels that contribute to oxidative stress and necrosis,49 which could explain the increased fibrosis in our chronic KASE animals. On the other hand, increased cardiac fibrosis is also linked to arrhythmogenesis, dysfunction of ion channels, and changes in ion homeostasis.51 Future studies should investigate the potential use of cardioprotective drugs, including ion channel modulators and antifibrotic medications, to help prevent the development of cardiovascular disease in chronic epilepsy.
While evident in mRNA level, further studies are required to confirm the protein expression and the underlying mechanism of cardiac channelopathy in epilepsy. Another limitation is that the animal studies were limited to the males. This may lead to a lack of understanding of potential sex-based differences in biological responses and outcomes. Although there is no clear evidence to suggest that the risk of cardiac abnormalities in epilepsy is higher in one gender, the incidence of SUDEP was reported to be 1.4-fold greater in male patients compared with female patients in a combined cohort of 289 cases,52 while the prevalence of cardiovascular disease in the general population is slightly higher in females.53
5 CONCLUSIONS
This study compared the cardiac structure and ion channel/exchanger expression in two different animal models of acquired TLE, at both the subacute post-SE and the chronically epileptic timepoints. The results demonstrated similar alterations in cardiac structure in two animal models of TLE at both timepoints, despite the difference in species and modes of epilepsy induction. This suggests that the changes are likely a direct or indirect effect of the seizures. Abnormal autonomic output to the heart may increase adrenergic signaling in cardiomyocytes and damage the myocardium, leading to cardiac remodeling, and consequently, modulation of downstream effectors and transcription factors may differentially regulate cardiac electrical stability.15 We described different cardiac ion channel/exchanger mRNAs expression between models, which is often the case among other studies and patient groups.15 Overall, our study characterized cardiac abnormalities at the subacute timepoint post-SE and in chronic epilepsy in two different animal models, which are evident at both the structural and molecular levels and might contribute to the elevated risk of cardiac-related mortality in epilepsy. Future studies should aim to confirm the effects of sympathetic activation and catecholamine-involved pathways in stress-induced cardiomyopathy in epilepsy and investigate the genetic predisposition to cardiac channelopathy, as many ion channels are co-expressed in the brain and heart. Furthermore, the use of cardioprotective drugs to prevent seizure-induced sympathetic activation or cardiac fibrotic malformation may also help prevent the development of cardiovascular disease in chronic epilepsy.
AUTHOR CONTRIBUTIONS
Conception and design: Liu, O'Brien, Casillas-Espinosa. Acquisition of data: Liu, Gomes, Thergarajan, Ali, Casillas-Espinosa. Analysis and interpretation of data: Liu, Gomes, Thergarajan. Statistical analysis: Liu. Drafting the article: Liu. Critically revising the article: Liu, Sivathamboo, Gomes, Thergarajan, Ali, Perucca, Jones, O'Brien, Casillas-Espinosa. Reviewed submitted version of manuscript: All authors. Supervision: Sivathamboo, Powell, Perucca, Jones, O'Brien, Casillas-Espinosa.
ACKNOWLEDGMENTS
P.M. Casillas-Espinosa is supported by an Early Career Fellowship from the National Health and Medical Research Council (APP1087172), Department of Defense USA Epilepsy Research Program (EP200022), and Department of Health and Aged Care (MRFF) Stem Cell Therapies Mission grant (MRF2015957). P. Perucca is supported by an Emerging Leadership Investigator Grant from the Australian National Health and Medical Research Council (APP2017651), The University of Melbourne, Monash University, the Weary Dunlop Medical Research Foundation, Brain Australia, and the Norman Beischer Medical Research Foundation. T.J. O'Brien is supported by a Program Grant (APP1091593) and an Investigator Grant (APP1176426) from the National Health and Medical Research Council of Australia and the Victorian Medical Research Acceleration Fund. Open access publishing facilitated by The University of Melbourne, as part of the Wiley - The University of Melbourne agreement via the Council of Australian University Librarians.
CONFLICT OF INTEREST STATEMENT
The authors report no competing interests related to the current work. We confirm that we have read the Journal's position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
Open Research
DATA AVAILABILITY STATEMENT
The full dataset and statistical analysis codes will be made available on reasonable request from any qualified researcher.