Left-sided epileptiform activity influences language lateralization in right mesial temporal sclerosis
Abstract
Objective
To investigate the association between left epileptiform activity and language laterality indices (LI) in patients with right mesial temporal sclerosis (MTS).
Methods
Twenty-two patients with right MTS and 22 healthy subjects underwent fMRI scanning while performing a language task. LI was calculated in multiple regions of interest (ROI). Data on the presence of left epileptiform abnormalities were obtained during prolonged video-EEG monitoring.
Results
After correction for multiple comparisons, LI was reduced in the middle temporal gyrus in the left interictal epileptiform discharges (IED+) group, compared with the left IED− group (p < 0.05).
Significance
Using a responsive reading naming fMRI paradigm, right MTS patients who presented left temporal interictal epileptiform abnormalities on video-EEG showed decreased LI in the middle temporal gyrus, indicating decreased left middle temporal gyrus activation, increased right middle temporal gyrus activation or a combination of both, demonstrative of language network reorganization, specially in the MTG, in this patient population.
Plain Language Summary
This research studied 22 patients with right mesial temporal sclerosis (a specific type of epilepsy) comparing them to 22 healthy individuals. Participants were asked to perform a language task while undergoing a special brain imaging technique (fMRI). The findings showed that patients with epilepsy displayed a change in the area of the brain typically responsible for language processing. This suggests that their brains may have adapted due to their condition, altering the way language is processed.
Key points
- Twenty-two patients with right mesial temporal sclerosis and 22 healthy subjects underwent a reading responsive naming (RRN) fMRI paradigm.
- Lateralization indices for the fMRI task were calculated in five frontal and temporal regions of interest.
- Left interictal epileptiform discharges were associated with lower lateralization indices in the middle temporal gyrus in an RRN fMRI language task (p = 0.02).
- Occurrence of left interictal discharges in right MTS patients was associated with language network reorganization in the middle temporal gyrus.
1 INTRODUCTION
Mesial temporal sclerosis (MTS) patients more frequently exhibit atypical language lateralization compared to epilepsy patients with lesions in other brain areas.1 Language laterality determination in this group of patients is relevant to identify a population at high risk for verbal memory and naming decline after epilepsy surgery.2
Investigations of language laterality in homogeneous MTS populations are rare. The shift in left-to-right language representation was associated with the presence of left IED in a homogeneous population of left MTS patients.3
Long-term EEG monitoring usually displays high concordance between the hippocampal sclerosis side on structural MRI and the side of epileptiform activity.4 However, approximately 20%–30% of patients with unilateral hippocampal sclerosis on MRI may present bilateral independent epileptiform activity5-7 even in the absence of identifiable bilateral hippocampal lesions on high-resolution MRI.
Epileptic activity in the left hippocampus could conceivably disrupt normal and adaptive language functioning mechanisms. The appearance of contralateral epileptiform activity in patients with right MTS may interfere with the functioning of language network in the left hemisphere.
In this study, we investigated if the presence of left interictal and ictal epileptiform activity in a right MTS patient population would be associated with left-to-right shift of language representation. To our knowledge, the role of left interictal and ictal activity in the contralateral hemisphere in a homogeneous patient population with right MTS has not been previously evaluated.
2 METHODS
2.1 Ethics
All participants were recruited prospectively and signed the informed consent approved by the Institutional Review Board.
2.2 Participants
A series of patients with the diagnosis of medically refractory epilepsy secondary to unilateral right MTS were consecutively enrolled in this study, after evaluation by a neurologist (C.L.J. and R.V.) from the Epilepsy Program at Hospital das Clínicas da Faculdade de Medicina da Universidade de Sao Paulo, Brazil. We included patients with unilateral right hippocampal sclerosis confirmed by high-resolution 3T-MRI (volume loss of hippocampal structures on T1-weighted images and/or increased signal on T2-weighted or fluid-attenuated inversion recovery [FLAIR] images in the same structures), without other lesions on MRI, except occasional small nonspecific white matter hyperintense signal lesions on T2 or FLAIR sequences.
Patients with bilateral hippocampal sclerosis, other temporal or extratemporal lesions (including small-calcified lesions) on structural MRI, or with extratemporal epileptiform activity on routine EEG were excluded. All included patients underwent a right anteromesial temporal lobectomy by the same experienced neurosurgeon.
The control group consisted of friends or family members accompanying the patients, who fulfilled the following inclusion criteria and were evaluated on the day of the fMRI study by one of two researchers (J.P.A. or B.M.C.): aged between 18 and 55 years, education level of 8 years or more, right-handed dominance determined by a score higher than 50 on the Edinburgh Handedness Inventory, absence of intellectual disability or psychiatric conditions hindering the completion of functional magnetic resonance imaging and cognitive assessment, absence of diseases affecting the central nervous system except for primary headache, and absence of clinical conditions that could interfere with cognitive abilities, such as alcoholism and illicit drug use. The age cutoff for early precipitant insult was established as 6 years.
All participants were native Brazilian Portuguese speakers and had normal or fully corrected vision. Twenty-five patients were assessed for eligibility. Three patients were excluded from the study: one refused to participate and two had an active psychiatric comorbidity that prevented cooperation on fMRI.
2.3 Video-EEG monitoring
All patients underwent noninvasive video-EEG monitoring. We used the modified 10–20 system, as approved by the American EEG Society in 1990, in all patients. An extension of the 10–20 System was used to designate the 10% electrode placements. Positions were 10% inferior to the standard frontal-temporal electrodes and additional anterior-temporal electrodes.
EEG analysis was performed by two neurophysiologists (V.P. and H.C.-L.) blinded to all other patient data. On completion of an independent review of interictal and ictal data, the two reviewers on cases reached a consensus agreement when categorization differed between the analyses.
Since automatic seizure detection software was not available in our service, all records were manually reviewed for seizure onsets. Additionally, we chose to perform an interictal epileptiform activity count, carried out by the two independent board-certified neurophysiologists (V.P. and H.C.-L.), in 1 h periods during wakefulness (possibly smaller IED count) and sleep (possibly higher IED count), in the initial period of the recording (before ASM reduction or withdrawal, reflecting real life conditions), and in the last recording day (reflecting ASM tapering or withdrawal, and after occurrence of epíleptic seizures). Patients with extratemporal interictal activity were not included. Only spikes and sharp waves were considered IED; the location and frequency of interictal epileptiform discharges (IEDs) were assessed with visual analysis. The patients were classified according to the presence or absence of temporal left IED in two groups: left IED [+] and left IED [−].
For ictal-EEG analysis, the antiseizure drug dose was tapered on an individual basis, considering seizure frequency and risk of tonic–clonic generalized seizures. Ictal epileptiform activity was defined as all rhythmic epileptiform activity clearly distinct from background EEG activity, evolving in frequency and morphology and associated with clinical manifestations. Patients were classified into two groups: left seizure [+] and left seizure [−].
- At least one seizure with ictal onset in the left temporal region.
- Initially bilateral ictal onset.
- Initial right ictal EEG onset, followed by lateralization switch to independent left temporal lobe seizure activity.
Patients were classified as left-seizure [−] after the recording of at least three seizures with exclusive ictal onset in the right temporal region, without switching to the left temporal region as the seizure evolved.
According to interictal classification, of the 22 right MTS patients, seven were classified as left IED [+] and 15 as left IED [−]. According to ictal classification, 12 patients were classified as left seizure [+], and 10 patients as left seizure [−].
2.4 Functional magnetic resonance imaging (fMRI)
2.4.1 Image acquisition and analysis
fMRI was performed at least 48 h after the last self-reported seizure. All subjects were trained in the fMRI tasks outside the scanner, using the same experimental setup (exactly the same stimulation procedure adopted for fMRI acquisition), presented on a laptop screen. Structural and functional MRI images were acquired in the same session with a 3T scanner and an eight channels SENSE Head Coil (Achieva, PHILIPS Healthcare, Best, The Netherlands). The subject's head was stabilized inside the coil with lateral foam padding and adhesive tape over the forehead to ensure immobilization during the exam. Structural MRI consisted of a localizing three-plane sequence of the anatomic structures (total time 40 s), followed by a T2-weighted sagittal sequence to localize the anterior and posterior commissures (ACPC) (total time 20 s). T1-weighted sagittal volumetric scan, with Fast Field Echo (3DT1-FFE) tridimensional technique acquisition parameters, were 1 mm3 isotropic voxel dimension, repetition time (TR) = 7.0 ms, echo time (TE) = 3.2 ms, matrix = 240 × 240, the field of view (FOV) = 240 × 240, 180 slices, acceleration factor (SENSE) = 1.5 (total time 6 min). FLAIR axial images were collected to evaluate for possible structural parenchymal lesions and consisted of 20 slices, slice thickness = 4.5 mm, gap = 0.5 mm, TR = 11 000 ms, inversion time (IT) = 2.800 ms, TE = 130 ms, voxel = 0.65 × 0.86 × 4.5 mm3, matrix = 352 × 210, FOV = 230 × 183 mm2 (total time 4 min). Functional imaging used blood oxygenation level-dependent (BOLD) contrast detection with T2*-weighted sequences. Acquisition used gradient echo planar imaging (GRE-EPI). Image acquisition was ACPC oriented, with 40 slices, slice thickness = 3 mm, gap = 0.3 mm, TR = 4 s, TE = 30 ms, isotropic voxel = 3 mm3, matrix = 80 × 78, FOV = 240 mm2, SENSE = 2 and flip angle = 90°.
Images were anonymously coded and analyses were performed blindly to clinical data. Pre- and postprocessing to determine 3D activation maps employed the fMRIB Software Library (FMRIB, Analysis Group, Oxford, UK, http://www.fmrib.ox.ac.uk/fsl), with movement correction and realignment, slice timing correction, spatial smoothing with 5 mm filter, temporal filtering of the acquired time-series, normalization to MNI152 space (Montreal Neurological Institute), high-pass temporal filtering with sigma 100 s to control for BOLD signal stimulation-associated fluctuations. Individual activation maps were obtained with the general linear model (GLM)8, 9 with a statistical significance level of 0.05. FMRI Expert Analysis tool was used to generate z (Gaussianized T/F) statistic images thresholded with voxel cut-offs at z Woolrich >2.3 (corrected) and cluster significance threshold at p = 0.05. The regression model also included three movement vectors, computed during image registration, to further reduce the effects of head movement on task response estimation. The initial four volumes of each functional run were automatically discarded to allow for T1 saturation effects and were not included in the reported volumes.
2.4.2 Reading response naming task
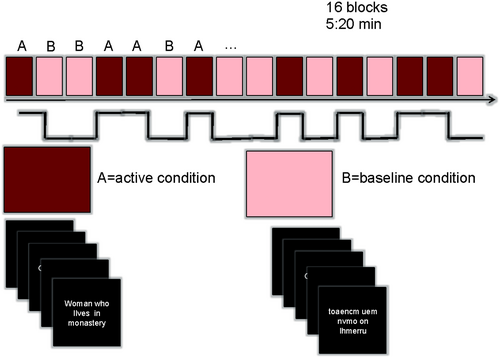
Subjects underwent a Reading Response-Naming (RRN) paradigm developed in Brazilian Portuguese. In this task, participants named an object described in a five-word sentence contrasted by a baseline visual condition consisting of the same letters scrambled in five nonwords forming an unreadable sentence. Cognitive demands in contrast between active and baseline conditions in this RRN were visual word form processing, lexical-semantic processing, speech comprehension (semantic and syntactic processing), word retrieval, and covert (silent) articulatory planning for the production of speech sounds. This paradigm encompasses both semantic and productive demands and is associated with a more extensive activation of anterior and posterior language-related areas,10 since, in TLE, frontal and temporal language-related regions can present language reorganization11 (Figure 1).
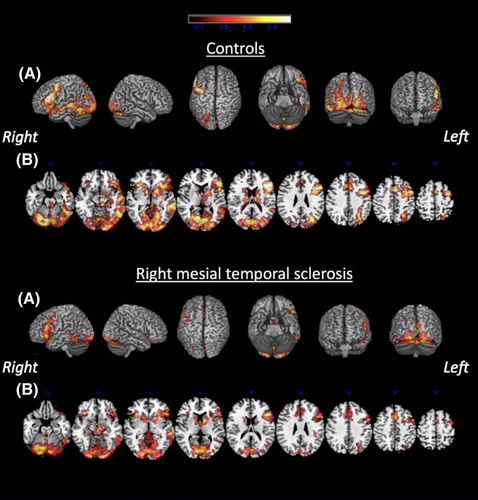
In healthy volunteers and patients, the contrast (RRN) > control condition (C) showed greater activation of the left hemisphere in the middle frontal gyrus (MFG), inferior frontal gyrus (IFG), middle temporal gyrus (MTG), inferior temporal gyrus (ITG) and bilaterally with left predominance in the fusiform gyrus (FusG) (Figure 2).
2.4.3 Regional language lateralization
Language laterality index (LI) was determined for each region of interest using with LI toolbox and a bootstrapping method.12 LI were obtained in a continuum range between +1 (complete left-sided lateralization) and −1 (complete right-sided lateralization). Region of interest (ROIs) (MFG, IFG, MTG, ITG, and FusG) were automatically defined for each hemisphere based on the segmentation available in the FreeSurfer 5.3.13
2.5 Statistical analysis
We performed continuous variable analysis with t test (two groups) or one-way ANOVA (more than two groups). For nonparametric variables, the Mann–Whitney U test and Kruskal–Wallis test were used. Post-hoc correction for multiple analyses with Tukey's HSD test was used for comparisons between each group and Bonferroni for multiple regions. Fisher's exact test was used for descriptive analysis of categorical variables. We used Medcalc Statistical software, version 15.2 (Medcalc Software, Ostend, Belgium; http://www.medcalc.org; 2015) with a statistical significance level of 0.05.
3 RESULTS
We evaluated 22 patients with unilateral right MTS (12/22 or 54.5% men, 33.8 ± 10.2 years, 11 ± 1.7 education years) and 22 healthy subjects (10/22 or 45.5% men, 31.8 ± 10.1 years, 12 ± 2.7 education years) (Table 1). Fifteen patients had a prior history of an early precipitant insult (EPI): febrile seizure (n = 9), unprovoked single seizure (n = 4), meningoencephalitis (n = 1), and minor cranial trauma (n = 1).
| R-MTS (22) | Controls (22) | p-Value | |
|---|---|---|---|
| Age (years) | 33.8 ± 10.2 | 31.8 ± 10.1 | 0.7 |
| Gender (women/total) | 12/22 (54.5) | 14/22 (58.3) | 0.8 |
| Education (years) | 11.0 ± 1.7 | 12.0 ± 2.7 | 0.6 |
| Edinburgh Handedness score | 81.0 ± 14.8 | 82.0 ± 10.7 | 0.1 |
| History of early precipitating insult | 15/22 (68.2) | ||
| Age at epilepsy onset (years) | 11.6 ± 7.4 | ||
| Epilepsy duration (years) | 22.3 ± 11.1 | ||
| Average number of seizures/month | 6.1 (5.4) | ||
| Number of ASM in use before surgery | 2.6 (0.7) | ||
| Pathologically confirmed hippocampal sclerosis | 22 |
- Note: R-MTS = Right mesial temporal lobe epilepsy secondary to hippocampal sclerosis; age at epilepsy onset was characterized as the age of first unprovoked seizure for all patients. Values are presented as n/n (%) or mean ± SD. T-test (two groups) was used for continuous variables and Fisher's exact test was used for categorical variables.
All patients presented the right IEDs in the interictal period. No extratemporal IEDs were recorded. Seven patients (31.8%) were classified as left IED [+]. All (100%) patients with left IED [+] reported a history of an early precipitant injury (EPI), compared to 46.6% of the group of left IED [−] cases group (p < 0.01).
We did not observe extratemporal-onset seizures, seizures that could not be properly classified, or psychogenic seizures. Twelve (54.5%) patients had at least one seizure with left temporal involvement on ictal EEG and were classified as left seizure [+] group. No differences were noted between the left seizure [+] and the left seizure [−] groups in terms of clinical and demographic variables or number of recorded seizures.
In the left seizure [−] group (n = 10), six patients had all seizure onsets ipsilaterally to the lesion and four patients experienced at least one seizure onset on the right, with later involvement of the left temporal lobe, maintaining ipsilateral predominance.
In the left seizure [+] group (n = 12), four patients had at least one seizure with an electroencephalographic onset in the left temporal lobe, and the remaining eight patients experienced at least one seizure with bilateral onset without predominance or bilateral onset with left predominance.
The average number of recorded seizures was 4.7 ± 3.7, range of 2—in 2 patients in the left seizure [+] group—to 18.
Noninvasive video-EEG monitoring had a minimum duration of 2 days (mean 5.7 ± 2.8 days, median 5, range 2–20).
3.1 Functional magnetic resonance imaging (fMRI)
3.1.1 fMRI behavior data analysis
fMRI behavior data were available for 22 patients and 22 controls. There were no differences between patients and controls in mean reaction times (680 ms ± 103 and 650 ± 123, respectively) and percentage of total responses (90.8 ± 4.0 and 94.3 ± 5.3, respectively). The right MTS group (80.2% ± 12.9%) showed a smaller percentage of correct responses compared with healthy subjects (91.7% ± 6.1%, p < 0.0001).
3.1.2 Left epileptiform activity and language lateralization
Considering interictal activity, we observed a significant reduction in LI in the left IED [+] group compared with the control group in the MTG (0.32 vs. 0.77) and in the ITG (0.30 vs. 0.69) regions and compared with the left IED [−] group in the MTG region (0.32 vs. 0.84), on one-way multiple comparison ANOVA with Tukey correction. After Bonferroni correction for multiple analysis for all five regions (MFG, IFG, MTG, ITG, and FusG), only the difference between IED [+] and IED [−] groups in the MTG remained significant (p = 0.02) (Figure 3).
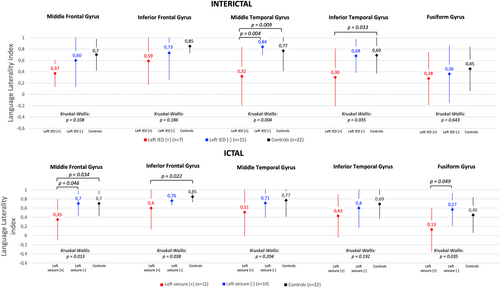
Regarding ictal activity, on one-way ANOVA with Tukey correction, LI was significantly smaller for the left seizure [+] group compared with the control group in the MFG (0.35 vs. 0.7) and the IFG (0.6 vs. 0.85) regions and compared with the left seizure [−] group in the MFG (0.35 vs. 0.7) and FusG (0.13 vs. 0.45) regions. These differences did not remain significant after correction for Bonferroni correction for multiple analyses of the five regions.
There was no difference between the median frequency of right IED in the IED [+] patient group (93 discharges/h, IQR 332) and the IED [−] patient group (41 discharges/h, IQR 744), p = 0.377.
The median frequency for left IED in the IED [+] patient, group was 56 discharges/h (IQR 171) and, as expected, zero discharges/h for the IED [−] patient group, p ≤ 0.001.
4 DISCUSSION
In this study, we found that left interictal epileptiform activity in patients with right mesial temporal sclerosis was associated with lower LI in the MTG, indicating decreased left MTG activation, increased right MTG activation, or a combination of both in this subgroup of right MTS patients.14 These findings suggest that left (contralateral) temporal epileptiform interictal activity (in the absence of an identifiable left hemisphere lesion) may interfere with language network organization in the MTG in right-MTS patients.
Atypical language representation (right or bilateral) is more commonly seen in patients with epilepsy than in the normal population.11, 15, 16 Focal epilepsy has been associated with changes in lateralization patterns and relocation of language regions.11 A growing body of evidence supports the notion that chronic epileptiform abnormalities cause focal cognitive deficits and cognitive network reorganization. Interictal discharges are associated with transient cognitive fluctuations,17, 18 reflecting regional functional disturbance in the area originating spikes, such as impaired verbal performance associated with left-sided interictal discharges.17 In one study, using a covert word generation task in a left MTS population,19 the frequency of interictal abnormalities has been associated with left-to-right language shift. Learning and memory, including semantic memory, play a pivotal role in language acquisition, as well as in lateralization and organization patterns.20 Acquired left hippocampal disease may disrupt normal language acquisition and organization of the language network, resulting in the left-to-right shift of language representation.21
Previous investigations of factors associated with language lateralization in TLE patients have focused on left TLE patient populations.14, 22-24 Language-processing deficits have also been described in right TLE patients. The presence of right TLE can negatively influence a word-list generation, confrontation naming, and categorization decision performances.25 Those findings have been attributed to right hemisphere language processing disturbance related to word recognition.26 A study with 301 patients with right TLE used a memory specialization index to identify strengths and weaknesses in verbal versus visual memory for those patients and showed a significant decrease in visual memory.27 The impact of bilateral epileptiform activity in MRI-defined unilateral hippocampal sclerosis on language dysfunction5-7 and language reorganization patterns has received little attention. Scalp EEG propagation patterns to the contralateral hemisphere can reflect either independent contralateral temporal lobe epileptiform activity or propagation patterns.28 Alternatively, a severely injured hippocampus may be unable to generate ictal activity in the ipsilateral temporal neocortex, resulting in ictal activity spread to contralateral temporal structures (“burnout” hippocampus theory).6
Our findings indicate that right-MTS patients with contralateral (left) epileptiform activity display decreased language lateralization indices in the right MTG, and, possibly other regions, compared to right-MTS patients without detectable left epileptiform activity. Classification criteria for bilateral or contralateral IED patterns on scalp EEG in unilateral MRI-defined hippocampal sclerosis patients vary across studies. In the present study, interictal categorization included any spike and sharp waves over the left temporal region, detected with prolonged video-EEG monitoring. In this study, approximately 30% of patients presented contralateral IED, which is consistent with reported figures, ranging from 30% to 50%.7, 29 The presence of left interictal activity was associated with lower language LIs, in the middle temporal gyrus, indicative of left-to-right language shift. Although LI values were significantly lower compared to controls, values were still above 0.2, the usual cutoff for atypical language dominance.11 Since the right hemisphere also presented interictal epileptiform discharges in all patients, it is conceivable that language network reorganization in the left IED [+] patient group may have been influenced by epileptiform activity in both hemispheres. We were unable to find other studies that evaluated the influence of left IED in language network reorganization in populations with right-hemisphere epilepsy.
We did not find LI differences between the left IED [−] group and controls. The differences were only seen in the presence of left IEDs. For left seizure onset or involvement in right MTS patients, we found decreased LI values in the inferior and middle frontal gyri, and in the fusiform gyri, but this difference did not remain significant after correction for multiple corrections. Our small sample lacked the power to detect a significant difference between the left IED [−] group and controls.
In this study, we included only right-handed patients. The degree of left-handedness has been shown to be a marker of atypical (rightward) language lateralization in temporal lobe epilepsy, in the context of seizure features that potentially lead to combined language and handedness reorganization, such as left hemisphere focus, early or intermediate age at seizure onset. We included only right-handed patients to avoid the confounding factor of left hemisphere language dominance in patients with a genetic factor for left-handedness.24
Considering the data available for analysis, a cut-off for the seizure classification (left seizure + or −) was established as three ipsilateral seizure onset (left seizure [−]), which may have resulted in a misclassification in a minority of cases. It is conceivable that, if more seizures had been recorded, a seizure onset on the left side might have been captured. Additionally, the chances of not detecting seizures were minimized by the fact that all recordings were manually reviewed to assess for seizure occurrence, increasing the ability to detect subclinical seizures. Interestingly, of the ten seizure [−] patients in this study (all ictal onsets on the right), all 10 also had IED exclusively on this right, indicating that ictal and interictal data were concordant, and suggesting a small possibility that a seizure [−] patient was erroneously classified as seizure [+]. If a patient disclosed seizure onset contralaterally to the MTS (i.e., on the left side), the patient would automatically be classified as seizure [+], and recording of additional seizures would not change classification. For these cases, a minimum of three seizures was therefore not required.
5 CONCLUSIONS AND IMPLICATIONS
In conclusion, in this study of right-handed patients with temporal lobe epilepsy associated with unilateral right mesial temporal sclerosis, left interictal temporal epileptiform activity was associated with lower language lateralization indices in the middle temporal gyrus, indicating language network reorganization in this patient group, with decreased activation of the left MTG, increased activation of the right MTG, or a combination of both.
Future studies should evaluate if the left-to-right shift of language representation in these patients is associated with preserved performance in tests that measure language abilities. Additionally, successful epilepsy surgery, with seizure cessation in the left and right hemispheres may be associated with improved postoperative language performance in this patient population, if the deleterious influence of epileptiform discharges on the left hemisphere is eliminated with surgery.
AUTHOR CONTRIBUTIONS
Conceived and designed the analysis: JPA, BMC, VP, KTC, EA, LHC. Collected the data: JPA, BMC, VP, KTC, HC-L, CL. Performed the analysis: JPA, BMC, VP, CLJ, RV, LZP. Wrote the paper: JPA, BMC, VP, KTC, HC-L, CL, CLJ, RV, PRA, EA, LZP, LHC.
ACKNOWLEDGMENTS
This study was funded by the São Paulo Research Foundation (FAPESP) (grant 2005/5464-9). The authors thank Wen Hung Tzu (M.D. Neurosurgeon of Epilepsy Program, Clinical Hospital, University of Sao Paulo School of Medicine, Sao Paulo, Brazil) and the patients and families who participated in this study.
CONFLICT OF INTEREST STATEMENT
None of the authors has any conflict of interest to disclose. We confirm that we have read the Journal's position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.