Prospective study of cenobamate on cognition, affectivity, and quality of life in focal epilepsy
Abstract
Objective
Cenobamate is a recently approved antiseizure medication that proved to be safe and effective in randomized controlled trials. However, little is known about its impact on some areas frequently affected by epilepsy. For this reason, we explored the effects of cenobamate on cognitive performance, as well as on negative affectivity and quality of life in a sample of patients with drug-resistant epilepsy.
Methods
Two prospective cohort studies were carried out. In Study 1, 32 patients (22 men and 10 women) underwent a baseline (T0) and a short-term (T1) neuropsychological assessment after 3 months of cenobamate administration. In Study 2, 22 patients (16 men and 6 women) from the T1 sample also underwent a baseline and a follow-up evaluation (T2) 6 months after T0.
Results
No significant differences were found in cognitive variables, negative affectivity, and quality of life either in Study 1 or Study 2. Similarly, based on the reliable change index, it was found that most patients showed no changes in these variables.
Significance
These results suggest that cenobamate is a safe antiseizure medication in terms of cognition, negative affectivity, or quality of life since no adverse events have been found after 3 and 6 months of treatment.
Plain Language Summary
Cenobamate is a new antiseizure medication. In patients with epilepsy, cenobamate seems to not affect cognition, anxiety, depression, or quality of life.
Key points
- The effects of adjunctive CNB treatment after 3 and 6 months on cognitive performance, negative affect, and QOL were explored.
- No differences were found in cognitive, emotional, or QOL variables after 3 and 6 months of CNB administration.
- Based on RCIs, most patients remained stable in their cognitive performance, negative affectivity, and QOL both in Study 1 and Study 2.
- In conclusion, CNB treatment appears to be safe for cognition, negative affectivity, and QOL in patients with drug-resistant epilepsy.
1 INTRODUCTION
Cenobamate (CNB), a tetrazole alkyl carbamate derivative, is a new antiseizure medication (ASM) with dual antiseizure activity: blocking the persistent component of sodium currents, enhancing and prolonging the inactivation of the transient component of sodium currents, and potentiating GABA A receptors to decrease neuronal excitability.1, 2
In the USA, CNB is indicated for the treatment of focal seizures in adult patients.3 In Europe, CNB has been approved for the adjunctive treatment of focal-onset seizures, with or without secondary generalization, in adult patients with epilepsy who have not achieved adequate seizure control despite treatment with at least two ASMs.4 The efficacy of CNB in seizure control has been established in two randomized, double-blind controlled trials.5, 6 Long-term effectiveness and tolerability have been confirmed in open-label studies.7-9 Finally, real-world data information regarding the use of this medication has been reported in a few observational studies.10, 11
In previously mentioned studies, dose-related adverse events have been found. Chung et al. (2020) showed that 76.1% of patients who were treated with 200 mg/day of CNB reported adverse events during the 12-week treatment period. In the same line, Krauss et al. (2020) found that, after 18 weeks of treatment, the percentage of patients reporting adverse effects increased with dose, with 65% of patients taking 100 mg/day, 76% of those taking 200 mg/day and 90% of those taking 400 mg/day. These adverse events were mild to moderate in severity and related to the central nervous system.12
To our knowledge, no studies have comprehensively addressed the effects of CNB on cognitive functioning in people with epilepsy. In animal models, a single administration of 10 and 30 mg/kg intraperitoneally CNB (corresponding to 0.81 and 2.44 mg/day in humans) has been found to have anxiolytic effects in the elevated zero maze and impaired recognition and object location memory.13 In the same study, mice that received chronic treatment of 5 mg/kg (equivalent to 0.41 mg/kg in humans) after a period of adaption of 10 days showed impaired recognition and spatial learning performance.13
Despite these interesting results, only one human study has examined this topic with a cognitive screening tool. The results showed that 72% of patients who took CNB doses that ranged from 50 to 250 mg/day for approximately 3 months (with a mean of 125 mg/day) had stable scores in the EpiTrack screening tool, while 16% of the patients even improved.14 However, although this test is a sensitive tool for exploring the cognitive effects of ASMs, it is an instrument focused exclusively on attention and executive functions.15 Therefore, other cognitive domains remained unexplored, such as memory or language, equally affected in patients with temporal lobe epilepsy.16
Finally, as far as we know, there are also no studies that have addressed the effects of CNB on comorbidities often associated with epilepsy, such as negative affectivity (ie, anxiety and depression) or poor quality of life (QOL), despite their increased recognition by the International League Against Epilepsy (ILAE) in recent years.17 For these reasons, the present study aims to determine the possible effects of adjunctive CNB treatment with doses ranging from 50 to 250 mg/day on cognition performance, negative affectivity, and QOL in people with drug-resistant epilepsy after three and six months of administration.
2 METHODS
2.1 Participants and experimental design
This is a longitudinal and prospective cohort study in which participants were recruited from the Refractory Epilepsy Unit of the Hospital Universitario y Politécnico La Fe between December 2021 and June 2023. This report followed STROBE guidelines,18 and the study was conducted following the Declaration of Helsinki and approved by the Ethics Committee of the hospital. All participants provided informed consent.
The inclusion criteria were as follows: (a) patients with a diagnosis of drug-resistant epilepsy; (b) age of at least 18 years; and (c) patients with focal aware seizures, focal impaired awareness seizures, and/or focal to bilateral tonic–clonic seizures. Excluded were patients who: (a) were older than 70 years; (b) with a recognized and diagnosed severe intellectual disability, in whom the assessment could not be performed with a minimum of reliability; and (c) were not fluent in Spanish. Participants who were enrolled before the 15th of September 2022 were part of the Expanded Access Program (EAP), before reimbursement authorization.
Demographic and clinical data were extracted from clinical charts. Epilepsy types were independently classified following the classification of epilepsy from the ILAE by two experienced epileptologists (MG and KH) and based on available seizure semiology, EEG data, neuropsychological test results, as well as functional and structural imaging data.17 Any disagreements were resolved through consensus after a thorough review.
In the initial sample, thirty-five patients underwent a neuropsychological assessment before starting CNB treatment (baseline, T0). However, three patients discontinued the treatment before the follow-up assessment: two men due to the onset of a rash and one woman due to dizziness. As a result, the final sample consisted of thirty-two patients who also performed a follow-up assessment after the introduction of adjunctive treatment with CNB (short-term evaluation, T1). This assessment was performed after a titration period, with doses ranging from 50 to 200 mg, usually 3 months after T0 (mean = 96.47 days, SD = 22.12). Moreover, to study possible effects with higher doses and after reaching a stable dose, twenty-two patients of those who undertook the T1 assessment also underwent a long-term assessment (T2), which was usually after 6 months of T0 (mean = 193.05 days, SD = 33.17). CNB doses ranged from 150 to 250 mg. The same neuropsychological battery was applied to every assessment. To organize the results and provide an overview of the effects of CNB after a period of gradual increase and after reaching a stable dose, comparisons between T0 and T1 will be presented in Study 1 and comparisons between T0 and T2 in Study 2.
2.2 Pharmacological treatment
CNB titration was performed according to the SmPC. In all patients, CNB was started at 12.5 mg/day and increased every 2 weeks to 25, 50, 100, 150, and 200 mg/day. The initial target dose was 200 mg/day, but lower and higher maintenance dosages were allowed. To determine the drug load of other ASMs (ie, without considering CNB dose), the defined daily dose (DDD) was used following the ATC index of the WHO Collaborating Centre for Drug Statistics Methodology.19
2.3 Seizure frequency
Seizure frequency was assessed during a structured interview in which information provided by both the patient and the relative was collated. Furthermore, patients were advised to keep a seizure diary, which was used in T1 and T2 assessments, to obtain greater accuracy of the data collected.
2.4 Neuropsychological assessment
A comprehensive assessment was designed following the recommendations of the E-PILEPSY consortium20 that provided data from different domains. It lasted approximately 2 hours, and tests were always administered in the same order. All participants completed the full assessment.
2.4.1 Attention and executive functions
The EpiTrack15 is a screening tool particularly sensitive to the adverse cognitive effects of ASMs.15 It assesses response inhibition, attention, cognitive tracking, processing speed, verbal fluency, and working memory through six subtests: an interference test, Trail-Making Test A and B, a maze test, word fluency, and digit span backward. Based on the results of the subtests, a total age-adjusted EpiTrack score was calculated, ranging from 9 to 49 points, with higher scores indicating better performance.
2.4.2 Naming functions
The Boston Naming Test (BNT)21 in its 60-item version was used to assess visual confrontation naming. Semantic and phonemic cues were provided to patients in the case of no response or incorrect response. The total score was computed as the number of cards correctly named without phonemic cues, 60 being the maximum score.
2.4.3 Memory
The Spanish version22 of the Wechsler Memory Scale-Third Edition (WMS-III)23 was used to evaluate both immediate and delayed memory (scalar scores ranging from 4 to 76 and from 5 to 95, respectively). To obtain an immediate memory score, the following tests were used: Faces I, Family Scenes I, Logical Memory I, and the Verbal Paired Associates I. Regarding the delayed memory index, Faces II and Family Scenes II were used, together with Logical Memory II, Verbal Paired Associates II, and their recognition tests. These tests provide specific indices of immediate auditory and verbal memory and delayed auditory and verbal memory (for all, scalar scores from 2 to 38) and auditory-related indices as delayed auditory recognition (scalar scores from 1 to19). For all, higher scores indicate better performance.
2.4.4 Trait anxiety
It was assessed using the State–Trait Anxiety Inventory (STAI).24 The trait anxiety scale (STAI-T) evaluates relatively stable aspects of anxiety and is composed of 20 items rated on a four-point scale ranging from 0 (“hardly never”) to 3 (“almost always”), being 60 the maximum score. Higher scores indicate higher anxiety.
2.4.5 Depression
The beck depression inventory-II (BDI-II)25 was used to assess depression with 21 items rated on a four-point scale, with scores ranging from 0 to 63. Higher scores indicate higher depression levels.
2.4.6 QOL
Quality of Life in Epilepsy Inventory (QOLIE-31),26 in its Spanish version,27 was used to assess QOL and includes 31 items distributed in seven scales: seizure worry; overall QOL; emotional well-being; energy; cognitive self-rating; medication effects; and social functioning, ranged from 0 to 100. A QOL composite score was computed using a weighted average of subscales (including seizure worry and medication effects, which were scored on an inverse scale), with higher scores indicating better QOL.
2.5 Statistical analyses
Descriptive statistics were computed for demographic and clinical variables. We explored differences depending on the time of the evaluation (ie, T0, T1, or T2) on clinical variables such as the number of ASMs or DDD using repeated measures ANOVAs. To explore the differences in seizure frequency, the Wilcoxon signed rank test for repeated measures was used since this variable was not normally distributed. Furthermore, repeated measures ANOVAs were also performed to check changes in cognitive performance, negative affectivity, and QOL. In these analyses, the time of the evaluation was taken as a within-subject factor, using the DDD at T1 as a covariate in Study 1 and the DDD at T2 in Study 2. In both studies, age was included as a covariate, given the use of direct scores in the neuropsychological evaluation, and also the DDD of antidepressants, since their concomitant use can interact with CNB.
To study the relationships between seizure control, negative affectivity, and QOL, Pearson's correlations were performed. The percentage of seizure reduction in T1 and the change between T0 and T1 in the BDI-II, the STAI-T, and the QOLIE's total score was used for Study 1, replicating the same analysis with the results of T2 in Study 2.
Moreover, meaningful cognitive changes were assessed using the Reliable Change Index (RCI).28 The RCI identifies reliable changes in cognitive performance that cannot be explained by typical test–retest variations, such as practice effects or regression to mean.29 Due to the lack of a control group, the RCIs were derived from the SD and reliability data for each test,30, 31 except for the EpiTrack. This test provides RCIs, considering a significant improvement with gains of ≥4 points and a significant deterioration in cases of loss of ≥3 points.15 Data used to calculate RCIs and the reliable change criterion to exceed the 95th centile of the RCIs are provided in Table 1.
| Test-retest reliability | SD | RCI criterion | |
|---|---|---|---|
| BNT21, 32 | 0.86 | 3.47 | ±3.60 |
| TMT A | 0.90 | 10.40 | ±9.12 |
| TMT B | 0.90 | 28.20 | ±24.72 |
| WMS-III23 | |||
| Immediate memory | 0.85 | 14.50 | ±15.57 |
| Delayed memory | 0.87 | 14.60 | ±14.59 |
| Delayed auditory recognition | 0.62 | 14.40 | ±24.61 |
| Letter–number sequencing | 0.71 | 2.60 | ±3.88 |
| BDI-II33 | 0.87 | 7.76 | ±7.76 |
| STAI-T34 | 0.89 | 10.39 | ±9.55 |
| QOLIE-3126 | 0.89 | 16.00 | ±14.71 |
- Abbreviations: BDI-II, Beck Depression Inventory-II; BNT, Boston Naming Test; QOLIE-31, Quality-Of-Life Epilepsy Inventory; RCI, Reliable Change Index; STAI-T, Trait Anxiety Scale; TMT, Trail Making Test; WMS-III, Wechsler Memory Scale-III.
Finally, to explore whether seizure freedom predicted cognitive, emotional, and QOL changes, a binary logistic regression model was performed. In this model, seizure freedom (Yes/No) at T1 for Study 1 and T2 for Study 2 was considered as a predictor and dichotomous variables based on the RCI were used as dependent variables (No change/Improvement versus Worsening).
Statistical analyses were carried out using SPSS 25.0 and two-tailed tests with P set to 0.05 considered significant.
3 RESULTS
3.1 Clinical changes after CNB administration at 3 and 6 months
In Study 1, the sample consisted of 32 patients (22 men and 10 women; mean age = 39.63, SD = 14.55) who underwent a neuropsychological assessment at T0 and T1, after 3 months of CNB administration. CNB doses ranged from 50 to 200 mg/day. Most patients were taking 200 mg/day of CNB (59.4%), followed by 150 mg/day (18.8%) and 100 mg/day (18.8%), and 50 mg/day (3.1%). During these 3 months, patients were in the titration period.
After 3 months of CNB administration, a decrease in seizure frequency at T1 was found (P < 0.001), from a mean of 29.53 seizures per month at T0 (SD = 65.00) to a mean of 17.97 in T1 (SD = 53.63). More specifically, 71.9% of patients showed a reduction of at least 25% and, among them, 28.1% showed seizure freedom. The remaining 28.1% of patients either experienced a reduction of less than 25% or had no change or increase in their seizure frequency (9.4% for each of them). This improvement was reached even when the number and the DDD of other ASMs, apart from CNB administration, significantly decreased from T0 to T1 (F1, 31 = 5.74, P = 0.023, n2P = 0.16 and F1, 31 = 15.50, P < 0.001, n2P = 0.33, respectively). Changes in clinical variables are shown in Table 4.
Among patients of Study 1, 22 of them (16 men and 6 women; mean age = 39.05, SD = 15.14) participated in Study 2 and underwent a follow-up evaluation (T2) 6 months after T0. CNB doses at T2 ranged from 150 to 250 mg/day. Most patients (68.2%) were taking 200 mg/day of CNB at T2, followed by 150 mg/day (18.2%) and 250 mg/day (13.6%). All of them were taking a stable dose.
After 6 months of CNB administration, a decrease in seizure frequency was found (P = 0.001), from a mean of 38.32 seizures per month (SD = 77.01) in T0 to 20.73 (SD = 47.49) in T2. Specifically, seizure frequency decreased by at least 25% in 72.7% of patients in Study 2, with 13.6% of these patients showing a 100% reduction. Furthermore, 13.6% of the patients showed no changes in seizure frequency, and 13.6% showed an increase. Patients in T2 also took a lower number of ASMs and DDD than in T0 (F (1, 21) = 8.35, P = 0.009, n2P = 0.29 and F (1, 21) = 14.78, P = 0.001, n2P = 0.41).
Sample characteristics of Studies 1 and 2 are shown in Table 2. Furthermore, concomitant ASMs at T0, T1, and T2 are listed in Table 3. Finally, the description of the CNB administration, the changes in DDD of other ASMs, and the evolution in seizure frequency in both studies are shown in Table 4.
| Study 1 (n = 32) | Study 2 (n = 22) | |
|---|---|---|
| Age (years) | 39.63 ± 14.55 | 39.05 ± 15.14 |
| Sex | ||
| Male | 22 (68.8%) | 16 (72.7%) |
| Female | 10 (31.3%) | 6 (27.3%) |
| Educational level | ||
| Primary | 13 (40.6%) | 8 (36.4%) |
| Secondary | 9 (28.1%) | 7 (31.8%) |
| Lower university | 3 (9.4%) | 3 (13.6%) |
| University | 7 (21.9%) | 4 (18.2%) |
| Academic/employment insertion | ||
| Yes | 21 (65.6%) | 14 (63.6%) |
| No | 11 (34.4%) | 8 (36.4%) |
| Household members | ||
| Parents | 12 (37.5%) | 8 (36.4%) |
| Partner and/or sons | 16 (50%) | 13 (59.09%) |
| Living alone | 4 (12.5%) | 1 (4.6%) |
| Epilepsy type | ||
| FLE | 6 (18.8%) | 5 (22.7%) |
| TLE | 20 (62.5%) | 11 (50%) |
| PCE | 4 (12.5%) | 4 (18.2%) |
| Multifocal | 2 (6.3%) | 2 (9.1%) |
| Side of the seizure focus | ||
| Left | 13 (40.6%) | 8 (36.4%) |
| Right | 15 (46.9%) | 10 (45.5%) |
| Bilateral | 4 (12.5%) | 4 (18.2%) |
| Age at epilepsy onset (years) | 15.12 ± 11.50 | 13.64 ± 8.74 |
| Epilepsy duration (years) | 24.56 ± 16.01 | 25.41 ± 16.29 |
| MRI findings | ||
| HS | 1 (3.1%) | 0 (0%) |
| FCD | 8 (25%) | 7 (31.8%) |
| Cavernoma | 1 (3.1%) | 0 (0%) |
| Normal | 14 (43.8%) | 10 (45.5%) |
| Encephalomalacia | 3 (9.4%) | 3 (13.6%) |
| Hypothalamic hamartoma | 1 (3.1%) | 1 (4.6%) |
| Nonspecific pathologya | 4 (12.5%) | 1 (4.6%) |
| Previous brain surgery | ||
| Yes | 15 (46.9%) | 10 (45.5%) |
| No | 17 (53.1%) | 12 (4.6%) |
| Seizure type | ||
| FAS | 4 (12.5%) | 2 (9.1%) |
| FIAS | 11 (34.4%) | 6 (27.3%) |
| FBTCS | 1 (3.1%) | 0 (0%) |
| FAS + FIAS | 4 (12.5%) | 3 (13.6%) |
| FAS + FBTCS | 2 (6.3%) | 2 (9.1%) |
| FIAS + FBTCS | 8 (25%) | 7 (31.8%) |
| FAS + FIAS + FBTCS | 2 (6.3%) | 2 (9.1%) |
- Abbreviations: FAS, focal aware seizure; FBTCS, focal to bilateral tonic–clonic seizures; FIAS, focal impaired awareness seizure; FCD, focal cortical dysplasia; FLE, frontal lobe epilepsy; HS, hippocampal sclerosis; MRI, magnetic resonance imaging; PCE, posterior cortex epilepsy; TLE, temporal lobe epilepsy.
- a Nonspecific pathology, such as white matter changes or atrophy, refers to findings on a cerebral MRI that are not specific to a particular neurological disorder.
| Study 1 (n = 32) | Study 2 (n = 22) | |||
|---|---|---|---|---|
| T0 | T1 | T0 | T2 | |
| Carbamazepine | 14 (43.8%) | 11 (34.4%) | 10 (45.5%) | 8 (36.4%) |
| Valproic acid | 5 (15.6%) | 6 (18.8%) | 5 (22.7%) | 5 (22.7%) |
| Clobazam | 7 (21.9%) | 7 (21.9%) | 5 (22.7%) | 5 (22.7%) |
| Levetiracetam | 7 (21.9%) | 6 (18.8%) | 5 (22.7%) | 5 (22.7%) |
| Lacosamide | 14 (43.8%) | 12 (37.5%) | 10 (45.5%) | 9 (40.9%) |
| Eslicarbazepine | 5 (15.6%) | 4 (12.5%) | 3 (13.6%) | 3 (13.6%) |
| Zonisamide | 4 (12.5%) | 4 (12.5%) | 2 (9.1%) | 2 (9.1%) |
| Topiramate | 1 (3.1%) | 1 (3.1%) | 1 (4.5%) | 1 (4.5%) |
| Lamotrigine | 10 (31.3%) | 11 (34.4%) | 8 (36.4%) | 8 (36.4%) |
| Clonazepam | 3 (9.4%) | 4 (12.5%) | 3 (13.6%) | 1 (4.5%) |
| Perampanel | 12 (37.5%) | 12 (37.5%) | 8 (36.4%) | 4 (18.2%) |
| Brivaracetam | 14 (43.8%) | 13 (60.6%) | 8 (36.4%) | 7 (31.8%) |
| Oxcarbazepine | 1 (3.1%) | 1 (3.1%) | 1 (4.5%) | 1 (4.5%) |
| Diazepam | 1 (3.1%) | 0 (0%) | 1 (4.5%) | 0 (0%) |
| Pregabalin | 2 (6.3%) | 1 (3.1%) | 2 (9.1%) | 1 (4.5%) |
| Lorazepam | 1 (3.1%) | 1 (3.1%) | 1 (4.5%) | 1 (4.5%) |
| Cannabidiol | 1 (3.1%) | 1 (3.1%) | 1 (4.5%) | 1 (4.5%) |
| Vigabatrin | 1 (3.1%) | 1 (3.1%) | 1 (4.5%) | 1 (4.5%) |
| SSRI | 4 (12.5%) | 4 (12.5%) | 3 (13.6%) | 3 (13.6%) |
| SNRI | 1 (3.1%) | 1 (3.1%) | 1 (4.5%) | 1 (4.5%) |
- Abbreviations: SNRI, Selective Serotonin and Norepinephrine Reuptake Inhibitor; SSRI, Selective Serotonin Reuptake Inhibitor; T0, baseline assessment; T1, short-term assessment; T2, long-term assessment.
| Study 1 (n = 32) | Study 2 (n = 22) | |||||
|---|---|---|---|---|---|---|
| T0 | T1 | P | T0 | T2 | P | |
| Number of ASMs | 3.25 ± 0.98 | 2.94 ± 1.05 | 0.023 | 3.41 ± 1.05 | 2.82 ± 1.06 | 0.009 |
| Total DDD of concomitant ASMs | 3.84 ± 1.14 | 3.25 ± 1.24 | 0.0001 | 4 ± 1.18 | 3.14 ± 1.28 | 0.001 |
| Total DDD including CNB | — | 4.00 ± 1.56 | 0.360 | — | 4.10 ± 1.26 | 0.634 |
| CNB dose (mg/day) | — | 172.73 ± 45.58 | — | 197.73 ± 28.77 | ||
| 50 | — | 1 (3.1%) | — | 0 (0%) | ||
| 100 | — | 6 (18.8%) | — | 0 (0%) | ||
| 150 | — | 6 (18.8%) | — | 4 (18.2%) | ||
| 200 | — | 19 (59.4%) | — | 15 (68.2%) | ||
| 250 | — | 0 (0%) | — | 3 (13.6%) | ||
| Seizures per month | 29.53 ± 65.00 | 17.97 ± 53.63 | 0.0001 | 38.32 ± 77.01 | 20.73 ± 47.49 | 0.001 |
| Seizure reduction (%) | — | 45.13 ± 66.53 | — | 28.70 ± 83.32 | ||
| Seizure changes | ||||||
| < 25% decrease | — | 26 (81.3%) | 17 (77.3%) | |||
| ≥ 25% decrease | — | 23 (71.9%) | — | 16 (72.7%) | ||
| ≥ 50% decrease | — | 19 (59.4%) | — | 11 (50.0%) | ||
| ≥ 75% decrease | — | 14 (43.8%) | — | 6 (27.3%) | ||
| 100% decrease | — | 9 (28.1%) | — | 3 (13.6%) | ||
| Increase | — | 3 (9.4%) | — | 3 (13.6%) | ||
| No changes | — | 3 (9.4%) | — | 2 (9.1%) | ||
- Note: P for Total DDD including CNB corresponds to the differences between the DDD at T0 (without CNB) and T1 (including CNB; Study 1) and between the DDD at T0 (without CNB) and T2 (including CNB; Study 2).
- Abbreviations: ASM, antiseizure medication; CNB, cenobamate; DDD, defined daily dose; T0, baseline assessment; T1, short-term assessment; T2, long-term assessment.
3.2 Differences in cognition, negative affectivity, and QOL changes at 3 and 6 months
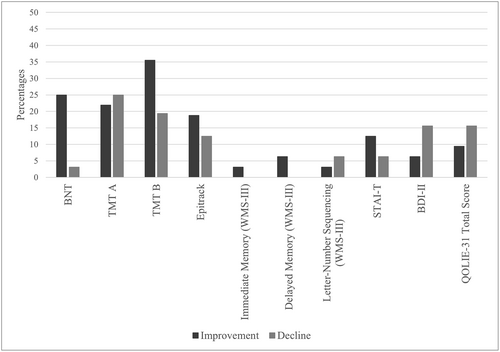
In Study 1, no significant differences were found in cognitive, emotional, or QOL variables. Considering reliable changes from T0 to T1 in terms of global cognitive performance (ie, contemplating all cognitive variables), 86.7% of patients showed no reliable changes, 18.95% improved, and 11.1% had a decline.
By cognitive domains, 71.9% of patients showed no reliable changes in visual confrontation naming, while 25% showed an improvement and 3.1% a deterioration. Regarding attention and executive functions, 68.7% of patients showed no changes, followed by 18.8% who showed an improvement and 12.5% who had a deterioration. Concerning immediate memory, 96.9% of patients showed no reliable changes and 3.1% showed an improvement, while in delayed memory 93.7% showed no changes and 6.3% improved.
Concerning negative affectivity, 78.1% of patients had no reliable changes in depressive symptoms, although 6.3% of the patients showed an improvement and 15.6% worsened. Additionally, in trait anxiety, 81.2% of patients had no changes, 12.5% showed an improvement and 6.3% had a decline.
Regarding QOL, 75% of patients showed no changes, followed by 15.6% who experienced a decline, and 9.4% who improved.
Reliable changes from T0 to T1 are shown in Figure 1.
Finally, it is noteworthy that the percentage of seizure reduction was not related to the changes in the BDI-II, the STAI-T, or the QOLIE's total scores. Moreover, seizure freedom at T1 did not predict significant changes in any cognitive, emotional, or QOL variables.
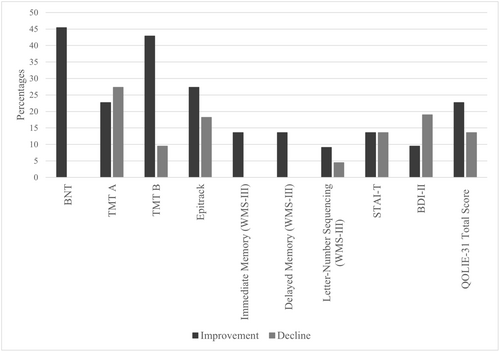
Regarding Study 2, no significant differences were found in cognition, negative affectivity, or QOL. Considering all cognitive measures together, 66.6% of patients showed no reliable changes. However, 24.9% showed an improvement in cognitive performance from T0 to T2, and 8.5% showed a worsening.
Differentiating by domains, 54.6% of patients showed no reliable changes in visual confrontation naming, followed by 45.4% who showed an improvement. Regarding attention and executive functions, 54.5% showed no reliable changes between T0 and T2, 27.3% had an improvement, and 18.2% worsened. In immediate memory, 86.4% showed no changes, and 13.6% had an improvement. Likewise, in delayed memory, 86.4% of patients showed no reliable changes and 13.6% of patients showed an improvement.
The mean and SD of the different cognitive variables of Studies 1 and 2 are shown in Table 5.
| Study 1 (n = 32) | Study 2 (n = 22) | |||||
|---|---|---|---|---|---|---|
| T0 | T1 | p | T0 | T2 | p | |
| BNT | 42.38± 2.24 | 43.97 ± 2.11 | 0.362 | 42.05 ± 3.08 | 44.77 ± 2.98 | 0.066 |
| Epitrack | 27.34 ± 1.31 | 27.94 ± 1.26 | 0.282 | 27.27 ± 1.90 | 28.59 ± 2.00 | 0.825 |
| Interference | 22.03 ± 1.96 | 22.25 ± 2.37 | 0.484 | 22.32 ± 2.77 | 23.32 ± 3.79 | 0.352 |
| TMT A | 59.84 ± 13.38 | 62.00 ± 14.83 | 0.638 | 69.73 ± 19.44 | 75.55 ± 22.82 | 0.931 |
| TMT B | 157.84 ± 16.98 | 160.87 ± 22.35 | 0.982 | 147.25 ± 21.68 | 121.00 ± 11.48 | 0.525 |
| Maze Test | 45.41 ± 6.06 | 42.59 ± 4.54 | 0.162 | 49.09 ± 8.80 | 51.91 ± 1.14 | 0.545 |
| Verbal fluency | 18.38 ± 1.72 | 18.91 ± 1.55 | 0.744 | 19.18 ± 2.33 | 20.41 ± 2.21 | 0.270 |
| Immediate memory | 3.53 ± 0.17 | 3.72 ± 0.19 | 0.051 | 3.68 ± 0.21 | 3.73 ± 0.28 | 0.936 |
| WMS-III | ||||||
| Immediate auditory memory | 13.03 ± 1.16 | 15.28 ± 1.02 | 0.764 | 13.73 ± 1.48 | 16.73 ± 1.49 | 0.314 |
| Immediate visual memory | 14.38 ± 1.24 | 18.13 ± 1.92 | 0.697 | 14.05 ± 1.63 | 18.50 ± 1.71 | 0.623 |
| Immediate memory | 27.09 ± 2.03 | 31.72 ± 2.03 | 0.540 | 27.32 ± 2.67 | 33.82 ± 3.01 | 0.071 |
| Delayed auditory memory | 13.81 ± 1.01 | 15.25 ± 0.97 | 0.320 | 14.59 ± 1.34 | 17.36 ± 1.23 | 0.233 |
| Delayed visual memory | 14.69 ± 1.14 | 16.78 ± 1.34 | 0.713 | 14.77 ± 1.47 | 18.05 ± 1.79 | 0.938 |
| Delayed memory | 35.53 ± 2.46 | 39.66 ± 2.62 | 0.868 | 37.09 ± 3.28 | 44.14 ± 3.67 | 0.637 |
| Delayed auditory recognition | 7.34 ± 0.62 | 7.66 ± 0.62 | 0.177 | 7.23 ± 0.83 | 8.77 ± 0.87 | 0.939 |
| Letter–number sequencing | 6.69 ± 0.60 | 6.97 ± 0.49 | 0.641 | 6.41 ± 0.76 | 7.27 ± 0.81 | 0.438 |
| BDI-II | 9.16 ± 1.33 | 11.94 ± 1.81 | 0.399 | 8.62 ± 1.66 | 9.86 ± 2.02 | 0.825 |
| STAI-T | 25.38 ± 1.83 | 25.16 ± 2.47 | 0.948 | 26.41 ± 2.15 | 23.41 ± 2.06 | 0.952 |
| QOLIE-31 | 57.25 ± 3.22 | 55.86 ± 3.38 | 0.182 | 55.04 ± 3.67 | 58.56 ± 3.90 | 0.261 |
| Seizure worry | 46.52 ± 5.38 | 55.97 ± 4.89 | 0.656 | 42.77 ± 7.12 | 51.32 ± 5.63 | 0.373 |
| Overall QOL | 65.49 ± 3.80 | 65.31 ± 3.89 | 0.971 | 65.24 ± 4.86 | 66.25 ± 2.89 | 0.357 |
| Emotional well-being | 65.00 ± 3.78 | 60.13 ± 4.25 | 0.432 | 68.91 ± 4.12 | 68.00 ± 3.53 | 0.593 |
| Energy | 61.56 ± 3.28 | 57.19 ± 4.07 | 0.506 | 63.86 ± 3.25 | 61.05 ± 5.14 | 0.385 |
| Cognition self-rating | 56.46 ± 5.41 | 52.52 ± 5.28 | 0.136 | 52.47 ± 6.60 | 56.47 ± 6.95 | 0.120 |
| Medication effects | 56.96 ± 4.79 | 58.59 ± 5.03 | 0.268 | 54.55 ± 5.89 | 57.44 ± 6.37 | 0.401 |
| Social functioning | 51.69 ± 4.34 | 53.50 ± 3.98 | 0.350 | 45.55 ± 5.21 | 50.77 ± 4.68 | 0.758 |
- Abbreviations: BDI-II, Beck Depression Inventory-II; BNT, Boston Naming Test; QOLIE-31, Quality-Of-Life Epilepsy Inventory; QOL, Quality of Life; STAI-T, Trait Anxiety Scale; T0, baseline assessment; T1, short-term assessmentT2, long-term assessment; TMT, Trail Making Test; WMS-III, Wechsler Memory Scale-III.
Regarding negative affectivity and QOL, 71.5% of patients showed no reliable changes in depressive symptoms, 9.5% had an improvement, and 19% worsened. In terms of trait anxiety, no reliable changes were found for 72.8% of patients, followed by 13.6% who showed an improvement, and 13.6% who showed a decline. Finally, for QOL, 63.7% of patients showed no reliable changes, 22.7% showed an improvement in QOL scores and 13.6% experienced a deterioration.
Reliable changes between T0 and T2 are shown in Figure 2.
In Study 2, no relationships were found between the percentage of seizure reduction and the changes in the BDI-II, STAI-T, and QOLIE's total scores. Furthermore, seizure freedom at T2 also did not predict changes in cognitive, emotional, or QOL variables.
4 DISCUSSION
As far as we know, this is the first study to examine the possible effects of CNB on different cognitive domains, as well as on negative affectivity (ie, anxiety and depression) and QOL in the same sample of patients with epilepsy. Our results suggest that CNB is a safe pharmacological treatment in terms of cognitive performance, negative affectivity, and QOL since no related adverse effects were found after 6 months of adjunctive treatment.
Regarding cognition, no significant differences were found in attention, executive functions, or visual confrontation naming after CNB treatment, with all patients showing an improvement or no change in their memory scores. These results are of interest to this population, due to the high prevalence of patients with memory deficits35 and considering that some ASMs can reduce memory performance.36 Furthermore, patients remained stable in most cases in both Studies 1 and 2, suggesting that CNB treatment seems not to produce time or dose-dependent effects on cognition variables.
Although there are no studies that comprehensively assess cognition in humans after CNB treatment, Schuetz et al., (2022) explored the effects of CNB on cognition using the EpiTrack after 3 months, showing that most patients remained stable (72%), followed by 16% who improved and 12% who showed a deterioration. Similar results have been found in Study 1, since 68.7% of patients remained stable, 18.8% improved and 12.5% showed a deterioration. Examining whether other variables might be influencing changes in EpiTrack, we found that 5 patients out of 8 taking concomitant lamotrigine after 3 months of CNB administration showed a significant improvement at T2, with only one patient showing a deterioration. Following this result, lamotrigine has been shown to have positive effects on cognition, especially on executive functions.37 However, concomitant treatment with sodium channel blockers drugs in patients taking CNB is associated with a higher incidence of adverse effects,5 so studies with larger samples are needed.
In this regard, it is noteworthy that these changes could be influenced by concomitant ASMs. The DDD of other ASMs significantly decreased in both studies, and previous literature has shown that higher drug load is negatively related to cognitive performance.38-40 Nevertheless, when the CNB load was also considered, the DDD increased from T0 to T1 and T2, but not significantly. Therefore, we suggest that, although decreasing the load of other drugs may have contributed to better cognitive performance, the cognitive changes are more likely to be due to the introduction of CNB into pharmacological treatment and its clinical effects.
Regarding depressive symptoms, although most patients showed no changes, five patients showed a worsening after 3 months, and four at 6 months. Depression is one of the most common comorbidities in epilepsy,41 being an important variable to be considered in this population. In this line, it has been found that CNB could lead to suicidal behavior and ideation,3 so the monitoring of patients with concomitant antidepressant drugs and CNB treatment is recommended.42
In the case of trait anxiety, the percentage of patients who worsened was doubled in Study 2 (13.6%) compared to Study 1 (6.3%). However, as Study 2 had a smaller sample, this difference was due to only one subject. Thus, 19 of the 22 participants in Study 2 showed no change in anxiety level or even an improvement. Congruently, Chung et al. (2020) found in a randomized, double-blind, placebo-controlled study that anxiety was an infrequent adverse event, which was reported only in 0.9% of patients who were taking CNB.5
Concerning QOL, we found a higher percentage of patients who improved their QOL scores in T2 (22.7%) compared to T1 (9.4%). Studying the changes in the different subscales, we found that the most pronounced differences were found in the seizure worry subscale. This suggests that the increase in QOL could be related, at least in part, with the reduction of the seizure frequency, since other studies have found that it is a strong predictor of QOL.43 Nevertheless, in our study no significant relationships were found between the percentage of seizure reduction and QOL, which could be due to the limited sample.
In this line, it was found a significant reduction in the seizure number after three and 6 months of CNB administration. More specifically, the average percentage of seizure reduction in Study 1 was 45.1%, with 28.1% achieving seizure freedom. Analyzing these results over a longer period (ie, 6 months), a lower mean seizure reduction rate of 28.7% was observed. However, the percentage of patients with a reduction of at least 50% was quite similar in both studies (59.4% in Study 1 and 50% in Study 2). These results are in line with those obtained in clinical trials, in which 40–69.9% of patients were found to show response rates of ≥50% at 3 months,6, 9 but also with nonclinical trials, where 42% of patients showed a seizure reduction of at least 50% at 3 months.14 In the same context, Villanueva et al. (2023) found an average percentage of seizure reduction of 45.9% after 3 months of CNB treatment, closely related to our results, with 58.8% of patients with a response rate ≥ 50%, increasing until 65% after 6 months of treatment.
Given the real-world nature of this study, the results obtained about the effects of CNB are particularly relevant to the clinical setting. However, this study is not exempt from limitations: firstly, the results, especially those of Study 1, should be considered with caution, as many patients were still in the titration period. Therefore, we consider that the results of Study 2 provide more robust data, as patients reached the target dose. Secondly, patients in Study 2 performed the EpiTrack three times. However, due to the lack of data to categorize reliable changes based on the results of a third assessment, the proposed criteria for change in the second administration were used. Therefore, improvements in the EpiTrack could be related to the fact of having performed this test three times. Thirdly, although intra-subject differences have been assessed, taking their baseline performance as a reference, a control group would provide more information. Fourth, using three covariates with a limited sample, especially in Study 2, could bias the results. A larger and more homogeneous sample could help also to determine possible significant differences, although 32 patients with two assessments should be enough to detect changes clinically relevant. Finally, although this study comprises a follow-up of half a year, it would be useful to follow up throughout longer periods.
5 CONCLUSION
In conclusion, CNB adjunctive treatment seems to be an effective ASM without any long-term adverse events in cognition, negative affectivity, and QOL. Specifically, it has been found that the vast majority of patients remained stable in their cognitive performance and in negative affectivity and QOL. Therefore, we conclude that CNB could be an ASM of choice in drug-resistant epilepsy patients, given the apparent safety of the drug and the absence of adverse effects found.
AUTHOR CONTRIBUTIONS
VV and KH conceptualized the manuscript. MG and KH established the diagnosis of drug-resistant epilepsy, recruited the patients for neuropsychological evaluation, and provided clinical data. JC-A, AL-G, and PT-P participated in the neuropsychological assessment and interpretation of the data. JC-A managed the literature search, undertook the statistical analyses, and wrote the original draft. JC-A, EG-B, IC-L, KH, and VV wrote, reviewed, and edited the manuscript. All authors contributed to the article and approved the submitted version.
ACKNOWLEDGMENTS
This work was supported by the project PID2020-118992RB-I00 funded by MCIN/AEI/10.13039/501100011033. J.C.A. was supported by the Generalitat Valenciana (Valencian Government) under grant [number ACIF/2021/094].
CONFLICT OF INTEREST STATEMENT
Author V. Villanueva has received honoraria and/or research funds from Angelini Pharma, Bial, Eisai, Jazz Pharmaceuticals, Neuraxpharm, Novartis, Nutricia, Takeda, UCB Pharma, and Xenon. The remaining authors have no conflicts of interest. We confirm that we have read the Journal's position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
Open Research
DATA AVAILABILITY STATEMENT
Data can be available to other researchers by contacting the corresponding author, KH, upon reasonable request.